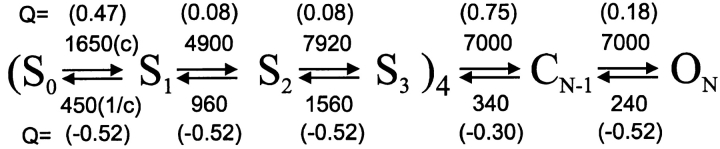Opening the Shaker K channel should be easy. Release the membrane potential that normally holds it closed, and massive stored molecular energies snap it rapidly and inexorably into its open configuration. Unlocking the inner secrets of this voltage-dependent channel's gating mechanism has proven to be much more difficult. An article by Ledwell and Aldrich in this issue has opened that door significantly.
An impressive array of experimental techniques and intellectual powers have been used to attack this problem over the past half century. Techniques such as the voltage clamp for measuring macroscopic and gating currents, and the patch clamp for small cells and single channels were developed to study voltage-dependent channels. Molecular cloning and mutagenesis, cysteine-site availability, limiting slope measurements, fluorescence labeling, rescue mutations, as well as temperature, pressure, water, and ionic alterations have all been brought to bear on the problem. An acceleration of progress is apparent through the concerted efforts of many groups.
Over the past year, The Journal of General Physiology has hosted the latest round of discussion concerning the activation mechanism of voltage-dependent K channels (e.g., Cha and Bezanilla, 1998; Loots and Isacoff, 1998; Rodríguez et al., 1998; Roux et al., 1998; Schoppa and Sigworth, 1998a–c; Smith-Maxwell et al., 1998a,b; Zheng and Sigworth, 1998; Bao et al., 1999). A fascinating picture is emerging. Building on extensive previous work (Bezanilla et al., 1994; Hoshi et al., 1994; Zagotta et al., 1994), Schoppa and Sigworth (1998a–c) started off with a detailed analysis of macroscopic, gating, and single channel currents in two forms of the channel. The “wild-type” channel, modified to remove fast inactivation, is the basic starting type for all the studies presented here. In addition, they examined the V2 mutant, which slows activation. Their concluding model, though differing in detail from the models of others, presents what has become a consensus view.
When closed, the channel's positively charged S4 segments (one in each of the channel's four subunits) are cocked toward the cytoplasmic side of the membrane. Upon depolarization, these S4 charges are pulled through the membrane's electric field in a series of steps that proceed essentially independently in each subunit. After this unlatching, it appears that the subunits act cooperatively to produce the final steps of opening a central conductive pore. Schoppa and Sigworth's (1998c) 3+2 model quantitatively describes these steps, as shown here in Scheme I.
Three independent steps are shown for each of the four subunits, followed by two cooperative steps. At 0 mV, each reaction is fast in the forward direction, and slow to reverse (the rate per second at 0 mV is shown with each step above). Note, however, that most of the voltage dependence (i.e., the effective charge, Q, that determines how the unidirectional reaction rates change with voltage) is in the reverse reactions. At the resting potential, the reverse reaction rates will be greatly increased, strongly enhancing the probability that the channel is in one of its closed states.
When looking at these types of kinetics, we've grown used to thinking in terms of rates, charges, and the reactions necessary to open a voltage-dependent channel. What has been emphasized less, even if known by all, is that channels desperately want to be open. When no membrane field is applied (e.g., at 0 mV), they essentially “fall” into the open state, and barring inactivation or other flickers, would stay there. Seen from this perspective, the challenge is to keep the channel closed reliably!
Recent work of Bao et al. (1999) highlights this point. When most of the charge is mutated out of the Shaker S4 (specifically charges in the 1, 2, 4, and 7 positions), the primary functional deficit is the ability of voltage to close the channel. The pore conducts normally, and appears to retain vestiges of C-type inactivation, pore gating, etc., but this activity is now uncoupled from voltage.
