Calcium sparks were discovered in single isolated rat cardiac myocytes by Cheng et al. (1993) while imaging fluorescence from the calcium indicator fluo-3 (Minta et al., 1989) with a confocal microscope. Cardiac muscle calcium sparks are associated with an approximate doubling of the resting fluo-3 fluorescence (ΔF/F = 1.0) and occupy a tiny region of the cell ∼2 μm in diameter. A simple equilibrium calculation of the likely change in calcium underlying the spark suggested that the local calcium peaked at ∼300 nM in 10 ms (Cheng et al., 1993). However, this figure underestimates the true change in calcium because of the limited kinetics and dynamic range of fluo-3, as well as blurring by the microscope. From a recent paper by Smith et al. (1998), we can estimate that the true change in calcium underlying the spark (averaged by microscope blurring in a region 0.5 μm across) may be >10 μM. Calcium sparks occur at a frequency of 1.6 s−1 in line scan images (Cheng et al., 1993), which survey ∼70–100 sarcomeres. However, calcium sparks can also be produced by membrane depolarization and therefore reflect the process of excitation–contraction (E–C) coupling at individual junctions between the t-tubular system and the sarcoplasmic reticulum that occur at the z-line (Shacklock et al., 1995; Cheng et al., 1996). Spark sites are therefore separated by ∼1.8 μm longitudinally in resting cardiac cells. In the transverse direction, calcium spark sites have a more variable spacing and sites that are closer to each other are more likely to coactivate (Parker et al., 1996).
The whole-cell calcium transient can be explained by the spatio-temporal summation of calcium sparks (Cannell et al., 1994, 1995). Hence, the whole-cell sarcoplasmic reticulum (SR) calcium release flux should be the average probability of SR release (P Spark) multiplied by the average local SR release flux (J Rel), but these variables are not separable in conventional whole-cell photometric measurements. However, recording calcium sparks overcomes this problem because this method provides a direct measure of P Spark (which is proportional to the number of sparks detected in the confocal imaging volume per unit time) as well as J Rel (which can be estimated from the spatio-temporal properties of the calcium spark). An initial estimate of J Rel by Cheng et al. (1993) suggested that the calcium spark could be explained by a calcium flux of ∼4 pA for 10 ms. Subsequent detailed mathematical modeling of the calcium removal processes suggests that the average flux of calcium associated with a spark is ∼3 pA (Blatter et al., 1997). Relating these fluxes to the number of SR release channels involved is problematic because there is considerable uncertainty in the value of the open probability of the SR calcium release channels during E–C coupling as well as the calcium flux passed by the channel under physiological conditions. Nevertheless, such small fluxes immediately suggested that the calcium spark was due to either a single channel or a small number of channels gating in concert (Cheng et al., 1993; Blatter et al., 1997). More recently, it has been proposed that a single SR release channel may conduct <0.5 pA under near physiological conditions (Mejia- Alvarez et al., 1998), indicating that the spark is almost certainly due to a cluster of SR release channels gating in concert. Such a measurement of the single SR release channel current only places a lower bound on the number of channels involved because we do not know the time course of the open probability of the SR calcium release channels during the rise of the calcium spark. The time course of the SR calcium release channel gating should be reflected in the actual time course of the SR calcium release flux during a spark. One approach to obtain this information is to use a mathematical description of the factors that should determine the spatio-temporal properties of the spark and back calculate the calcium flux required to produce the observed calcium spark (Lukyanenko et al., 1998). Although Lukyanenko et al. (1998) showed that they were able to measure a reduced rate of SR release channel inactivation in the presence of FK506 (which alters SR release channel gating), it is important to note that the accuracy of this type of method is uncertain since the diffusional properties of the various calcium buffers present in the cytosol are not precisely known. Therefore, it is not firmly established whether the SR calcium release declines exponentially during a calcium spark with a time constant that depends on the magnitude of the release (Lukyanenko et al., 1998). Although using mathematical models to estimate the SR calcium release flux from calcium spark records is a powerful approach for obtaining information on the time course of SR calcium release underlying sparks, major problems reside in the low signal-to-noise ratio associated with calcium sparks, even if the detailed properties of the calcium buffers in the cytosol become known. An alternative approach would be to use a calcium indicator in conjunction with a high concentration of a slow calcium buffer (such as EGTA) when the indicator signal can become a direct measure of the flux (Pape et al., 1995; Song et al., 1998).
Although P Spark is proportional to the number of sparks detected over a fixed time interval, during normal E–C coupling too many sparks occur for them to be unequivocally counted, although it has been estimated that P Spark increases by a factor of ∼104 during an action potential (Cannell et al., 1994). To overcome this problem, experimenters either limit the voltage range over which they measure P Spark or limit calcium influx via the L-type calcium channel (or dihydropyridine receptor [DHPR]) by either reducing external calcium levels and/or using a calcium channel antagonist or EGTA to strongly buffer internal calcium (Song et al., 1998). The fact that reducing the DHPR current causes a large decrease in the probability of spark production shows that the local calcium in flux via DHPRs is a major trigger for SR release (Cannell et al., 1994, 1995; López-López et al., 1995). In the rest of this perspective, we will only discuss SR calcium release triggered by DHPRs as the potential role of the Na/Ca exchanger in triggering sparks under physiological conditions is unknown.
