Abstract
Pharmacological inhibition or genetic disruption of cyclooxygenase (COX)-1 or COX-2 exacerbates the inflammatory and functional responses of the lung to environmentally relevant stimuli. To further examine the contribution of COX-derived eicosanoids to basal lung function and to allergic lung inflammation, transgenic (Tr) mice were generated in which overexpression of human COX-1 was targeted to airway epithelium. Although no differences in basal respiratory or lung mechanical parameters were observed, COX-1 Tr mice had increased bronchoalveolar lavage fluid PGE2 content compared with wild-type littermates (23.0 ± 3.6 vs 8.4 ± 1.4 pg/ml; p < 0.05) and exhibited decreased airway responsiveness to inhaled methacholine. In an OVA-induced allergic airway inflammation model, comparable up-regulation of COX-2 protein was observed in the lungs of allergic wild-type and COX-1 Tr mice. Furthermore, no genotype differences were observed in allergic mice in total cell number, eosinophil content (70 vs 76% of total cells, respectively), and inflammatory cytokine content of bronchoalveolar lavage fluid, or in airway responsiveness to inhaled methacholine (p > 0.05). To eliminate the presumed confounding effects of COX-2 up-regulation, COX-1 Tr mice were bred into a COX-2 null background. In these mice, the presence of the COX-1 transgene did not alter allergen-induced inflammation but significantly attenuated allergen-induced airway hyperresponsiveness, coincident with reduced airway leuko-triene levels. Collectively, these data indicate that COX-1 overexpression attenuates airway responsiveness under basal conditions but does not influence allergic airway inflammation.
Cyclooxygenase (COX)3 catalyzes the committed step in the biosynthetic pathway of prostanoid formation from arachidonic acid. Both COX-1 and COX-2, the constitutive and inducible COX isoforms, respectively, are expressed in the lung and are important in the pulmonary response to allergic and nonallergic inflammatory stimuli (1, 2). In normal mouse lung, immunohistochemical analysis indicates that COX-1 is constitutively expressed at high levels in airway smooth muscle cells, airway epithelial (Clara) cells, and alveolar type II cells (3). Similarly, expression of COX-1 in human lung is abundant and widespread (4). Conversely, COX-2 expression is low and is limited primarily to airway Clara cells in naive mice (3) and in humans (4), but is up-regulated in numerous cell types in response to inflammatory stimuli and in certain pathological conditions.
COX-derived prostanoids include PGE2, PGD2, PGF2α, PGI2 (prostacyclin), and thromboxane A2, all of which can exert receptor-mediated effects in the airways and/or vasculature of the lung. For example, PGE2 and PGI2 possess bronchodilatory activity (5, 6), whereas PGD2 and PGF2α are bronchoconstrictive (6, 7). As such, COX products have received considerable attention regarding their potential roles in various lung diseases, with a particular emphasis on asthma and allergic inflammation. In humans, inhaled PGE2 inhibits the early- and late-phase pulmonary responses to inhaled allergen (8, 9) and attenuates aspirin- and exercise-induced bronchoconstriction in susceptible patients (10, 11). However, the interactions and cumulative effects of other COX-derived prostanoids with PGE2 in asthma and allergic inflammation are complex and not completely understood (12). In a murine model of allergic lung inflammation induced by sensitization and challenge with OVA, genetic deficiency of COX-1 or COX-2 increases airway inflammation and decreases lung compliance, whereas COX-1 deficiency also results in increased lung resistance (2). The administration of a selective COX-1 or COX-2 inhibitor to wild-type (WT) mice similarly increases airway inflammation and hyperresponsiveness following OVA (13). Collectively, these observations suggest that a COX product(s) is protective in allergic lung inflammation and that the exaggerated inflammatory and functional responses are, at least in part, a result of its impaired production.
To further examine the roles of COX and COX-derived prostanoids in the lung under normal and inflammatory conditions, we generated transgenic (Tr) mice that overexpress human COX-1 specifically in airway Clara cells. Expression and localization of the transgene were confirmed by molecular and immunohisto-chemical techniques and its functionality was assessed by quantifying prostanoid levels in bronchoalveolar lavage (BAL) fluid. Functional effects of the transgene on respiration and pulmonary mechanics were evaluated using noninvasive and invasive techniques. Finally, the inflammatory and functional responses of COX-1 Tr mice were compared with WT littermates in an established model of allergic airway inflammation.
Materials and Methods
All studies were conducted in accordance with principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee.
Construction and identification of COX-1 Tr mice
The coding region of human COX-1 cDNA (GenBank accession no. M59979) was cloned into the HindIII-NotI sites of the pcDNA2.1-CC10-bovine growth hormone (bGH) vector (provided by Dr. J. A. Whitsett, Children’s Hospital Medical Center, Cincinnati, OH). This vector contains the murine Clara cell 10-kDa protein (CC10) promoter to drive Clara cell-specific expression of the transgene and bGH/poly(A) sequences to enhance transgene mRNA stability. The linearized transgene was microinjected into pronuclei of single-cell C57BL/6 mouse embryos that were implanted into pseudopregnant mice. Identification of founders was performed by PCR analysis of genomic DNA isolated from tail biopsies. PCR analysis was performed using the following oligonucleotide primer pairs: CC10F1, 5′-CCTACAGTTCCACGACCTCTGGGT-3′, and COX1R1, 5′-GGCACAGAGGGCAGAATACGA-3′ (835-bp amplicon); CC10F2, 5′-CATACCCTCACATTACAACATCAG-3′, and COX1R2, 5′-CACAGAGGGCAGAATACGAGT-3′ (570-bp amplicon). All mice were propagated as heterozygous Trs by breeding with WT C57BL/6 mice. All animal studies described herein were approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences.
Analysis of transgene expression
RNA was isolated from various tissues using an RNeasy Midi kit (Qiagen) and tissue expression of the transgene was analyzed by RT-PCR (Superscript one-step RT-PCR System; Invitrogen Life Technologies). First-strand cDNA was synthesized from 1.0 µg of total RNA at 50°C for 30 min, followed by 2 min at 94°C. The PCR was then performed for 35 cycles, each consisting of 20 s at 94°C for denaturation, 30 s at 55°C for annealing, and 30 s at 72°C for extension. The following primers were designed on the basis of the cDNA sequences: 5′GTCTCTTGCTCCGGTTCT-3′ (sense) and 5′-GGAAGTGGGTGAAAGAGG-3′ (antisense) for human COX-1 and 5′-GCCTTCTTTGCACAACAC-3′ (sense) and 5′-GCAGGAAATAGCCACTCA-3′ (antisense) for murine COX-1. The expected sizes of the PCR products were 243 bp for human COX-1 and 460 bp for murine COX-1. PCR products were resolved on 1.2% agarose gels and visualized with ethidium bromide staining.
COX-1 protein levels in lung tissue were determined by immunoblotting. Lysates were prepared from frozen lung tissue as described previously (1), and immunoblotting for COX-1 was performed using a murine anti-sheep mAb (no. 160110; Cayman Chemical) that also recognizes human and murine COX-1. Equivalent amounts of lung lysate protein from COX-1 Tr and WT mice were separated on 10% Tris-glycine gels and transferred to nitrocellulose membranes. Membranes were immunoblotted with the primary Ab at a dilution of 1/500, followed by a HRP-conjugated bovine anti-mouse polyclonal Ab (Santa Cruz Biotechnology) at a dilution of 1/3000. Bound Abs were detected and visualized with an enhanced chemiluminescent detection system (Pierce).
Immunostaining for COX-1 was performed on formalin-fixed, paraffin-embedded lung tissue using the same murine anti-sheep mAb as above (Cayman Chemical). The detailed immunostaining protocol can be found on the National Institute of Environmental Health Sciences Laboratory of Experimental Pathology website (〈http://dir.niehs.nih.gov/dirlep/immuno/protocols.htm〉).