Consider the forces that are applied in dragging the channel back from its open state. The average force on a single charge in a voltage gradient, like that inside a membrane, is easily calculated by F = q d V/dx, where volts, V, are defined as J/C, a single charge q is 1.6 × 10−19 C, and dx is (at greatest) the width in meters of the hydrophobic portion of bilayer or protein. A reasonable minimum estimate of d V/dx at −100 mV potential is 2.5 × 107 V/meter, which will give a force per charge of at least 4 pN. This seems a trivial force until one compares it to the nearly identical range of forces generated by each myosin molecule in a muscle (Huxley and Tideswell, 1996; Guilford et al., 1997). Indeed, the work done in moving the channel's 12–14 effective charges through the membrane field during gating is equivalent to the energy derived from splitting four ATP molecules. As the channel closes during hyperpolarization, all this force is brought to bear on the molecular machinery. Work is done pulling back on internal springs, “cocking” the channel for the next quick release.
These concepts, though general, serve to focus further questions for understanding channel function. How do the forces that result from hyperpolarization cause the disassembly of the open state? Do all charges participate equally in delivering these forces, or are the steps truly sequential, with only a few charges exerting force during any one step? Is there a subset of the charges in the S4 that also drives the final, channel-wide rearrangements surrounding the pore opening? Are the reaction steps that close the channel simply the reverse of the channel's opening steps?
Unfortunately, the wild-type channel is not always conducive to the study of such detailed mechanistic questions. The rates and voltage dependencies of its various reactions are closely tuned and often quite fast. Even by studying its kinetics under conditions (e.g., at extremes of voltage) that bias measured rates toward single reaction steps, as Schoppa and Sigworth (1998a–c) have done, the Shaker channel but grudgingly unlocks its secrets.
A series of studies by Smith-Maxwell et al. (1998a,b) and Ledwell and Aldrich (1999) have helped to bypass this problems and answer some of the questions. They noted in a pair of articles published here last year (Smith-Maxwell et al., 1998a,b) that several relatives of the Shaker channel have distinctly different patterns of gating. In particular, the Shaw form of the channel has less voltage dependence to its gating, requires much more positive potentials to open, and has slower, monoexponential, kinetics. To determine if these properties were linked to alterations in the S4 structure, they swapped the whole Shaw S4 sequence into a Shaker channel. In the first article, they showed that this swap confers a number of Shaw-like properties into Shaker. The Shaker-Shaw-S4 chimera channel activates slowly with monoexponential kinetics, and requires positive potentials to open significantly. Using kinetic analysis and tandem dimer expression, they concluded that the primary alteration was in the step closest to opening, where subunits have their primary cooperativity.
Smith-Maxwell et al. (1998b) showed that, of the 11 different amino acids in the Shaw S4 (including neutralization or reversal of the first, second, and seventh charges), mutating just three noncharged residues (V369I, I372L, and S376T, called “ILT”) of the wild-type Shaker channel into the Shaw equivalents made a critical difference. This ILT mutation gave the wild-type channel properties that were virtually identical to the Shaw-S4 chimera. They further demonstrated with quantitative modeling that virtually all of their results could be explained by raising the barrier height, slowing the final cooperative opening transition, and by increasing the charge that powers this reaction step. These results, along with those of Schoppa and Sigworth's V2 mutation, provide evidence for a functional distinction between early independent gating transitions and late cooperative ones.
But, if the ILT charge-neutral mutation gave rise to behavior virtually identical to the Shaw-S4 chimera, what is the role of the altered charge in Shaw? Do these charges, which have been implicated in other studies (e.g., Bao et al., 1999) as being important in determining the Shaker channel's voltage dependence, become irrelevant in Shaw?
Ledwell and Aldrich (1999), published in this volume, answers these questions. The work compares the gating properties of the ILT and Shaw-S4 mutant channels, with particular emphasis on establishing the properties of the now separable early and late transitions.
The ILT channel was quantified and modeled in terms of its macroscopic currents, its gating currents, and its activation delays (i.e., sigmoidicity or Cole-Moore shift). Most of the gating charge moves quickly and at negative voltages. There is also now a clearly distinguishable fraction of gating charge (∼13%) that is associated with the slowed opening steps at positive potentials. The ILT channel also shows the familiar activation delays that seem to be the hallmark of channels with extensive S4 movement.
Interestingly, the Shaw-S4 mutant was found to lack these early voltage-dependent transitions almost completely. Despite extensive efforts, no pulse protocol or voltage range could be found that caused either activation delays or fast charge movement in the Shaw-S4 mutant channel. Instead, its gating charge was slow and flowed at more positive potentials, like the 13% subset in the ILT channel. Returning three charges (located where the first, second, and seventh charges of Shaker are found in the wild-type S4 region) to the Shaw-S4 rescued the early charge-moving steps in the mutant channel. The Shaw-S4 channel demonstrates, in effect, the completely separate nature of the late transition, which can be seen to function in isolation in this channel.