At negative potentials where the open probability (P o) of a DHPR is very low, the voltage dependence of P Spark is the same as that of the DHPR P o (increasing e-fold for ∼7 mV), showing that a single DHPR can activate a spark (Cannell et al., 1995; Santana et al., 1996). A similar conclusion was reached by López-López et al. (1995), who found that the time course of calcium spark production during a voltage clamp pulse was similar to the expected single DHPR kinetics. Hence P Spark = k · P o at a fixed potential and k should depend on the amplitude of the calcium flux via DHPRs as well as the mean open time of the L-type calcium channel. At more positive potentials (in the presence of DHPR antagonists), P Spark has a bell-shaped voltage dependence whose peak is shifted to the left by ∼10 mV compared with the L-type calcium current. This result shows that the microscopic gain in E–C coupling is voltage dependent (Santana et al., 1996), which fits nicely with the earlier results and conclusions of Sipido and Wier (1991) who studied the voltage dependence of the rate of SR calcium release after accounting for voltage-dependent changes in L-type calcium current amplitude. The voltage dependence of P Spark can be explained by the SR release channels sensing the local calcium produced by the flux of calcium during the DHPR opening (which is voltage dependent) and P Spark depending on the square of the local calcium concentration (Santana et al., 1996). However, the latter analysis did not explicitly account for the time-dependent change in P o during the voltage-clamp pulse that will change P Spark (López-López et al., 1995). As noted above, P Spark should be a function (f) of the single L-type channel current (i) as well as a function (g) of the mean open time of the DHPR (τ) and the probability that an L-type channel in the junction is open (P o). P o can be eliminated by noting that the whole cell calcium current (I Ca) is given by the number of calcium channels in the cell (n) times the single channel current (i) and P o (Santana et al., 1996). Hence:
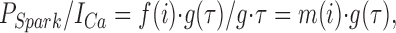 |
1 |
where h(i) is a new function of i. This equation can be integrated over the period of the voltage-clamp pulse that activates I Ca to give:
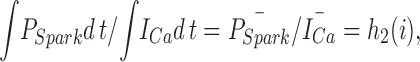 |
2 |
where h 2 is another function of i and the overbar signifies mean values over the period of the pulse. This approach assumes that τ is nearly constant during the pulse and any changes in the amplitude of the calcium current during the pulse arise from time-dependent changes in the mean closed time of the DHPR, which must not affect the ability of an L-type channel opening to elicit a spark. The latter assumption is made more reasonable by experiments and calculations that suggest that sparks are most likely to be activated by the first opening of a nearby DHPR (Cannell et al., 1994; Cannell and Soeller, 1997). The ratio of the integrals of P Spark and the L-type calcium current therefore provides a quantitative measure of the efficiency by which sparks can be activated by the local DHPR flux (i) (Gó- mez et al., 1997). h 2(i) has a similar voltage dependence to the measure P Spark/I Ca used by Santana et al. (1996), supporting the idea that P Spark has a nonlinear (possibly a square power) dependence on the local calcium level (which should be proportional to i; see Soeller and Cannell, 1997) in the junctional space between the t-tubule and the SR (Cannell et al., 1994; Santana et al., 1996). This conclusion arises from consideration of Eq. 1, where if f(i) = ki x (reflecting mass action and that the local calcium level is proportional to i), then P Spark/I Ca (or the ratio of their mean values) will contain i x − 1 . i x − 1 should decline approximately exponentially with voltage if x > 1, assuming that τ is a weak and monotonically increasing function of voltage (see Mazzanti et al., 1991). Since P Spark/I Ca and the ratio of their integrals shows such an exponential voltage dependence (see Santana et al., 1996; Gómez et al., 1997), this suggests that P Spark must depend on something like the square of the local calcium level produced by the activity of nearby DHPRs.
If P Spark is proportional to [Ca2+]2, then this result can explain how the ∼100-fold local changes in calcium that should develop during a DHPR opening (see Soeller and Cannell, 1997, for calculations) can produce such a massive increase in P Spark (estimated to be ∼104; see above). In addition, a square relation will ensure that adjacent spark sites are less likely to activate each other as diffusion over the distance of the sarcomere leads to the local [Ca2+] declining quite rapidly (Cannell and Allen, 1984; Wier and Yue, 1986). Since sparks do not normally spread between sarcomeres (but see Parker et al., 1996; Blatter et al., 1997), one would not expect SR calcium release to propagate throughout the cell (Trafford et al., 1993) and the spatial dissociation of spark sites helps limit regenerative behavior inherent in the calcium-induced calcium release mechanism (as described by Fabiato, 1983).