BAL and histological evaluation of lung tissue
Naive mice and mice used in the allergic inflammation model (see below) were killed with an overdose of sodium pentobarbital (80 mg/kg i.p.). BAL was performed with two 1.0-ml aliquots of HBSS; recovery was >80% for each mouse. Recovered BAL fluid from each mouse was processed and analyzed for total and differential cell counts by routine methods and for cytokines, eicosanoids, and/or protein content as appropriate. For histological evaluation, lungs were inflated and fixed with 4% paraformaldehyde. Sections (5–6 µm) were stained with H&E for analysis of lung architecture in naive mice and with periodic acid-Schiff for examination of inflammation in allergic mice.
Airway PG analysis
PG levels in BAL fluid were analyzed by liquid chromatography-tandem mass spectrometry. Briefly, 500 µl of BAL fluid was applied to Amprep Octadecyl C18 columns (Amersham Biosciences), followed by washes with water and then hexane. Extraction was performed with ethyl acetate that was collected in polypropylene tubes. The ethyl acetate was then evaporated under a stream of nitrogen gas, and the dried tubes were frozen and stored at −80°C until analysis. Online liquid chromatography of extracted samples was performed with an Agilent 1100 Series capillary HPLC. Separations were achieved using a Phenomenex Luna C18(2) column (5 µm, 150 × 2 mm), which was held at 40°C. The flow rate was 350 µl/min. Mobile phase A was 0.1% acetic acid in water. Mobile phase B was 0.1% acetic acid in 85:15 acetonitrile:methanol. Gradient elution was used and the mobile phase percent B was varied as follows: 15% B at 0 min, ramp from 0 to 2 min to 30% B, ramp from 2 to 5 min to 55% B, ramp from 5 to 12 min to 62% B, ramp from 12 to 14 min to 100% B, hold from 14 to 22 min at 100% B, ramp from 22 to 23 min down to 15% B, and hold 15% B from 23 to 27 min. Samples were solvated in 70 µl of 50% acetonitrile containing 105 pg PGE2-d4 as an internal standard. The injection volume was 20 µl. Samples were analyzed in triplicate.
Electrospray ionization tandem mass spectrometry was used for detection. Analyses were performed on an MDS Sciex API 3000 equipped with a TurboIonSpray source. Turbo desolvation gas was heated to 425°C at a flow rate of 7 L/min. All analytes were monitored simultaneously as negative ions in a multiple reaction monitoring scan with dwell times of 250 ms. Analytes were monitored at the following parent ion-product ion mass/charge ratio pairs and retention times (Rt): 6-keto PGF1α, 369.2:163.0 (Rt = 8.14 min); thromboxane B2, 369.2:169.0 (Rt = 8.86 min); PGF2α, 353.2:309.0 (Rt = 9.22 min); PGE2, 351.2:271.1 (Rt = 9.41 min); PGD2, 351.2:271.1 (Rt = 9.69 min); and PGE2-d4, 355.2:275.1 (Rt = 9.41 min).
The levels of the major COX PG product, PGE2, were also determined in BAL fluid, whole lung homogenates, and plasma of naive mice using a radioimmunoassay kit (Amersham Biosciences) according to the manufacturer’s instructions.
Noninvasive analysis of lung function and airway responsiveness by whole-body plethysmography
Respiratory parameters in spontaneously breathing naive mice were determined by barometric whole-body plethysmography (Buxco Electronics). Mice were placed in individual plethysmograph chambers and breathing frequency, tidal volume, minute ventilation, peak inspiratory flow, peak expiratory flow, and enhanced pause (Penh; a dimensionless parameter that is a function of total pulmonary airflow in mice) were determined and averaged over a 30-min period.
Invasive analysis of lung function and airway responsiveness in mechanically ventilated mice
Respiratory mechanics and airway responsiveness to aerosolized methacholine were determined in naive mice using invasive analysis with the FlexiVent mechanical ventilator system (SCIREQ) as described previously (14). Invasive analysis of respiratory mechanics and airway responsiveness was also performed on day 18 of the allergic airway inflammation model as described below.
Allergic airway inflammation model
Allergic inflammation was induced by sensitization and challenge with OVA essentially as described (15). Only male mice were used for study due to observed gender differences in respiratory parameters in naive mice (14) and reported gender differences in OVA-induced airway inflammation (16, 17). On days 0 and 1, male COX-1 Tr and WT littermate mice were administered 20 µg of OVA (grade V; Sigma-Aldrich) emulsified in 0.2 ml of aluminum hydroxide adjuvant by i.p. injection. Control (nonallergic) mice received adjuvant alone. On days 13–17, all mice were challenged for 30 min/day with 1% OVA in saline aerosol via a nose-only exposure chamber. On day 18, mice were subjected to invasive analysis of respiratory mechanics and airway responsiveness as described above and were subsequently sacrificed. BAL was performed as described above, and cells and cell-free BAL fluid supernatants were processed for total and differential cell counts and PG and cytokine analysis. BAL fluid cysteinyl leukotriene (cysLT) and leukotriene B4 (LTB4) levels in allergic mice were determined with enzyme immunoassay kits from Amersham Biosciences and Cayman Chemical, respectively, according to the manufacturers’ instructions. Following BAL, right lungs were removed and frozen at −80°C, and left lungs were inflated and fixed in 4% paraformaldehyde for histological evaluation.
COX-2 protein levels in whole lung lysates were quantified by immunoblotting using a rabbit polyclonal Ab (no. 160126; Cayman Chemical). Membranes were stripped with Restore Western Blot Stripping buffer (Pierce), and protein levels of GAPDH were subsequently determined with a goat polyclonal Ab (SC-20357; Santa Cruz Biotechnology). To account for variations in treatment groups and/or gel loading, relative band intensities, expressed as arbitrary units of COX-2 to GAPDH, were determined by densitometry with a ChemiImager 5500 system (Alpha Innotech Corporation). Whole lung lysate levels of PGD synthase protein and microsomal PGE synthase-1 protein in allergic mice were determined in a similar fashion using rabbit anti-murine polyclonal (no. 10004348; Cayman Chemical) and rabbit anti-human polyclonal (no. 160140; Cayman Chemical) Abs, respectively. BAL fluid PG levels were determined by liquid chromatography-tandem mass spectrometry as described above. BAL fluid levels of IL-1β, IL-4, IL-5, IL-6, and IL-10 were determined with a Bio-Plex mouse cytokine kit (Bio-Rad) using fluorescently labeled microsphere beads and a Bio-Plex suspension array system (Bio-Rad) according to the manufacturer’s instructions.
Generation of COX-2 null/COX-1 Tr mice
The following breeding strategy was used to generate COX-2-deficient mice harboring the airway-specific COX-1 transgene. COX-2 null males (Taconic Farms) were bred to COX-1 Tr females and ~50% of the F1 offspring were heterozygous at the COX-2 locus and also positive for the COX-1 transgene. Genotyping for the COX-2 allele was performed as described elsewhere (18), whereas genotyping for the COX-1 transgene was performed as described above. Female COX-2+/− COX-1 Tr mice were crossed to male COX-2−/− COX-1 WT (non-Tr) mice to generate F2 mice for study. F2 mice (~25%) were COX-2−/− COX-1 Tr (COX-2 null/COX-1 Tr) and ~25% were COX-2−/− COX-1 WT (COX-2 null/COX-1 WT). A similar scheme was used to generate COX-2+/+ COX-1 Tr and COX-2+/+ COX-1 WT mice. Male littermates were used in all studies because the COX-1 Tr mice are on a pure C57BL/6 background, whereas the COX-2 null mice are on a hybrid C57BL/6-SvEv background that has been intercrossed for >20 generations. Lung function and airway inflammation were assessed on day 18 of the allergic airway inflammation model as described above.
