Abstract
Swelling-induced activation of the outwardly rectifying anion current, ICl, swell, is modulated by intracellular ATP. The mechanisms by which ATP controls channel activation, however, are unknown. Whole cell patch clamp was employed to begin addressing this issue. Endogenous ATP production was inhibited by dialyzing N1E115 neuroblastoma cells for 4–5 min with solutions containing (μM): 40 oligomycin, 5 iodoacetate, and 20 rotenone. The effect of ATP on current activation was observed in the absence of intracellular Mg2+, in cells exposed to extracellular metabolic inhibitors for 25–35 min followed by intracellular dialysis with oligomycin, iodoacetate, and rotenone, after substitution of ATP with the nonhydrolyzable analogue AMP-PNP, and in the presence of AMP-PNP and alkaline phosphatase to dephosphorylate intracellular proteins. These results demonstrate that the ATP dependence of the channel requires ATP binding rather than hydrolysis and/or phosphorylation reactions. When cells were swollen at 15–55%/min in the absence of intracellular ATP, current activation was slow (0.3–0.8 pA/pF per min). ATP concentration increased the rate of current activation up to maximal values of 4–6 pA/pF per min, but had no effect on the sensitivity of the channel to cell swelling. Rate of current activation was a saturable, hyperbolic function of ATP concentration. The EC50 for ATP varied inversely with the rate of cell swelling. Activation of current was rapid (4–6 pA/pF per min) in the absence of ATP when cells were swollen at rates ≥65%/min. Intracellular ATP concentration had no effect on current activation induced by high rates of swelling. Current activation was transient when endogenous ATP was dialyzed out of the cytoplasm of cells swollen at 15%/min. Rundown of the current was reversed by increasing the rate of swelling to 65%/min. These results indicate that the channel and/or associated regulatory proteins are capable of sensing the rate of cell volume increase. We suggest that channel activation occurs via ATP-dependent and -independent mechanisms. Increasing the rate of cell swelling appears to increase the proportion of channels activating via the ATP-independent pathway. These findings have important physiological implications for understanding ICl, swell regulation, the mechanisms by which cells sense volume changes, and volume homeostasis under conditions where cell metabolism is compromised.
Keywords: cell volume, organic osmolytes, metabolic regulation, mechanotransduction
introduction
Maintenance and regulation of cell volume are fundamental physiological processes. In response to swelling, cells activate anion and cation channels that allow the passive loss of inorganic ions and organic solutes. Net solute and osmotically obliged water efflux functions to return cell volume towards its original value, a process termed regulatory volume decrease.
Swelling of vertebrate cells activates an outwardly rectifying anion current termed ICl, swell. The general characteristics of ICl, swell include an Eisenman type I anion permeability sequence (I− > Br− > Cl− > F−), modest outward rectification, voltage-dependent inactivation at potentials above ECl, inhibition by a wide variety of compounds, including conventional anion transport inhibitors, and block by extracellular nucleotides (Strange et al., 1996; Nilius et al., 1997; Okada, 1997). The degree of rectification, voltage sensitivity, and pharmacology of ICl, swell can vary somewhat between different cell types. It is not clear whether the differences observed reflect the existence of distinct channels or whether they are due to experimental and/or physiological variables.
The mechanism by which swelling activates ICl, swell is unknown (Strange et al., 1996; Nilius et al., 1997; Okada, 1997). However, two important physiological parameters have been shown to modulate channel activation. Changes in cytoplasmic ionic strength alter both the rate of channel activation and channel volume sensitivity (Emma et al., 1997; Cannon et al., 1998; Nilius et al., 1998), and swelling-induced activation requires the presence of intracellular ATP (reviewed by Strange et al., 1996; Nilius et al., 1997; Okada, 1997).
Intracellular ATP plays a crucial role in regulating many ion channels and transporters (e.g., Bryan and Aguilar-Bryan, 1997; Hilgemann, 1997). In most cases, the underlying cellular and molecular mechanisms of ATP-dependent regulation are incompletely understood. Hilgemann (1997) recently reviewed the mechanisms by which ATP can regulate channel/transporter function. These mechanisms include protein and lipid phosphorylation, ATP binding, ATP hydrolysis, modulation of cytoskeletal function, chelation of polyvalent cations, and modulation of membrane trafficking.
The mechanisms of ATP-dependent regulation of the ICl, swell channel are largely unknown. Most studies of ICl, swell have shown that nonhydrolyzable ATP analogues readily substitute for intracellular ATP, suggesting that the requirement for ATP involves binding rather than phosphorylation or hydrolysis reactions (reviewed by Strange et al., 1996; Nilius et al., 1997; Okada, 1997). However, studies in cultured mouse cortical collecting duct (Meyer and Korbmacher, 1996) and carotid body type I cells (Carpenter and Peers, 1997) indicate that phosphorylation of the ICl, swell channel and/or accessory proteins is required for swelling-induced activation.