In summary, cardiac E–C coupling is the result of the spatial and temporal summation of many “elementary” calcium sparks that are triggered via calcium influx across the surface membrane. This influx causes the local calcium in the junctional regions of the SR to increase, and the nonlinear relationship between the probability of activating a spark and the local calcium concentration causes a very large increase in the probability of spark occurrence and hence rate of calcium release by the SR. The idea that E–C coupling occurs in microdomains where local calcium levels are quite different (in both time course and spatial extent) from what is measured by conventional whole cell methods has essentially been proven by the discovery of calcium sparks. By considering what happens in the microdomain of the junctional space, new “local control” theories are being developed that provide an underpinning for how E–C coupling can achieve high gain and stability simultaneously (e.g., Stern, 1992; Cannell et al., 1995), which was always a problem for “common pool” models of E–C coupling (Stern, 1992). Despite the recent rapid progress in understanding cardiac E–C coupling, there are still many important questions to be addressed; e.g.: (a) Is the spark really an elementary event or do subsets of SR release channels in the junction sometimes open during normal E–C coupling? If they do, is this the basis of the smaller calcium sparks (termed calcium “quarks”) proposed by Lipp and Niggli (1996)? (b) What is the time course of SR release channel gating during the spark? How is SR calcium release terminated? Is the time course sensitive to physiological modulation? (c) To what extent can other calcium influx mechanisms (such as the Na/Ca exchange [e.g., see Levi et al., 1993] and T-type calcium channels [e.g., see Sipido et al., 1998]) trigger spark production? (d) Why do mathematical models of calcium sparks (e.g., Pratusevich and Balke, 1996; Smith et al., 1998) generate “sparks” that are spatially smaller than actually observed (i.e., ∼1 μm in diameter)? (e) What is the relationship between the ultrastructural organization of a junctional region and the properties of E–C coupling at that site?
Footnotes
Original version received 18 December 1998 and accepted version received 15 January 1999.
references
- Blatter LA, Huser J, Ríos E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+sparks in cardiac muscle. Proc Natl Acad Sci USA. 1997;94:4176–4181. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Allen DG. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984;45:913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]iduring excitation–contraction coupling in cardiac myocytes. Biophys J. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1050. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Soeller C. Numerical analysis of ryanodine receptor activation by L-type channel activity in the cardiac muscle diad. Biophys J. 1997;73:112–122. doi: 10.1016/S0006-3495(97)78052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]iwaves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Gómez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation–contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Levi AJ, Brooksby P, Hancox JC. A role for depolarisation induced calcium entry on the Na–Ca exchange in triggering intracellular calcium release and contraction in rat ventricular myocytes. Cardiovasc Res. 1993;27:1677–1690. doi: 10.1093/cvr/27.9.1677. [DOI] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol (Camb) 1996;492:31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Wiesner TF, Gyorke S. Termination of Ca2+ release during Ca2+sparks in rat ventricular myocytes. J Physiol (Camb) 1998;507:667–677. doi: 10.1111/j.1469-7793.1998.667bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, DeFelice LJ, Liu Y-M. Gating of L-type Ca2+channels in embryonic chick heart cells: dependence on voltage current and channel density. J Physiol (Camb) 1991;443:307–334. doi: 10.1113/jphysiol.1991.sp018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Alvarez R, Kettlun C, Ríos E, Stern M, Fill M. Unitary calcium currents through cardiac ryanodine receptors under physiological conditions. Biophys J. 1998;74:A58. doi: 10.1085/jgp.113.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescent chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- Pape PC, Jong DS, Chandler WK. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. J Gen Physiol. 1995;106:259–336. doi: 10.1085/jgp.106.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Zang WJ, Wier WG. Ca2+ sparks involving multiple Ca2+release sites along Z-lines in rat heart cells. J Physiol (Camb) 1996;497:31–38. doi: 10.1113/jphysiol.1996.sp021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratusevich VR, Balke CW. Factors shaping the confocal image of the calcium spark in cardiac muscle cells. Biophys J. 1996;71:2942–2957. doi: 10.1016/S0006-3495(96)79525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana LF, Cheng H, Gómez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+sparks and local control theories for cardiac excitation–contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Shacklock PS, Wier WG, Balke CW. Local Ca2+ transients Ca2+sparks originate at transverse tubules in rat heart cells. J Physiol (Camb) 1995;487:601–608. doi: 10.1113/jphysiol.1995.sp020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Carmeliet E, Van de Werf F. T-type Ca2+ current as a trigger for Ca2+release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. J Physiol (Camb) 1998;508:439–451. doi: 10.1111/j.1469-7793.1998.439bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Wier WG. Flux of Ca2+across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation– contraction coupling. J Physiol (Camb) 1991;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Keizer JE, Stern MD, Lederer WJ, Cheng H. A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys J. 1998;75:15–32. doi: 10.1016/S0006-3495(98)77491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J. 1997;73:97–111. doi: 10.1016/S0006-3495(97)78051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L-S, Sham JSK, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ‘Ca2+spikes' in rat cardiac myocytes. J Physiol (Camb) 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation–contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, O'Neill SC, Eisner DA. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflügers Arch. 1993;425:181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Wier WG, Yue DT. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. J Physiol (Camb) 1986;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]


