Abstract
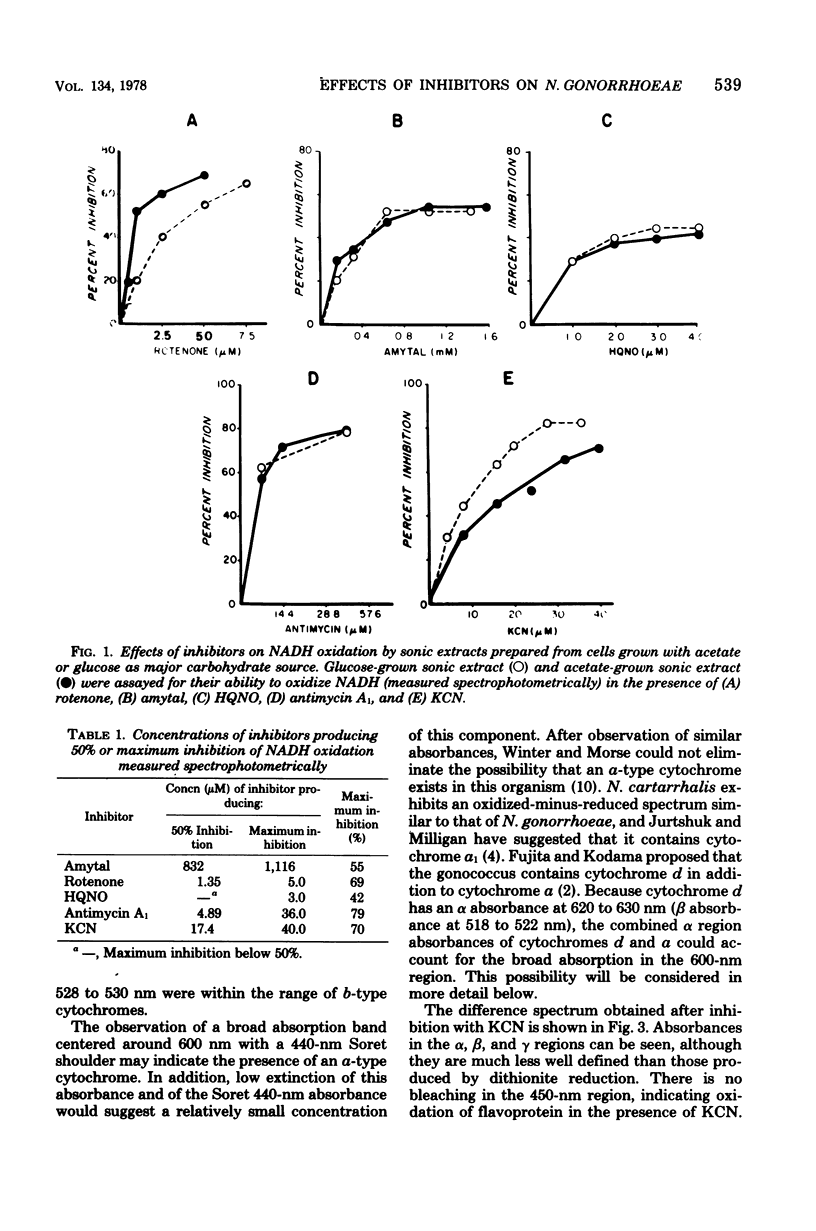
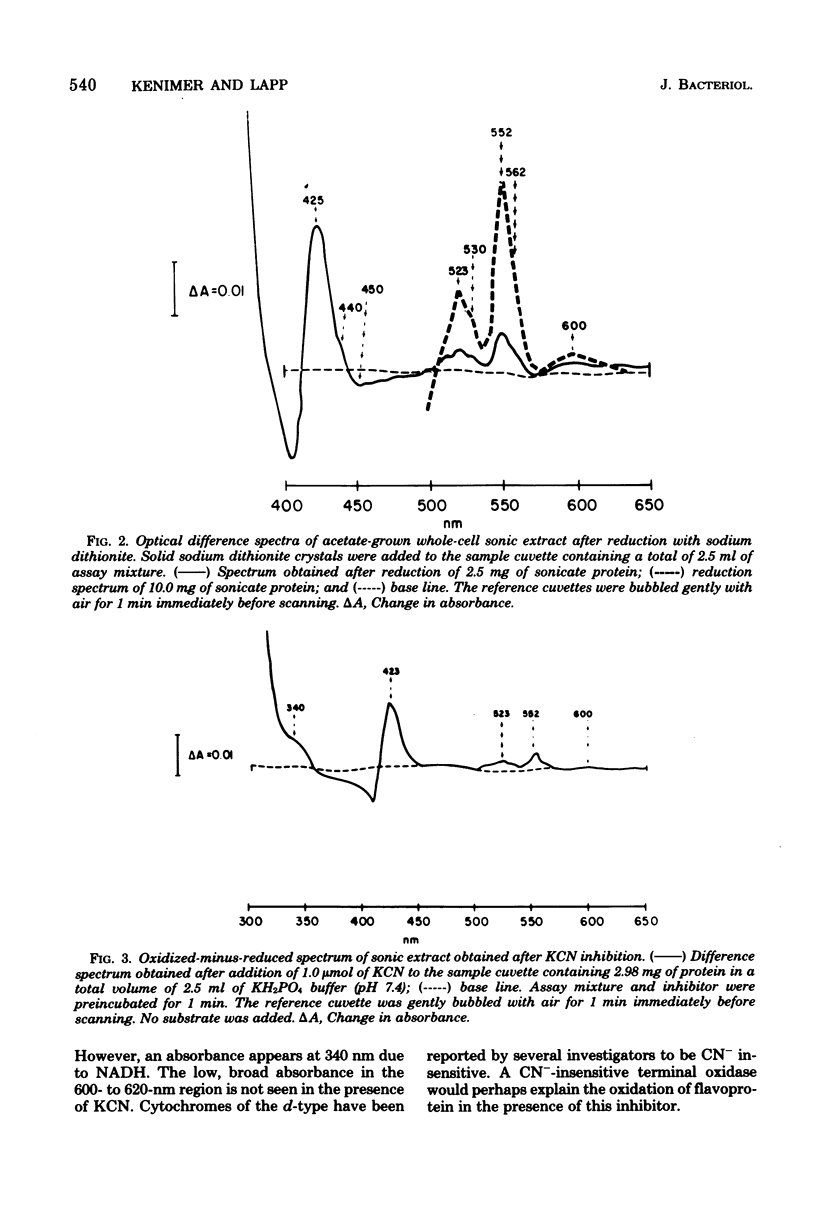
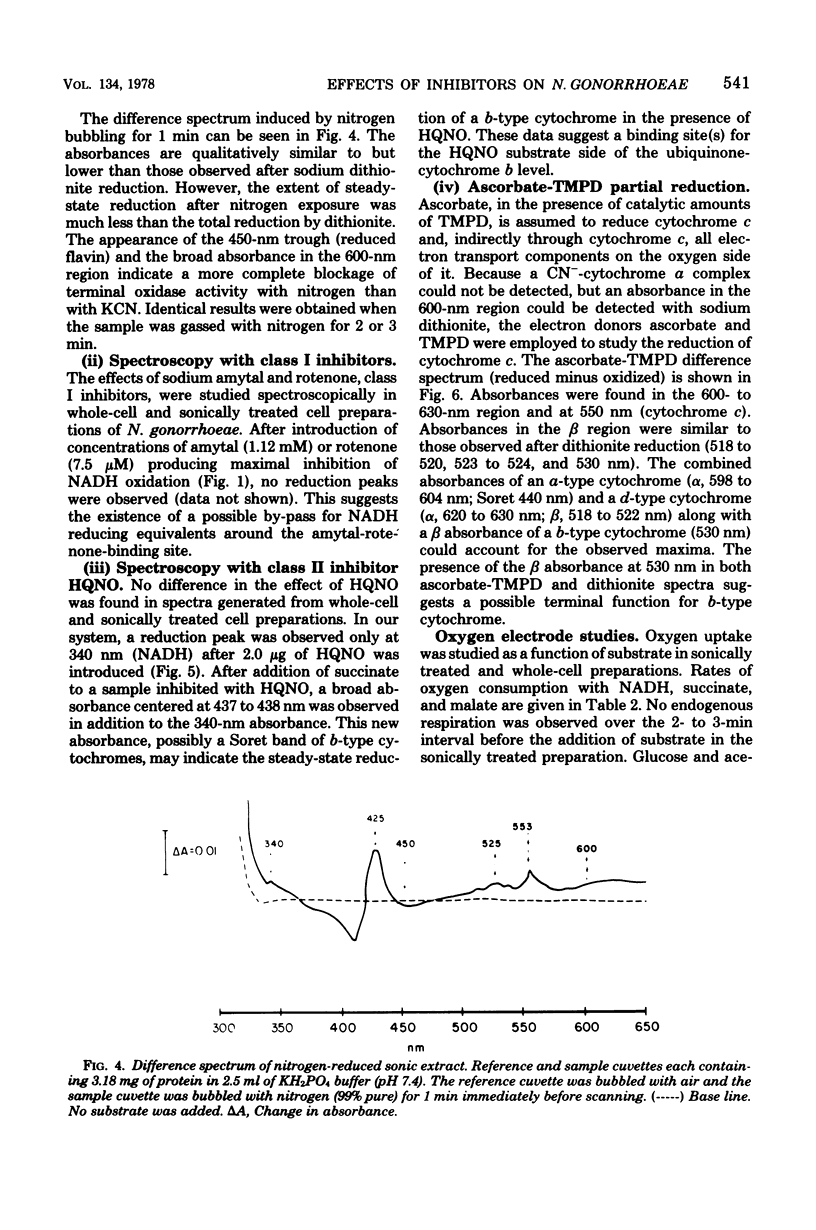
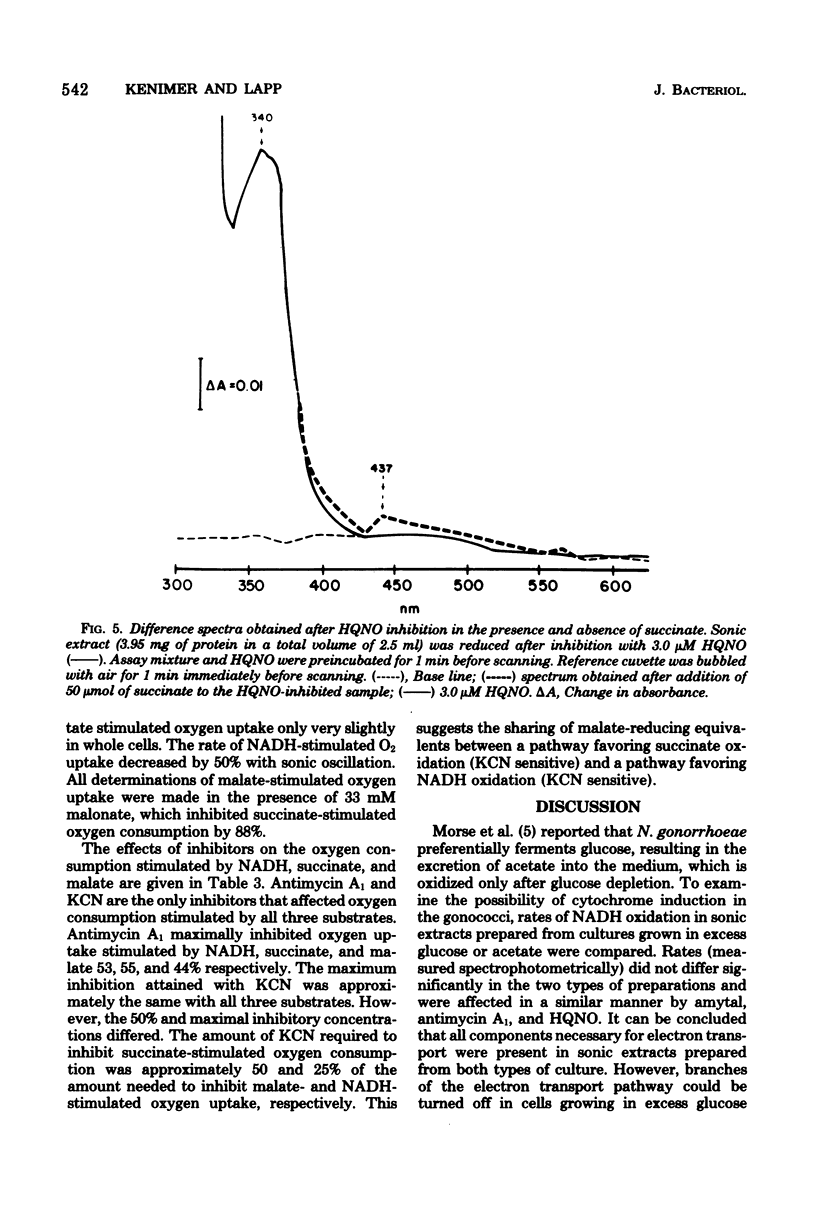
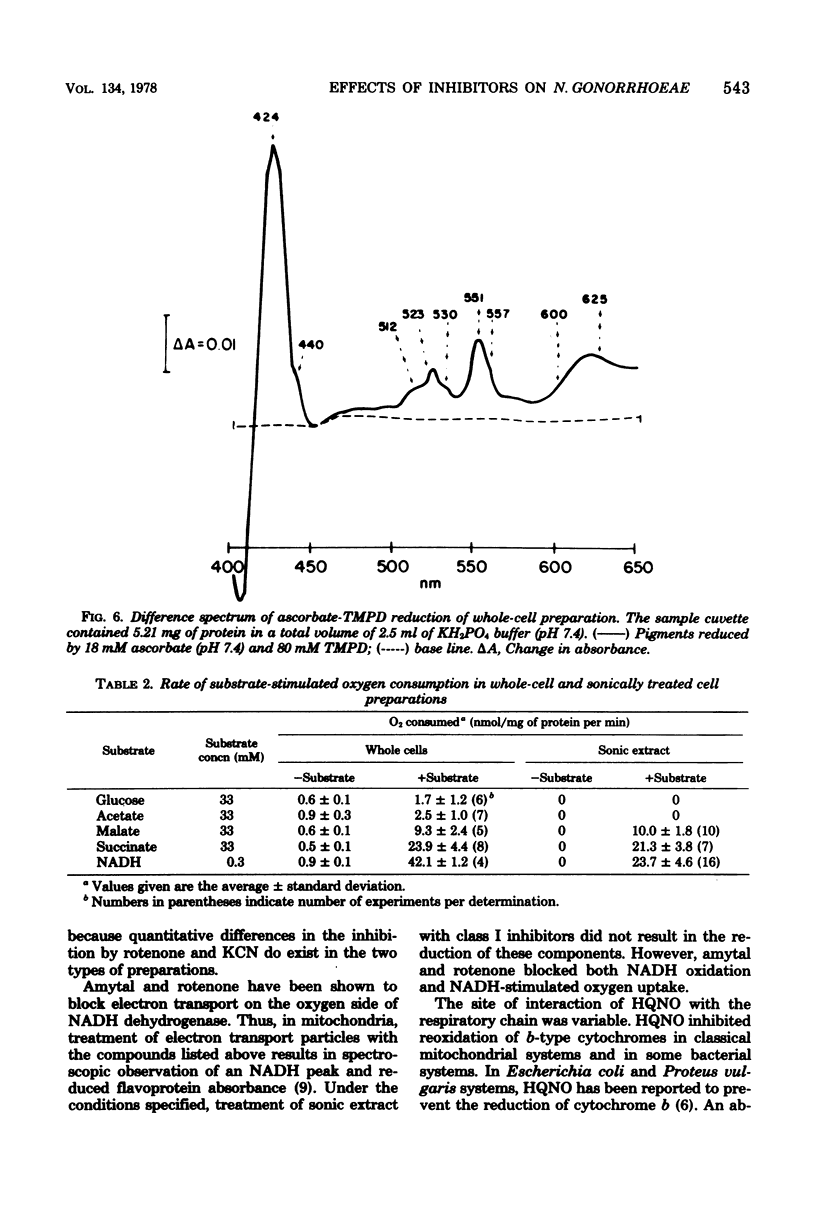
The electron transport system of Neisseria gonorrhoeae was partially characterized by using spectrophotometric, spectroscopic, and oxygen consumption measurements. The effects of selected electron transport inhibitors (amytal, rotenone, 2-heptyl-4-hydroxyquinoline, antimycin A1, and potassium cyanide [KCN]) on electron transfer in whole-cell and sonically treated whole-cell preparations of N. gonorrhoeae were examined. The oxidation of reduced nicotinamide adenine dinucleotide, measured as a decrease in absorbance at 340 nm, was inhibited by each of the compounds tested. Oxygen consumption stimulated by reduced nicotinamide adenine dinucleotide was also inhibited, whereas oxygen uptake stimulated by succinate and malate was inhibited by KCN alone, suggesting the presence of a KCN-sensitive terminal oxidase. Room temperature optical difference spectra indicate an operational electron bypass around the amytal-rotenone-binding site. Difference spectra in the presence of 2-heptyl-4-hydroxyquinoline suggest a possible site of interaction of this compound at the substrate side of cytochrome b. Reduced-minus-oxidized spectra of ascorbate-tetramethyl-p-phenylenediamine suggest the participation of b-, a-, and d-type cytochromes in terminal oxidase activity. Hence, N. gonorrhoeae appears to have an electron transport chain containing cytochrome c, two b-type cytochromes (one of which has an oxidase function), and possibly a- and d-type cytochromes. An abbreviated chain exists through which succinate and malate can be oxidized directly by a KCN-sensitive component.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Quantitation of the tetramethyl-p-phenylenediamine oxidase reaction in Neisseria species. Appl Microbiol. 1974 Dec;28(6):1079–1081. doi: 10.1128/am.28.6.1079-1081.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