Statistical analyses
Data are presented as group means ± SEM. Statistical comparisons among treatment groups were performed by randomized design two-way ANOVA followed by the Newman-Keuls post hoc test for more than two groups, or by an unpaired Student’s t test for two groups. In all cases, statistical significance was defined as p < 0.05.
Results
Generation and identification of COX-1 Tr mice
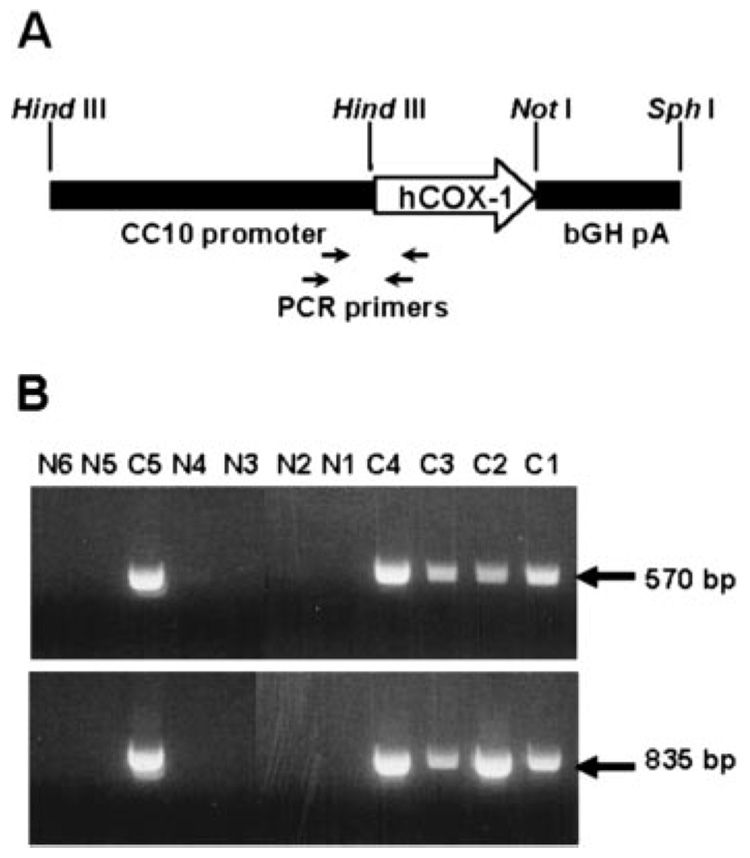
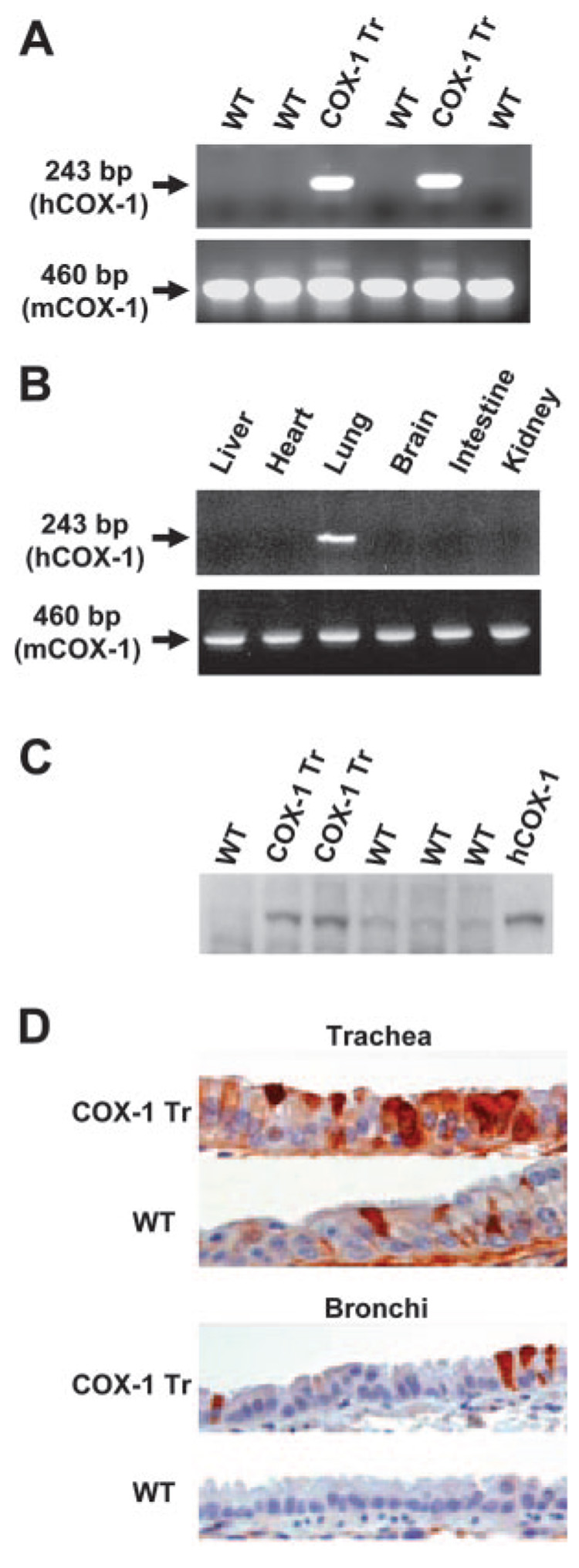
Human COX-1 expressed under control of the murine CC10 promoter (Fig. 1A) was used to generate five founder mice that contained the transgene, as identified by PCR analysis of tail biopsy DNA samples (Fig. 1B). One of the founders (line C4) was shown by RT-PCR and immunoblot blot analyses to overexpress the transgene, and this line was propagated for further study. Transgene-positive mice expressed human COX-1 mRNA specifically in lung tissue (Fig. 2, A and B) and had increased amounts of total COX-1 protein in lung tissue compared with WT littermates (Fig. 2C). Immunohistochemical analysis revealed increased staining for COX-1 in tracheal and bronchial epithelial cells of COX-1 Tr mice compared with WT littermates (Fig. 2D). Staining of alveolar epithelial cells did not differ between the genotypes (data not shown), consistent with a predominant airway expression of the transgene. No differences were observed between male and female COX-1 Tr mice with regard to expression of the transgene.
FIGURE 1. Generation and identification of COX-1 Tr mice.
A, Schematic representation of the Tr construct containing the CC10 promoter, COX-1 cDNA, and bGH intron/poly(A) sequences. The positions of PCR primers used to detect the transgene are shown. B, PCR identification of germline COX-1 Tr founders (C1–C5) and nongermline animals (N1–N6).
FIGURE 2. Analysis of transgene expression.
A, RT-PCR demonstrating human COX-1 expression in COX-1 Tr mouse lung and not in WT mouse lung. B, RT-PCR demonstrating lung-specific expression of human COX-1 in COX-1 Tr mice. C, Immunoblot demonstrating increased total COX-1 protein expression (~70 kDa) in COX-1 Tr vs WT mouse lung. The right-most lane contained recombinant human COX-1 as a standard. D, Immunohistochemical demonstration of increased COX-1 staining (red-brown) in COX-1 Tr vs WT trachea and bronchi.