As these results unfold, so too does a more complete picture of Shaker channel gating function. Only a subset of the gating charge (∼1.8 e o for the whole channel) appears to be involved directly with creating or destroying the channel's conductive state (i.e., the final transition between closed and open). Whereas in the wild-type channel this transition is fast and easy, it requires extra energy in both ILT and Shaw due to changes in S4's hydrophobic residues. Like a door that is misaligned and hangs on stiff hinges, the ILT and Shaw channel's opening and closing steps require extra force. The 1.8 effective charges, therefore, can be observed providing that force in both the forward and backward direction.
These late reactions are concerted or cooperative steps; i.e., the configuration of each subunit influences its neighbors. Intermediate states are unstable and the subunits tend to stay in precise lock-step with one another. Thus, the gating charge for this reaction step is likely to be distributed between the subunits. The location of this charge is still unknown, although the recent results of Bao et al. (1999) might cast eyes upon the fourth S4 charge.
And what of the remaining charges? It is now clear that the independent S4 movements don't contribute directly to the forces necessary to close the conducting pore. The massive energies dedicated here appear to be involved, rather, in bolting the door once closed, much like a series of dead bolts on an urban apartment entry. Opening the Shaker channel is easy—too easy. Our new perspective on gating function implies that keeping it shut reliably is the challenge for which much of S4's charges are adapted.
As the puzzle pieces (many of them published here in the past year) fall into place, a clearer picture of voltage-dependent channel gating is taking shape. The picture, in turn, may help to place further pieces. This cooperative interaction of many excellent laboratories may thus serve to unlock rapidly the secrets of this important gating function.
Scheme I.
Footnotes
The author thanks Dick Horn and Mark Nelson for their helpful discussions and comments.
references
- Bao H, Hakeem A, Henteleff M, Starkus JG, Rayner MD. Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shakerchannels. J Gen Physiol. 1999;113:139–151. doi: 10.1085/jgp.113.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Perozo E, Stefani E. Gating of ShakerK channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994;66:1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, Bezanilla F. Structural implications of fluorescence quenching in the Shaker K+channel. J Gen Physiol. 1998;112:391–408. doi: 10.1085/jgp.112.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM. Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophys J. 1997;72:1006–1021. doi: 10.1016/S0006-3495(97)78753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Shakerpotassium channel gating. I: Transitions near the open state. J Gen Physiol. 1994;103:249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Tideswell S. Filament compliance and tension transients in muscle. J Muscle Res Cell Motil. 1996;17:507–511. doi: 10.1007/BF00123366. [DOI] [PubMed] [Google Scholar]
- Ledwell JL, Aldrich RW. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J Gen Physiol. 1999;113:389–414. doi: 10.1085/jgp.113.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots E, Isacoff EY. Protein rearrangements underlying slow inactivation of the Shaker K+channel. J Gen Physiol. 1998;112:377–389. doi: 10.1085/jgp.112.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez BM, Sigg D, Bezanilla F. Voltage gating of Shaker K+channels. The effect of temperature on ionic and gating currents. J Gen Physiol. 1998;112:223–242. doi: 10.1085/jgp.112.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Olcese R, Toro L, Bezanilla F, Stefani E. Fast inactivation in Shaker K+channels. Properties of ionic and gating currents. J Gen Physiol. 1998;111:625–638. doi: 10.1085/jgp.111.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels. I. Characterization of voltage-dependent transitions. J Gen Physiol. 1998a;111:271–294. doi: 10.1085/jgp.111.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels. II. Kinetics of the V2 mutant channel. J Gen Physiol. 1998b;111:295–311. doi: 10.1085/jgp.111.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels. III. An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998c;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J Gen Physiol. 1998a;111:399–420. doi: 10.1085/jgp.111.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J Gen Physiol. 1998b;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Shakerpotassium channel gating. III. Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Sigworth FJ. Intermediate conductances during deactivation of heteromultimeric Shakerpotassium channels. J Gen Physiol. 1998;112:457–474. doi: 10.1085/jgp.112.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]