In the present investigation, we demonstrate that ICl, swell activation in N1E115 neuroblastoma cells does not involve ATP hydrolysis or phosphorylation reactions. Furthermore, we demonstrate that the ATP dependence of the ICl, swell channel is modulated by the rate of cell volume change. At high rates of swelling, ICl, swell activates rapidly and activation is insensitive to intracellular ATP concentration. Our results indicate that there are ATP-dependent and -independent mechanisms by which ICl, swell can be activated. In addition, they demonstrate that cells sense not only the magnitude of volume change, but also the rate at which volume is perturbed. These findings have important physiological implications for control of both cell volume and metabolic homeostasis, and they provide new insights into regulation of the ICl, swell channel.
materials and methods
Cell Culture
N1E115 mouse neuroblastoma cells were cultured in high glucose Dulbecco's modified eagle's medium (DMEM; GIBCO BRL) with 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 25 mM HEPES in the presence of 5% CO2/ 95% air. Cells were used between passages 30 and 45. The osmolality of the growth medium was measured by vapor pressure osmometry (5520; Wescor Inc.) and was 295–305 mOsm.
Patch Clamp Recordings
N1E115 cells were grown in 35-mm culture dishes and dissociated by brief treatment with Ca2+- and Mg2+-free modified Hank's solution. Dissociated cells were allowed to reattach to the poly-l-lysine–coated cover slip bottom of a bath chamber (R-26G; Warner Instrument Corp.) that was mounted onto the stage of an inverted microscope (TE 300; Nikon Inc.). Patch electrodes were pulled from 1.5-mm outer diameter borosilicate glass microhematocrit tubes (Fisher Scientific Co.) that had been salinized with dimethyl-dichloro silane (Sigma Chemical Co.). Electrodes were not fire polished before use.
The standard bath solution contained (mM): 70 N-methyl- d-glucamine (NMDG)1-Cl, 5 MgSO4, 1.3 CaCl2, 12 HEPES, 8 Tris, 5 glucose, 2 glutamine, 120 sucrose (pH 7.4; 300 mOsm).
Bath was reduced to 150–250 mOsm by reducing sucrose concentration. In one set of experiments, bath was reduced to 100 mOsm. For these studies, the NMDG-Cl concentration in the control (i.e., 300 mOsm) bath solution was reduced to 40 mM and sucrose concentration was increased to 200 mM.
Patch clamping was carried out using a pH 7.2 pipette solution that contained 125 mM CsCl, 20 mM HEPES, 6 mM CsOH, 1 mM EDTA, 40 μM oligomycin, 5 μM iodoacetate, and 20 μM rotenone. Sodium and K+-free bath and pipette solutions were used to minimize the contribution of cation currents to the whole cell conductance. To prevent spontaneous cell swelling, the osmolality of the pipette solution was hypotonic (280 mOsm) with respect to the bath. Metabolic inhibitors were added from concentrated stock solutions dissolved in DMSO. Final DMSO concentration in the pipette solutions was 0.15%. ATP and ATP analogues were added as sodium and lithium salts, respectively. Electrodes had DC resistances of 3–5 mΩ. Cells were used only if the series resistance was no greater than ∼150% of the pipette resistance and the reversal potential (Erev) was within ±3 mV of the calculated value.
An Axopatch 200A (Axon Instruments) patch clamp amplifier was used to voltage clamp N1E115 cells after gigaseal formation and attainment of whole cell access. Command voltage generation, data digitization, and data analysis were carried out on a 200 mHz Pentium computer (Dimension XPS M200s; Dell Computer Corp.) using a DigiData 1200 AD/DA interface with pClamp 6 software (Axon Instruments). Data were digitized at 5 kHz and filtered at 0.5 kHz using an eight pole Bessel filter (902; Frequency Devices). Electrical connections to the amplifier were made using Ag/AgCl pellets and 3 M KCl/agar bridges. Whole cell currents were measured by varying membrane potential from −80 to +80 mV at 80 mV/s every 5–15 s.
Cells were swollen by reduction of bath osmolality. At the end of a patch clamp recording, bath osmolality was returned to 300 mOsm and another cell was selected for study. Cells were exposed to hypotonicity approximately every 15–25 min and to at most four to five hypotonic shocks before they were removed and replaced with fresh cells. This number of repetitive hypotonic shocks had no effect on the rate or extent of current activation (data not shown).