Phenotypic characterization of COX-1 Tr mice
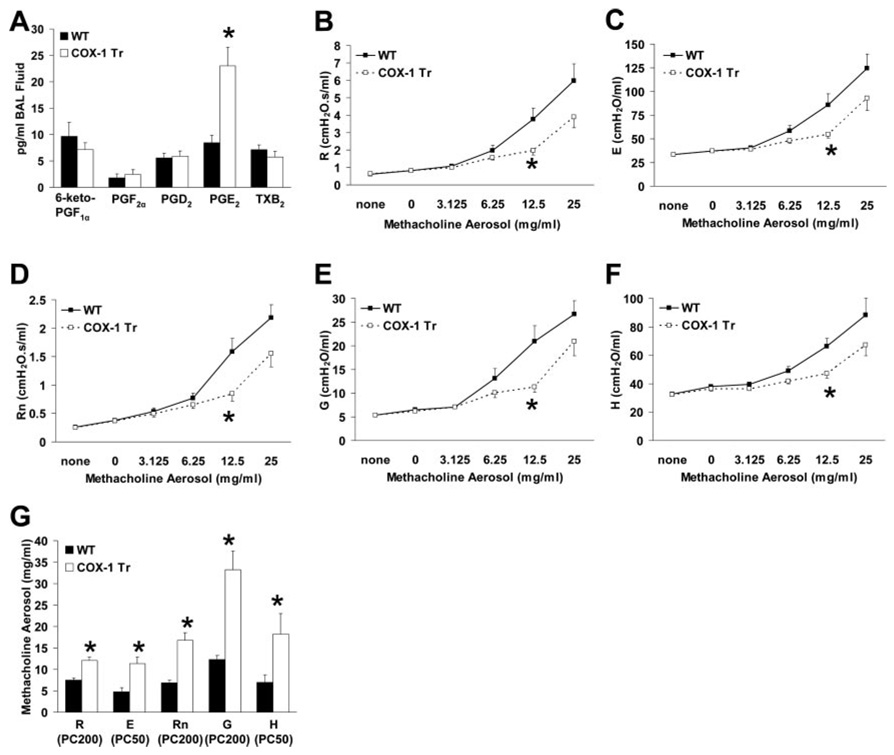
COX-1 Tr and WT littermates did not differ from one another with respect to appearance, growth rate, or disposition. Analysis of BAL fluid collected from naive mice did not reveal any differences between COX-1 Tr and WT littermates with regard to cell counts, cell differentials, or protein content (data not shown). However, BAL fluid PGE2 content was elevated nearly 3-fold in COX-1 Tr mice (p < 0.05), whereas levels of other prostanoids were not significantly altered (Fig. 3A). Analysis of BAL fluid by radioimmunoassay confirmed increased airway PGE2 in COX-1 Tr mice (57.3 ± 9.7 vs 19.5 ± 3.8 pg/ml for COX-1 Tr and WT, respectively; p < 0.05) and demonstrated no difference in whole lung homogenate or plasma PGE2 levels between genotypes (data not shown). Thus, the major prostanoid product of COX-mediated arachidonic acid metabolism was specifically elevated in the airways of COX-1 Tr mice, indicative of the functionality of the human COX-1 transgene.
FIGURE 3. Phenotypic characterization of naive COX-1 Tr mice.
A, BAL fluid PGE2 content was significantly elevated in COX-1 Tr mice, whereas levels of other PGs were not significantly altered (*, p < 0.05 vs WT; n = 11 WT and 15 COX-1 Tr). B–F, Airway responsiveness to aerosolized methacholine was reduced in COX-1 Tr mice as determined by parameters fit to the single compartment (B and C) and constant phase (D–F) models of respiratory mechanics (*, p < 0.05 vs WT; n = 14 WT and 13 COX-1 Tr). G, Calculated PC values of methacholine aerosol for R, E, Rn, G, and H were greater in COX-1 Tr mice than in WT mice, indicative of decreased sensitivity to methacholine (*, p < 0.05 vs WT; n = 14 WT and 13 COX-1 Tr).
Lung function in naive COX-1 Tr and WT littermate mice was assessed using invasive and noninvasive methods. We have previously observed (14) considerable gender differences in airway responsiveness to methacholine aerosol challenge in naive C57BL/6 mice; as such, lung function data presented herein was derived from studies conducted with male mice only. Similar results were obtained when studies were conducted with female mice (data not shown). Under resting conditions, breathing frequency, tidal volume, Penh, and other parameters that were assessed non-invasively did not differ between naive COX-1 Tr and WT mice (Table I). Traditional invasive techniques were used to examine both the single compartment and constant phase models of respiratory mechanics in COX-1 Tr and WT littermate mice. In the absence of any aerosol challenge, no differences were observed between genotypes as assessed by the single compartment model of the lung for total respiratory system resistance (R) or elastance (E) (Table II). Similarly, data derived from the constant phase model of the lung revealed no differences between genotypes for baseline Rn (Newtonian resistance, an indicator of central airways resistance), G (tissue damping, an indicator of peripheral airways and parenchymal resistance), or H (tissue elastance, an indicator of peripheral airways and parenchymal elastance) (Table II). Following aerosol administration of methacholine, all animals studied demonstrated increased responses in all of the measured parameters (Fig. 3, B–F); however, these responses were more pronounced in WT mice than in COX-1 Tr mice. Calculated provocative concentration (PC) 200 values (the concentration of methacholine aerosol required to increase baseline values by 200%) for R, Rn, G, and PC50 values (the concentration of methacholine aerosol required to increase baseline values by 50%) for E and H were significantly greater in COX-1 Tr mice than in WT mice (Fig. 3G), indicative of decreased sensitivity to methacholine. Collectively, these data indicate that COX-1 overexpression in the airways, and the concomitant increase in PGE2 levels, resulted in a beneficial effect on airway responsiveness and respiratory mechanics in naive mice following challenge with a cholinergic agonist.
Table I.
Baseline respiratory parameters in naive WT and COX-1 Tr mice assessed using noninvasive whole-body plethysmography
| Genotype | Mice (n) | f | Vt | MV | PIF | PEF | Penh |
|---|---|---|---|---|---|---|---|
| WT | 28 | 488 ± 13 | 245 ± 10 | 116 ± 6 | 8.6 ± 0.4 | 5.6 ± 0.3 | 0.56 ± 0.01 |
| COX-1 Tr | 29 | 475 ± 11 | 242 ± 10 | 113 ± 6 | 8.7 ± 0.4 | 5.5 ± 0.3 | 0.55 ± 0.01 |
f, Breathing frequency (breaths/min); Vt, tidal volume (µl/breath); MV, minute ventilation (ml/min); PIF, peak inspiratory flow (ml/s); PEF, peak expiratory flow (ml/s).
Table II.
