Abstract
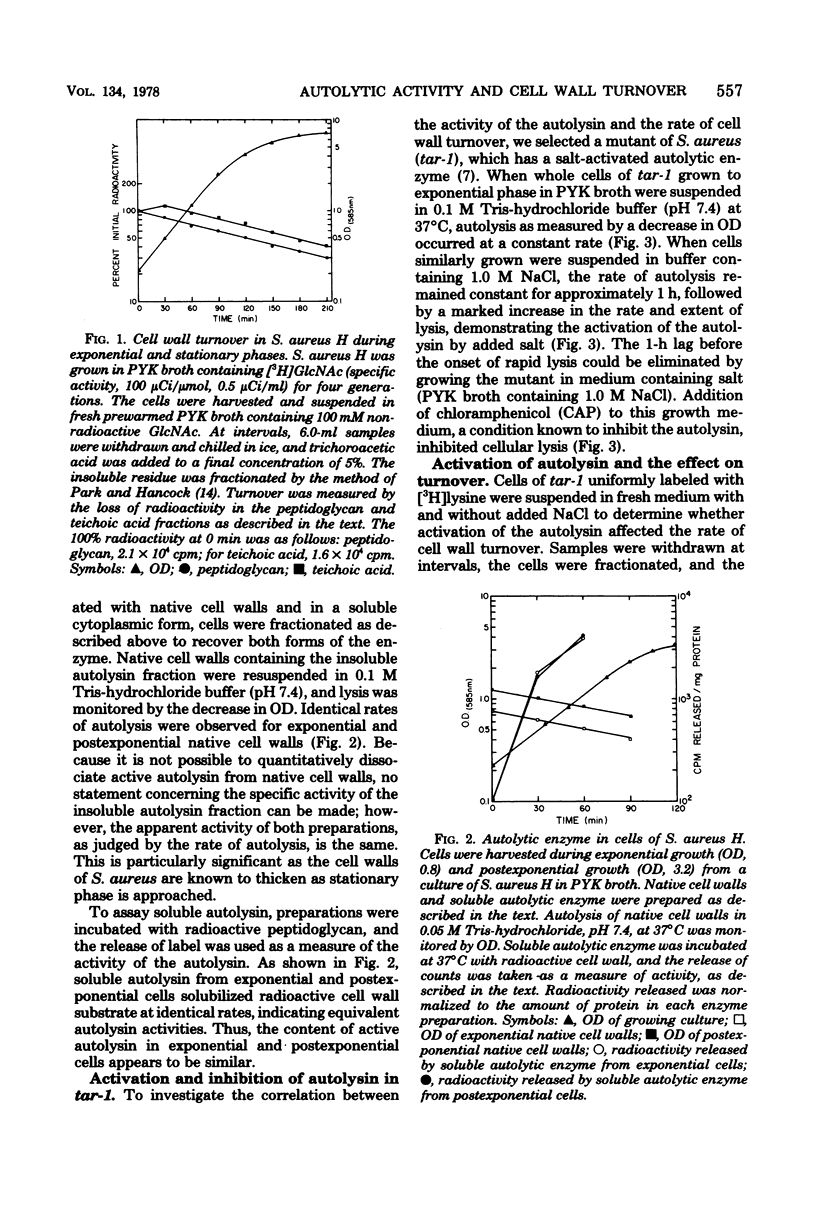
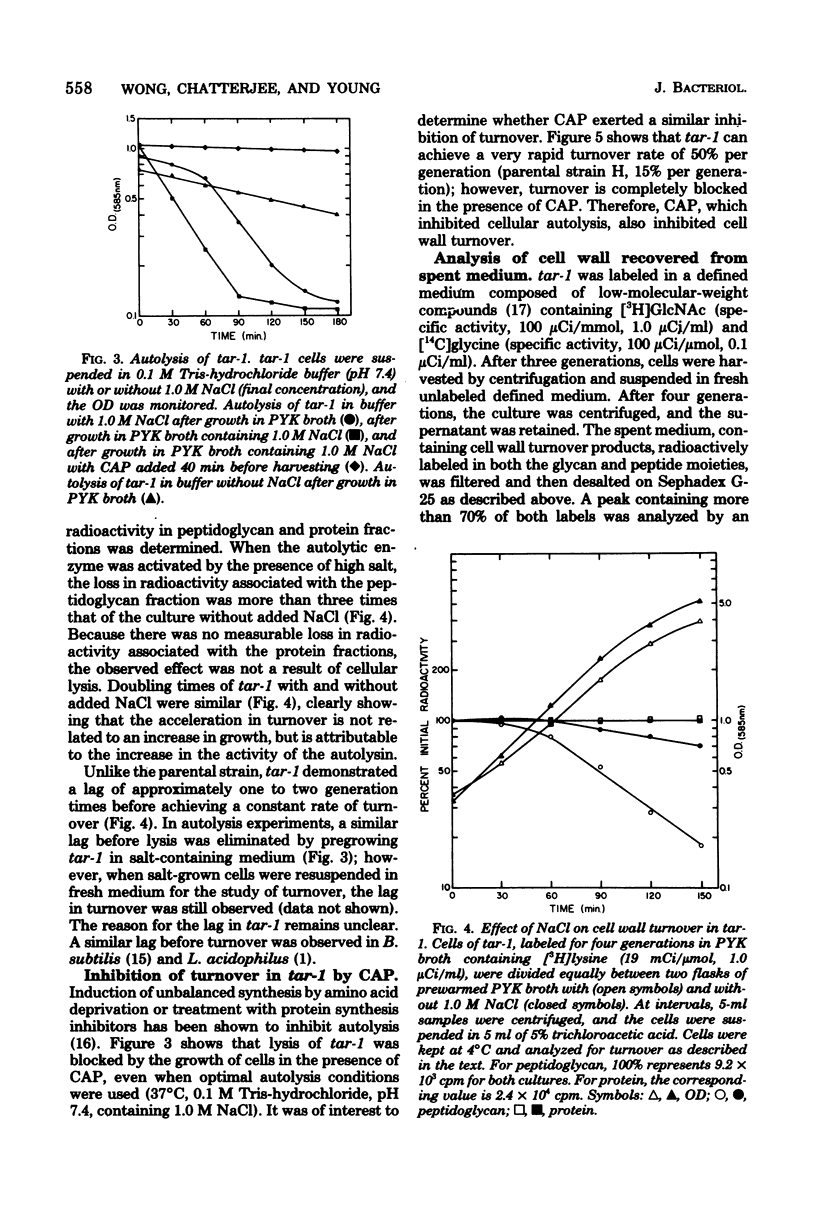
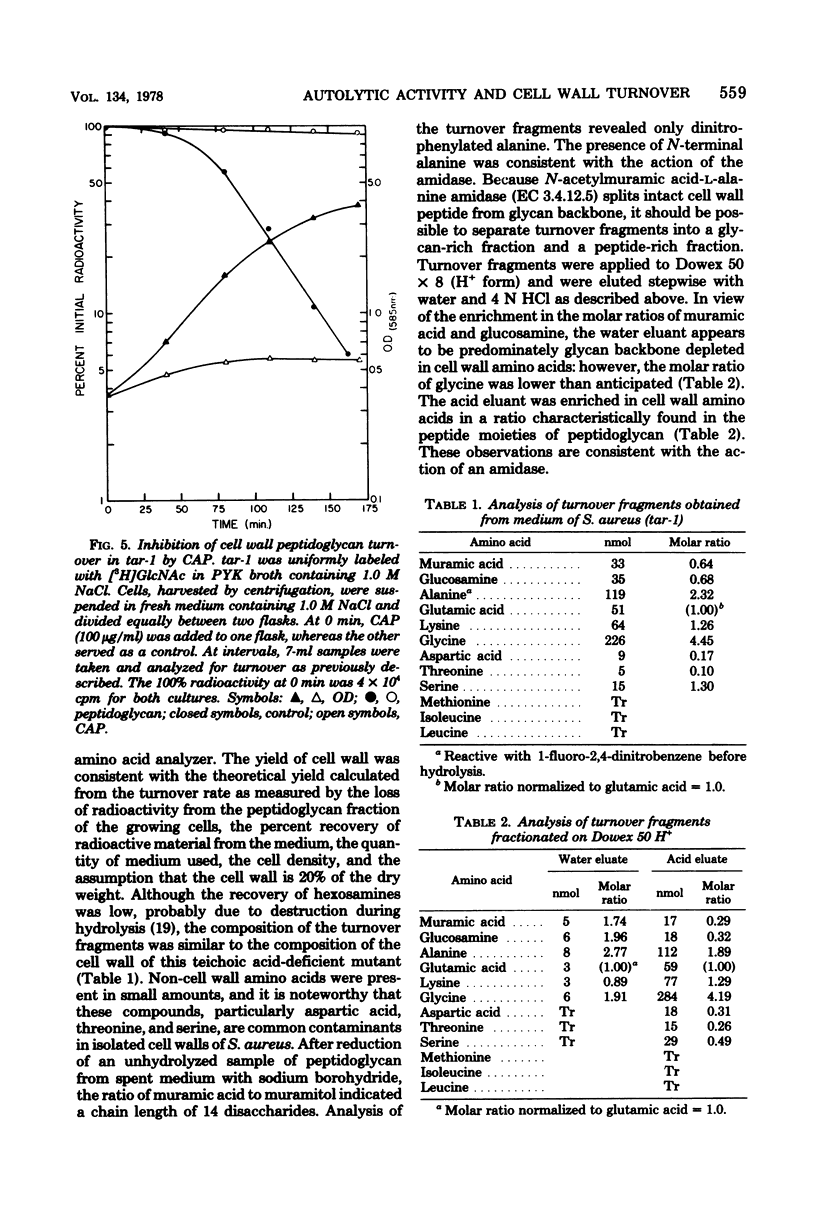
Cell wall turnover was examined in parent and mutant strains of Staphylococcus aureus. Peptidoglycan and teichoic acid were observed to undergo turnover in the wild-type strain during exponential growth; however, the rate of turnover did not decrease when the growth rate slowed, as the culture entered stationary phase. Isolated native cell walls and crude soluble autolytic enzyme were prepared from cells harvested during exponential and postexponential phases of growth. Native cell walls from both phases of growth autolyzed in buffer at identical rates; similarily, crude soluble enzyme from both preparations degraded radioactive cell walls at the same rate. Therefore, the activity of the autolysin in both exponential and