Whole cell current measured before induction of cell swelling is defined as “baseline current.” This current was recorded every 5 s for 60 s before the bath osmolality was reduced. These 13 data points were averaged to yield a mean baseline current. This current was subsequently subtracted from all data points within a given current record to correct for variability in resting current levels between different cells.
Measurement of Relative Cell Volume Changes
Whole cell currents and volume changes were measured simultaneously in single, patch clamped cells. Cells attached to the cover slip bottom of the patch clamp bath chamber were visualized by video-enhanced differential interference contrast microscopy (Strange and Spring, 1986) using a 63× oil-immersion objective lens (1.25 numerical aperture; Carl Zeiss, Inc.) and a 32× condenser lens (0.4 numerical aperture; Leitz). Optical sectioning (Strange and Spring, 1986) demonstrated that the cells maintained a spherical morphology for at least 60 min after attachment to the cover slip. Cells were routinely removed from the bath chamber and replaced with fresh ones every 30–45 min. Given that the cells have a spherical morphology, relative cell volume change can be determined as:
 |
where CSA is the cell cross-sectional area (CSA) measured at a single focal plane. In all CSA measurements described in this paper, we used focal planes located at the point of maximum cell diameter.
Cell images were recorded continuously throughout a patch clamp experiment using a super VHS video cassette recorder (SVO-2000; Sony Electronics, Inc.) and a CCD camera (C2400; Hamamatsu Photonics K.K.). Cross-sectional areas of single cells were quantified by digitizing recorded video images with an image processing computer board (MV-1000; MuTech) with 512 × 480 × 8 bit resolution and a 300 mHz Pentium II computer (Dimension XPS D300; Dell Computer Corp.). Digitized images were displayed on the computer monitor and cell borders were traced using a mouse and a computer-generated cursor. The CSA of a traced region was determined by image analysis software (Optimas; Bioscan, Inc.). This image acquisition and analysis system allows detection of changes in CSA with an accuracy of ±2–3%. Optical sectioning methods (Strange and Spring, 1986) have demonstrated that this approach reliably tracks relative cell volume increases up to 250% of control values (our unpublished observations).
During swelling, a small percentage of cells exhibited bleb formation. These cells were excluded from the analysis of both volume changes and current activation.
Cell volume typically increased in a linear fashion when bath osmolality was reduced. The rate of cell swelling was therefore determined by performing linear regression analysis on volume changes measured for 30–60 s after swelling was initiated.
Data Analysis
The effect of intracellular ATP concentration on ICl, swell was quantified using two parameters, the rate of current activation initiated by cell swelling and the cell volume set-point of the channel. Cell volume set-point is defined as the volume of the cell at which current activation is initiated.
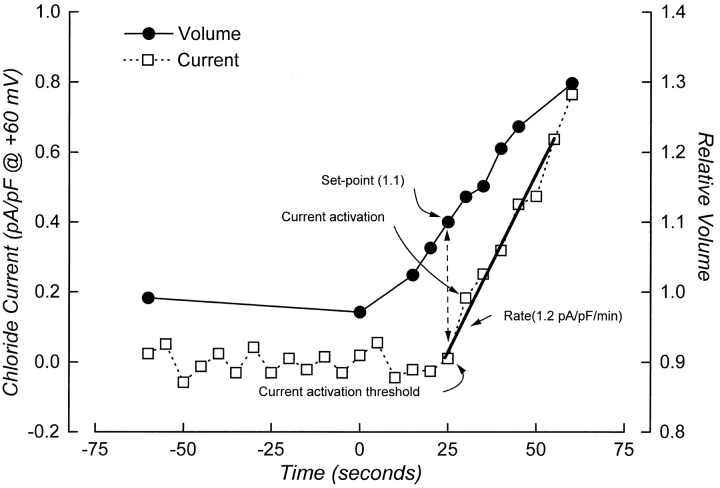
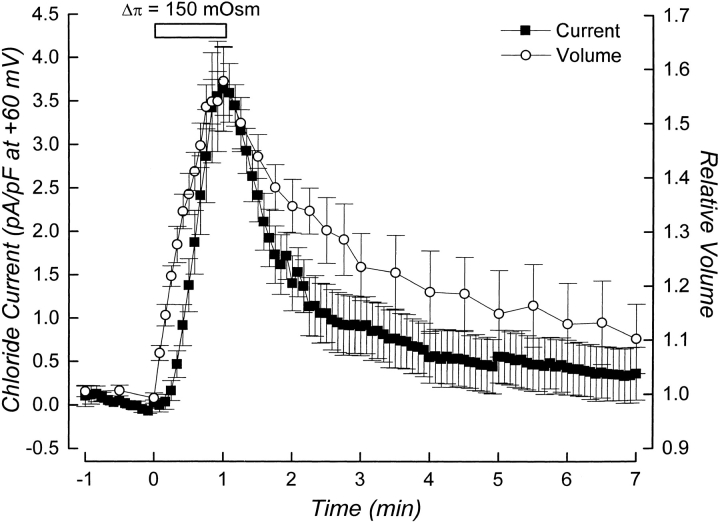
Rate of current activation and cell volume set-point were measured simultaneously in single, patch clamped cells. Fig. 1 shows volume and current measurements in a single cell and illustrates how the set-point and rate of current activation were quantified. Current activation is defined as the point at which there is a significant and continuous increase in current amplitude above the baseline current. Rate of current activation was quantified by performing linear regression analysis on whole cell currents measured for 30–60 s after activation was detected. The threshold for current activation is the intercept between the line defining rate of current activation and the current immediately before activation begins (see Fig. 1). The relative volume of the cell at the current activation threshold is defined as the cell volume set-point of the channel.