Baseline respiratory mechanics in mechanically ventilated naive WT and COX-1 Tr mice
| Genotype | Mice (n) | R (cmH2O · s/ml) | E (cmH2O/ml) | Rn (cmH2O · s/ml) | G (cmH2O/ml) | H (cmH2O/ml) |
|---|---|---|---|---|---|---|
| WT | 14 | 0.62 ± 0.04 | 33.5 ± 0.8 | 0.26 ± 0.02 | 5.4 ± 0.2 | 32.6 ± 0.7 |
| COX-1 Tr | 13 | 0.65 ± 0.03 | 33.4 ± 1.1 | 0.26 ± 0.02 | 5.4 ± 0.2 | 32.5 ± 1.0 |
Effect of COX-1 overexpression on allergic airway inflammation and responsiveness
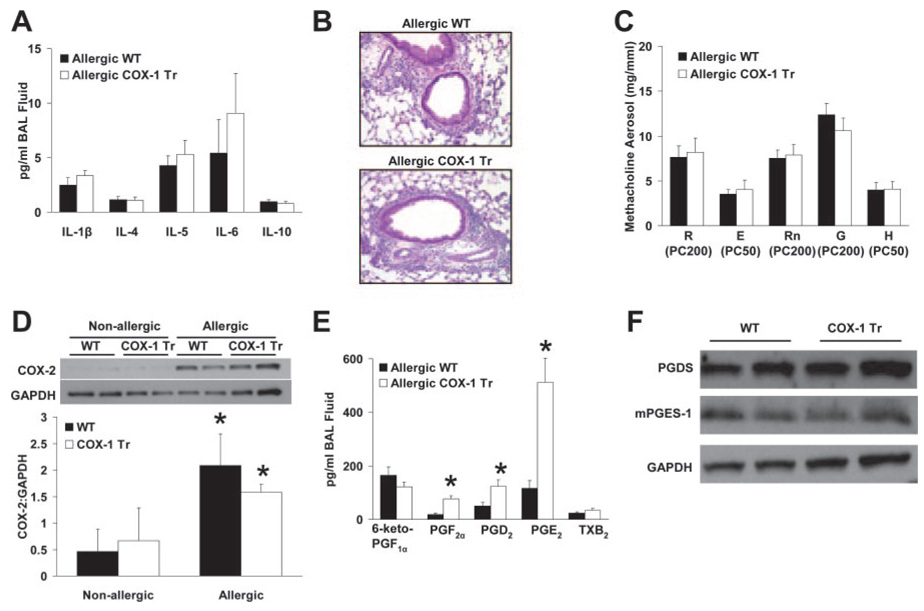
Due to the demonstrated importance of COX enzymes in mediating and resolving airway inflammatory responses (1, 2, 13, 19), we examined the responses of COX-1 Tr mice in an allergic model of airway inflammation induced by OVA sensitization and challenge. As expected, significant increases in BAL fluid total cell number and eosinophil content were observed in mice sensitized and challenged with OVA (i.e., allergic) in comparison to mice sensitized with adjuvant alone (i.e., nonallergic) (Table III). BAL fluid levels of IL-1β, IL-4, IL-5, IL-6, and IL-10 were at low or undetectable levels in nonallergic mice (data not shown) and were increased in allergic mice of both genotypes (Fig. 4A). However, no differences were found between allergic COX-1 Tr and WT littermate mice in any of the BAL fluid parameters measured (Table III and Fig. 4A), nor were any histological differences observed (Fig. 4B).
Table III.
BAL fluid inflammatory parameters in allergic WT and COX-1 Tr mice
| Genotype | Treatment | Mice (n) | Total Cells (×106/ml) | Eosinophils (%) |
|---|---|---|---|---|
| WT | Nonallergic | 4 | 0.14 ± 0.01 | 0.0 ± 0.0 |
| COX-1 Tr | Nonallergic | 5 | 0.13 ± 0.02 | 0.0 ± 0.0 |
| WT | Allergic | 13 | 1.10 ± 0.18* | 69.9 ± 3.6* |
| COX-1 Tr | Allergic | 14 | 1.45 ± 0.17* | 75.7 ± 3.0* |
p < 0.05 vs nonallergic group of the same genotype.
FIGURE 4. Airway inflammatory and functional responses in allergic COX-1 Tr mice.
A, BAL fluid levels of IL-1β, IL-4, IL-5, IL-6, and IL-10 did not differ between allergic WT and COX-1 Tr mice (n = 8 WT and 9 COX-1 Tr). B, Representative sections of lung from allergic WT and COX-1 Tr mice (periodic acid-Schiff stain) demonstrating areas of perivascular and peribronchiolar inflammatory cell influx. C, Airway responsiveness (measured as PC values) to inhaled methacholine did not differ between allergic WT and COX-1 Tr mice (n = 11 WT and 12 COX-1 Tr). D, Whole lung COX-2 protein levels were elevated in allergic WT and COX-1 Tr mice compared with nonallergic mice. Representative immunoblots from two mice per treatment group are shown for COX-2 (~72 kDa) and GAPDH (~40 kDa) (*, p < 0.05 vs nonallergic mice of the same genotype; n = 4 per group). E, BAL fluid PGF2α, PGD2, and PGE2 levels were increased in allergic COX-1 Tr mice (*, p < 0.05 vs allergic WT; n = 8 WT and 9 COX-1 Tr). F, Whole lung PGD synthase (~23 kDa) and microsomal PGE synthase-1 (~16 kDa) protein levels did not differ in allergic WT and COX-1 Tr mice. Representative immunoblots from two mice per treatment group are shown.
Respiratory mechanics and airway responsiveness to methacholine were determined in mechanically ventilated allergic mice. No differences were observed between allergic COX-1 Tr and WT mice with regard to baseline values of R, E, Rn, G, and H (Table IV), nor did these values differ from those observed in naive animals (Table II). Furthermore, allergic COX-1 Tr and WT mice responded similarly to methacholine aerosol, with comparable PC values found for all respiratory parameters assessed (Fig. 4C). These data demonstrate that allergic COX-1 Tr and WT mice did not differ in terms of respiratory mechanics and airway responsiveness following challenge with a cholinergic agonist.
Table IV.
Baseline respiratory mechanics in mechanically ventilated allergic WT and COX-1 Tr mice
| Genotype | Mice (n) | R (cmH2O · s/ml) | E (cmH2O/ml) | Rn (cmH2O · s/ml) | G (cmH2O/ml) | H (cmH2O/ml) |
|---|---|---|---|---|---|---|
| WT | 11 | 0.62 ± 0.03 | 31.0 ± 1.6 | 0.24 ± 0.01 | 5.4 ± 0.2 | 28.9 ± 1.6 |
| COX-1 Tr | 12 | 0.64 ± 0.01 | 32.4 ± 0.9 | 0.24 ± 0.01 | 5.4 ± 0.1 | 29.9 ± 1.0 |
COX-2 is known to be up-regulated in allergic airway inflammation and to generate beneficial (anti-inflammatory and bronchodilatory) and deleterious (proinflammatory and bronchoconstrictive) PGs that are involved in the regulation of inflammation and airway tone (2, 13). Thus, we speculated that the lack of differences between allergic WT and COX-1 Tr mice might have been due to COX-2 up-regulation and altered PG production masking the beneficial effect of the COX-1 transgene that was observed in naive mice. To test this possibility, COX-2 protein expression in whole lung homogenates from nonallergic and allergic mice was determined by immunoblotting and PG levels were measured in BAL fluid of allergic mice. Increased COX-2 protein was observed in lung homogenates from allergic mice compared with nonallergic mice, with no difference between COX-1 Tr and WT animals (Fig. 4D). Interestingly, the comparative profile of BAL fluid PGs in allergic COX-1 Tr vs WT mice differed from that observed in naive mice. Whereas absolute levels of all PGs were elevated in allergic compared with naive mice, PGF2β, PGD2, and PGE2 were significantly elevated in allergic COX-1 Tr mice compared with allergic WT mice (Fig. 4E), whereas only PGE2 levels differed between the genotypes in naive mice (Fig. 3A). Immunoblot analysis in whole lung homogenates of the synthases responsible for PGD2 and PGE2 production (PGD synthase and microsomal PGE synthase-1, respectively) did not reveal differences between allergic COX-1 Tr and WT mice (Fig. 4F), suggesting that differential lung expression of these proteins was not responsible for the observed differences in PG profiles. Furthermore, despite the differences in absolute levels of BAL fluid PGs, the ratios of anti- to proinflammatory PGs were similar between genotypes; the PGE2: PGD2 ratio was 3.8:1 and 5.8:1 in allergic WT and COX-1 Tr mice, respectively; whereas the PGE2:PGF2β ratio was 7.1:1 and 7.4:1. Thus, COX-2 up-regulation in both WT and COX-1 Tr mice, and the associated change in BAL fluid PG profiles, may have masked any potential benefit of the COX-1 transgene on airway inflammatory and functional parameters.