postexponential cells was similar. The autolysis of whole cells of a mutant tar-1 was enhanced by 1.0 M NaCl. When 1.0 M NaCl was present under growing conditions, the rate of cell wall turnover was greatly increased. The presence of chloramphenicol, which inhibits whole-cell autolysis, also inhibited turnover. Analysis of the cell wall material recovered from spent medium revealed products consistent with the known mode of action of the endogenous autolysin. It is concluded that cell wall turnover in S. aureus is independent of the stage of culture growth but is dependent instead on the activity of the autolysin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Chatterjee A. N., Doyle R. J., Streips U. N. A proposed functional role for bacterial N-acetylmuramyl-L-alanine amidases. J Theor Biol. 1977 Oct 7;68(3):385–390. doi: 10.1016/0022-5193(77)90067-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E. Structural difference between walls from hemispherical caps and partial septa of Bacillus subtilis. J Bacteriol. 1973 May;114(2):790–797. doi: 10.1128/jb.114.2.790-797.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Lindsay B. Relation between cell wall turnover and cell growth in Bacillus subtilis. J Bacteriol. 1977 May;130(2):610–619. doi: 10.1128/jb.130.2.610-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Arnheim N., Sternglanz R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J Biol Chem. 1973 Oct 25;248(20):7247–7252. [PubMed] [Google Scholar]
- KLOOS W. E., PATTEE P. A. A BIOCHEMICAL CHARACTERIZATION OF HISTIDINE-DEPENDENT MUTANTS OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:185–194. doi: 10.1099/00221287-39-2-185. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]