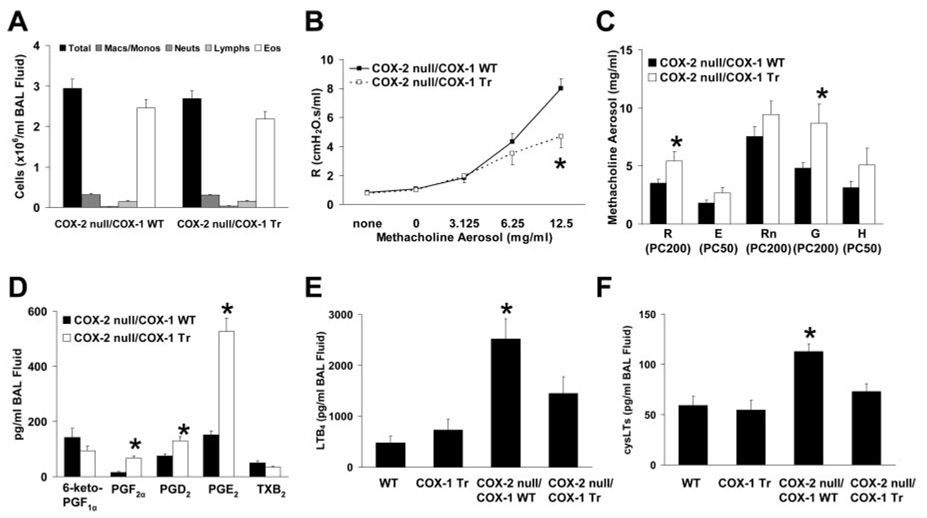
To study the influence of the COX-1 transgene on allergic airway responses in the absence of COX-2 up-regulation, we generated mice that were genetically deficient in COX-2 and that either possessed the human COX-1 transgene (COX-2 null/COX-1 Tr) or did not (COX-2 null/COX-1 WT). In these mice, allergic airway inflammation was not altered by the presence of the COX-1 transgene (Fig. 5A) but airway responsiveness, measured as R, was significantly reduced (Fig. 5B). Calculated PC values, shown in Fig. 5C, were greater in allergic COX-2 null/COX-1 Tr mice than in allergic COX-2 null/COX-1 WT mice for all parameters measured, indicative of decreased sensitivity to methacholine. In particular, PC200 values for R and G were significantly different (p < 0.05), whereas PC values for the other parameters also revealed a decreased sensitivity to methacholine in allergic COX-2 null/COX-1 Tr mice compared with allergic COX-2 null/COX-1 WT mice (p values ranged from 0.09 to 0.17). Analysis of BAL fluid PGs demonstrated that allergic COX-2 null/COX-1 WT and COX-2 null/COX-1 Tr mice had airway levels of PGF2α, PGD2, and PGE2 similar to those observed in allergic WT and COX-1 Tr mice, respectively (Fig. 5D; compare with Fig. 4E), indicating that COX-2 was not required for generation of these PGs in this setting and therefore did not likely underlie the lack of differences between allergic WT and COX-1 Tr mice. Therefore, we determined whether shunting of arachidonic acid from the COX metabolic pathway to the 5-lipoxygenase metabolic pathway may have occurred in allergic mice and contributed to the observed phenotypes. Although airway leukotriene levels did not differ between allergic WT and COX-1 Tr mice, allergic COX-2 null/COX-1 WT mice had increased LTB4 and cysLT levels compared with these groups (Fig. 5, E and F), indicative of shunting of arachidonic acid toward the 5-lipoxygenase pathway in the absence of COX-2. In contrast, BAL fluid leukotriene levels were lower in COX-2 null/COX-1 Tr mice (Fig. 5, E and F), suggestive of a reversal of the shunting of arachidonic acid back toward the COX pathway of metabolism in the presence of the COX-1 transgene.
FIGURE 5. Airway inflammatory and functional responses in allergic COX-2 null/COX-1 Tr mice.
A, BAL fluid inflammatory cell influx did not differ between allergic COX-2 null/COX-1 WT and COX-2 null/COX-1 Tr mice (n = 16 per genotype). B, Total respiratory system resistance in response to methacholine aerosol was reduced in allergic COX-2 null/COX-1 Tr mice (*, p < 0.05 vs COX-2 null/COX-1 WT; n = 16 per genotype). C, Airway responsiveness (measured as PC values) to inhaled methacholine was reduced in allergic COX-2 null/COX-1 Tr mice, as indicated by increased PC values for parameters fit to the single compartment (R and E) and constant phase (Rn, G, and H) models of respiratory mechanics (*, p < 0.05 vs COX-2 null/COX-1 WT; n = 16 per genotype). D, BAL fluid PGF2α, PGD2 and PGE2 levels were increased in allergic COX-2 null/COX-1 Tr mice (*, p < 0.05 vs allergic COX-2 null/COX-1 WT; n = 16 per genotype). BAL fluid LTB4 (E) and cysLT (F) levels were increased in allergic COX-2 null/COX-1 WT mice (*, p < 0.05 vs all other groups; n = 13–16 per genotype).
Collectively, these data indicate that the lack of any beneficial effect of the COX-1 transgene on allergic airway responses in COX-1 Tr mice was not the result of COX-2 up-regulation and differential airway PG profiles or of differential airway leukotriene profiles in allergic WT and COX-1 Tr mice. Conversely, the beneficial reduction in airway responsiveness observed in allergic COX-2 null/COX-1 Tr mice compared with COX-2 null/COX-1 WT mice may have been due, in part, to the lower airway leukotriene levels observed in the former.
Discussion
The importance of COX-1 and COX-2 in the resolution of airway inflammatory and functional responses to allergic stimuli has been demonstrated in murine models through the use of gene knockout strategies and by the administration of selective or nonselective pharmacological COX inhibitors (2, 13). To further study the contribution of COX-derived prostanoids to lung function and allergic airway inflammatory responses, we generated Tr mice in which human COX-1 was specifically targeted to airway epithelial cells using the murine CC10 promoter. In comparison to WT littermates, naive COX-1 Tr mice were shown to be normal in all aspects of baseline lung function and BAL fluid cellular characteristics, and histological examination did not reveal any evidence of morphological changes resulting from the presence of the transgene. Thus, constitutively increased airway COX-1 expression did not result in detectable alterations of lung function, cellularity, or structure under basal conditions. Interestingly, COX-1-deficient mice also do not demonstrate any alterations in baseline lung functional, cellular, or structural characteristics (2), suggesting that COX-1-derived prostanoids are not crucial regulators of lung function under normal circumstances. However, naive COX-1 Tr mice had significantly elevated BAL fluid PGE2 content and demonstrated decreased airway responses to aerosol administration of the bronchoconstrictor methacholine, indicative of a beneficial effect of the transgene and its prostanoid products on airway responsiveness to cholinergic stimulation.
In healthy humans, inhalation of PGE2 results in bronchodilation (6), whereas the analysis of Penh responses in conscious mice suggests that it exerts a bronchoconstrictive effect when administered alone in high doses and a bronchodilatory effect on airways challenged with a cholinergic agonist (20, 21). Interestingly, however, invasive analysis of respiratory mechanics does not support a constrictive effect of aerosolized PGE2 in naive mice (21), but reveals that PGE2 decreases methacholine-induced airway constriction (20). Similarly, we did not observe an alteration of basal airway tone in unchallenged naive COX-1 Tr mice compared to WT mice despite the increased airway PGE2 content, but found that methacholine-induced alterations of pulmonary mechanics were attenuated in COX-1 Tr mice. Our data support the concept that PGE2 is not a critical regulator of respiratory mechanics under basal conditions and are consistent with the results of a recent study (22), which used genetic approaches to alter pulmonary PGE2 levels in mice via deletion or lung-specific overexpression of microsomal PGE synthase-1 and showed that neither of these manipulations altered baseline lung resistance.
Having demonstrated that COX-1 Tr mice had increased airway levels of PGE2 and a blunted response to inhalational challenge with a cholinergic bronchoconstrictor, we next examined the responses of these mice to allergic airway inflammation induced by sensitization and challenge with OVA. Significant increases of total BAL fluid cells, eosinophils, and levels of a variety of ILs implicated in allergic lung inflammation were observed in allergic WT and COX-1 Tr mice, and histological evidence of allergic inflammation was also noted. However, none of these parameters differed between WT and COX-1 Tr mice. Furthermore, invasive analysis of lung function revealed no differences between allergic WT and COX-1 Tr mice in any of the respiratory parameters measured, including responsiveness to inhaled methacholine. Thus, airway-specific overexpression of COX-1 and the resulting increase of airway PGE2 levels observed at baseline were not sufficient to alter the inflammatory and functional responses to allergen sensitization and challenge in this model.
We surmised that the lack of any measurable differences in airway inflammation and responsiveness was likely due in part to the comparable up-regulation of COX-2 that was observed in the lungs of allergic WT and COX-1 Tr mice, which augmented PG production and thereby masked any favorable effect of the COX-1 transgene. Interestingly, the BAL fluid PG profile in allergic mice differed considerably from that observed in naive animals. Levels of the proinflammatory and bronchoconstrictive PGF2α and PGD2, in addition to the anti-inflammatory and bronchodilatory PGE2, were elevated in allergic COX-1 Tr mice compared with allergic WT mice, whereas in naive mice only PGE2 levels differed between the genotypes. Although positive feedback regulation of COX-2 expression by PGs has been demonstrated (23), we did not observe any differences in lung COX-2, PGD synthase, or mPGES-1 protein expression between WT and COX-1 Tr allergic mice. Furthermore, studies performed with COX-2 null/COX-1 WT and COX-2 null/COX-1 Tr mice indicated that the increased levels of PGE2, PGF2α, and PGD2 in allergic COX-1 Tr mice were not COX-2 dependent, since these levels did not change in the absence of COX-2. These observations suggest that mechanisms other than COX-2-mediated increases in pro and anti-inflammatory PGs were responsible for the altered airway PG profile and lack of beneficial outcome in allergic COX-1 Tr mice.
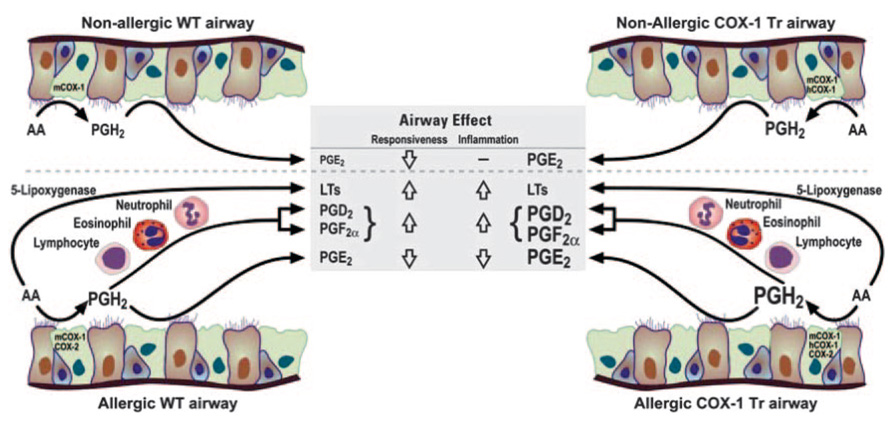
Given our observations, we propose the following scenario to explain the airway PG profiles and inflammatory and functional outcomes observed in this study (Fig. 6). Under nonallergic conditions, airway PGE2 was selectively increased in COX-1 Tr mice owing to the fact that PGE synthase is the predominant PG synthase in the naive airway (4) and/or is coupled most efficiently to COX-1 to generate PGE2 from COX-1-derived PGH2. The net effect of this was a decrease in airway responsiveness to methacholine in COX-1 Tr mice compared with WT mice. In the allergic airways of WT and COX-1 Tr mice, the availability of PGH2 was increased as a result of COX-2 up-regulation; however, PGH2 was maintained at a higher level in COX-1 Tr mice than in WT mice due to the sustained presence of the COX-1 transgene. Consequently, influx of inflammatory cells and transcellular metabolism of airway cell-derived PGH2 contributed to increased generation of PGE2, PGF2α and PGD2 (Fig. 6), the absolute levels of which were greater in COX-1 Tr mice than in WT mice due to the greater availability of PGH2 in the former. Our data suggest that transcellular metabolism of PGH2, a phenomenon believed to underlie altered PG levels in other experimental animal and human studies (24, 25), is the most likely explanation for the observed airway PG levels. In contrast to PG levels, the absolute levels of airway leukotrienes did not differ between allergic WT and COX-1 Tr mice; only when COX-2 was absent from the system did arachidonic acid become available for shunting to lipoxygenase-mediated metabolism, and this was partially reversed in the presence of the COX-1 transgene.
FIGURE 6. A proposed scheme to account for the airway eicosanoid profiles observed in nonallergic and allergic WT and COX-1 Tr mice.
In the nonallergic airway, PGE2 levels are selectively increased in COX-1 Tr mice due to the presence of the COX-1 transgene (hCOX-1) and preferential synthesis of PGH2 to PGE2 by airway epithelial cells; this leads to a decrease in airway responsiveness. In the allergic airway, increased PGH2 production as a result of COX-2 induction leads to transcellular metabolism and generation of PGD2 and PGF2α by inflammatory cells in addition to increased PGE2 production by airway epithelial cells; the net effect is a balanced production of pro- and anti-inflammatory PGs. Absolute levels of airway PGs are higher in allergic COX-1 Tr mice than in allergic WT mice due to the sustained presence of the COX-1 transgene and, therefore, maintenance of greater PGH2 production.
Since airway inflammation and responsiveness did not differ between WT and COX-1 Tr mice despite the observed differences in absolute airway PG levels, we speculate that the relative ratio of pro- and anti-inflammatory PGs within the airway, which were found to be similar between genotypes, was a more important determinant in the allergic response than were the absolute levels of these mediators. As such, it is doubtful that a higher level of expression or an alternate location for the COX-1 transgene within the airways would have influenced the allergic outcomes in the present study, as these PG ratios would likely have still remained similar between allergic mice of both genotypes. Additionally, it should be emphasized that airway COX-1, regardless of the level or location of expression, would not be expected to influence the sensitization to Ag administered by i.p. injection, as was the protocol used in this study. As such, the documented worsening of allergic airway responses in COX-1 null mice (2) can likely be ascribed to defects in the initial sensitization to Ag and in the subsequent adaptive immune responses to repeated airway challenges, indicating a critical role for nonairway COX-1 in the overall process. Indeed, COX-1 expression and activity is important in areas such as local lymph nodes where Ag presentation occurs and the thymus during immune system development (26). Thus, although we postulated that increasing COX-1 expression solely in the airways might have improved allergic responses, our data clearly indicate that this was not the case. Furthermore, whereas COX products, in particular PGE2, have been suggested to inhibit the development of allergen-induced airway inflammation and hyperresponsiveness through inhibition of the development of a Th2-mediated adaptive immune response (12, 15), the fact that the selective and beneficial increase of PGE2 in the naive COX-1 Tr airway was not maintained under allergic conditions underscores the inability of airway COX-1 overexpression to limit allergic airway inflammation.
Given the anti-inflammatory potential of PGE2 in the lung (27), it is reasonable to predict that airway inflammatory and functional outcomes in allergic airway disease may be reduced via strategies designed to selectively maintain increased airway levels of this prostanoid throughout the course of allergic inflammation. Alternatively, selective activation of certain PGE2 receptors that are thought to mediate the beneficial actions of this PG in the airways may prove beneficial. In this regard, a recent report (28) describing the inhibition of allergic airway inflammation and hyperresponsiveness in mice by a synthetic agonist specific for the PGE2 receptor EP4 suggests that this pathway may have therapeutic benefit. It is important to note that PG signaling is mediated by an array of receptors that are expressed on numerous cell types within the allergic airway and that the levels and expression patterns of these receptors are likely dynamically regulated during the course of an inflammatory response such as the one elicited in the model we used. Although it is beyond the scope of the present study to examine such events, it is likely that changes in the levels or pattern of expression of specific PG receptors on airway and/or inflammatory cells contributed to the inflammatory and functional outcomes observed herein.
In summary, we have demonstrated that constitutive overexpression of COX-1 in the murine airway increases PGE2 content and decreases responsiveness to cholinergic stimulation. Despite this beneficial effect on airway responsiveness in naive mice, the inflammatory and functional responses of the lung to an allergic stimulus are not altered in the presence of the COX-1 transgene, likely due in part to a balanced generation of pro- and anti-inflammatory PGs under allergic conditions. We propose that other genetic and/or pharmacologic strategies designed to alter airway levels of PGs (22, 29, 30) or to specifically target downstream signaling events may result in a more robust alteration of basal lung function and provide benefit in this and other models of lung disease in which diminished or altered COX activity are implicated.
Acknowledgments
We are grateful to Herman Price, Clark Colegrove, and Lynn Crawford for expert technical assistance; Sandy Ward for cell differential counting; and Drs. Donald Cook and Steven Kleeberger for helpful comments during preparation of this manuscript. This research was conducted in part at the National Institute of Environmental Health Sciences Inhalation Facility under contract to Alion Science and Technology. We acknowledge the NIH Transgenic Mouse Development Facility and Carl Pinkert (Contracts NO1-HD-53229 and NO1-DE-12634) for assistance with mouse production.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. J.W.C. is a recipient of a Research Fellowship Award from the Davies Charitable Foundation, and of a Senior Research Training Fellowship from the American Lung Association of North Carolina.
Abbreviations used in this paper: COX, cyclooxygenase; WT, wild type; BAL, bronchoalveolar lavage; Tr, transgenic; bGH, bovine growth hormone; Rt, retention time; cysLT, cysteinyl leukotriene; LTB4, leukotriene B4; PC, provocative concentration; CC10, Clara cell 10-kDa protein; Penh, enhanced pause.
Disclosures The authors have no financial conflict of interest.
References
- 1.Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2001;25:457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- 2.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J. Clin. Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am. J. Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ermert L, Dierkes C, Ermert M. Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clin. Cancer Res. 2003;9:1604–1610. [PubMed] [Google Scholar]
- 5.Hardy CC, Bradding P, Robinson C, Holgate ST. Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J. Appl. Physiol. 1988;64:1567–1574. doi: 10.1152/jappl.1988.64.4.1567. [DOI] [PubMed] [Google Scholar]
- 6.Mathe AA, Hedqvist P. Effect of prostaglandins F2α and E2 on airway conductance in healthy subjects and asthmatic patients. Am. Rev. Respir. Dis. 1975;111:313–320. doi: 10.1164/arrd.1975.111.3.313. [DOI] [PubMed] [Google Scholar]
- 7.Hardy CC, Robinson C, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N. Engl. J. Med. 1984;311:209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 8.Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am. J. Respir. Crit. Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am. Rev. Respir. Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Melillo E, Woolley KL, Manning PJ, Watson RM, O’Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1994;149:1138–1141. doi: 10.1164/ajrccm.149.5.8173753. [DOI] [PubMed] [Google Scholar]
- 11.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, Vaghi A, Folco GC, Bianco S, Robuschi M. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am. J. Respir. Crit. Care Med. 1996;153:572–575. doi: 10.1164/ajrccm.153.2.8564100. [DOI] [PubMed] [Google Scholar]
- 12.Carey MA, Germolec DR, Langenbach R, Zeldin DC. Cyclooxygenase enzymes in allergic inflammation and asthma. Prostaglandins Leukotrienes Essent. Fatty Acids. 2003;69:157–162. doi: 10.1016/s0952-3278(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 13.Peebles RS, Jr, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, Christman JW, Kang KH, Jarzecka K, Furlong J, et al. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 2002;165:1154–1160. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 14.Card JW, Carey MA, Bradbury JA, Degraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, Langenbach R, Zeldin DC. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am. J. Respir. Crit. Care Med. 2003;167:1509–1515. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand. J. Immunol. 2003;57:562–567. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 17.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 18.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves de Moraes VL, Boris Vargaftig B, Lefort J, Meager A, Chignard M. Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. Br. J. Pharmacol. 1996;117:1792–1796. doi: 10.1111/j.1476-5381.1996.tb15356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheller JR, Mitchell D, Meyrick B, Oates J, Breyer R. EP2 receptor mediates bronchodilation by PGE2 in mice. J. Appl. Physiol. 2000;88:2214–2218. doi: 10.1152/jappl.2000.88.6.2214. [DOI] [PubMed] [Google Scholar]
- 21.Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, McNeisch J, Valancius C, Panettieri RA, Jr, Penn RB, Koller BH. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am. J. Physiol. 2003;284:L599–L606. doi: 10.1152/ajplung.00324.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hartney JM, Coggins KG, Tilley SL, Jania LA, Lovgren AK, Audoly LP, Koller BH. Prostaglandin E2 protects lower airways against bronchoconstriction. Am. J. Physiol. 2006;290:L105–L113. doi: 10.1152/ajplung.00221.2005. [DOI] [PubMed] [Google Scholar]
- 23.Vichai V, Suyarnsesthakorn C, Pittayakhajonwut D, Sriklung K, Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm. Res. 2005;54:163–172. doi: 10.1007/s00011-004-1338-1. [DOI] [PubMed] [Google Scholar]
- 24.Boulet L, Ouellet M, Bateman KP, Ethier D, Percival MD, Riendeau D, Mancini JA, Methot N. Deletion of microsomal prostaglandin E2 PGE2 synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J. Biol. Chem. 2004;279:23229–23237. doi: 10.1074/jbc.M400443200. [DOI] [PubMed] [Google Scholar]
- 25.MacNab MW, Foltz EL, Graves BS, Rinehart RK, Tripp SL, Feliciano NR, Sen S. The effects of a new thromboxane synthetase inhibitor, CGS–13080, in man. J. Clin. Pharmacol. 1984;24:76–83. doi: 10.1002/j.1552-4604.1984.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 26.Rocca B, Spain LM, Pure E, Langenbach R, Patrono C, FitzGerald GA. Distinct roles of prostaglandin H synthases 1 and 2 in T cell development. J. Clin. Invest. 1999;103:1469–1477. doi: 10.1172/JCI6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Slipetz D, Tuck S, Boie Y, Wang I-M, Han Y, O’Neill G, Young R, Metters K. A novel EP4 receptor agonist inhibits airways hyperreactivity and inflammation in murine models of asthma. Proc. Am. Thor. Soc. 2006;3:A290. [Google Scholar]
- 29.Blaine SA, Meyer AM, Hurteau G, Wick M, Hankin JA, Murphy RC, Dannenberg AJ, Geraci MW, Subbaramaiah K, Nemenoff RA. Targeted overexpression of mPGES-1 and elevated PGE2 production is not sufficient for lung tumorigenesis in mice. Carcinogenesis. 2005;26:209–217. doi: 10.1093/carcin/bgh302. [DOI] [PubMed] [Google Scholar]
- 30.Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Invest. 1999;103:1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]