Figure 1.
Example of simultaneous current and volume measurements performed on a single, patch-clamped cell. The figure illustrates how the cell volume set-point of the channel and the rate of current activation were quantified. Current activation is defined as the point at which there is a significant and continuous increase in current amplitude above the baseline current. Rate of current activation was quantified by performing linear regression analysis on whole cell currents measured for 30–60 s after activation was detected. The threshold for current activation is the intercept between the line-defining rate of current activation and the current before activation begins. The relative volume of the cell at the current activation threshold is defined as the cell volume set-point of the channel. Cell was swollen by exposure to a 75-mOsm reduction in bath at time zero.
Statistical Analysis
Data are presented as means ± SEM. Statistical significance was determined using Student's two-tailed t test for unpaired, independent means. When comparing three or more groups, statistical significance was determined by one-way analysis of variance. P ≤ 0.05 indicated statistical significance.
Enzymes
Grade VI apyrase was purchased from Sigma Chemical Co. Alkaline phosphatase and creatine kinase were purchased from Boehringer Mannheim Biochemicals.
results
The ATP Requirement of ICl, swell Activation Does Not Involve Hydrolysis and/or Phosphorylation Reactions
In most cell types, ICl, swell activation is supported normally by nonhydrolyzable ATP analogues (reviewed by Strange et al., 1996; Nilius et al., 1997; Okada, 1997). However, in carotid body type 1 and mouse cortical collecting duct cells, phosphorylation reactions appear to be needed for channel activation (Meyer and Korbmacher, 1996; Carpenter and Peers, 1997). The reason for these contradictory findings is unclear and we therefore carried out a series of experiments to look more closely at the role of phosphorylation.
In their studies on cortical collecting duct cells, Meyer and Korbmacher (1996) exposed cells to extracellular metabolic inhibitors for 20–45 min before they were patch clamped. Such prolonged metabolic inhibition may result in dephosphorylation of the channel and/or other proteins. If the putative phosphorylation of these proteins is crucial to channel function, then intracellular ATP rather than nonhydrolyzable analogues would be required for swelling-induced activation. We therefore pretreated N1E115 neuroblastoma cells with 5 mM 2-deoxyglucose and 100 nM rotenone for 25–35 min, and then patch clamped them with the standard pipette solution containing (μM) 40 oligomycin, 5 iodoacetate, and 20 rotenone. Both 2-deoxyglucose and rotenone were present in the bath throughout the entire experiment. Cells were dialyzed for 4–5 min with the metabolic inhibitors before swelling was induced. Preliminary studies demonstrated that a dialysis period of 2–3 min was sufficient to maximally inhibit swelling-induced current activation, presumably by maximally reducing intracellular ATP levels.
In the presence of intracellular metabolic inhibitors and ATP or ATP analogues, an outwardly rectifying (data not shown) current was activated when cells were swollen by reduction of bath osmolality. With 73 and 125 mM Cl− in the bath and pipette solutions, respectively, the mean ± SEM Erev observed was 13.1 ± 0.3 mV (n = 109). This value is close to the value of 12.8 mV predicted from the Goldman-Hodgkin-Katz equation and the previously measured relative cation conductance (P cation/P Cl) of 0.03 (Jackson and Strange, 1993). These results indicate that the current activated by swelling is a Cl− current (i.e., ICl, swell).
Results of the metabolic inhibition studies are shown in Fig. 2 A. When exposed to intracellular metabolic inhibitors alone, and a Mg2+-free pipette solution containing 2 mM ATP, the mean ± SEM rate of chloride current activation measured at +60 mV was 3.95 ± 0.26 pA/pF per min (n = 28). This value was not significantly (P > 0.1) different from those obtained in cells pretreated for 25–35 min with 2-deoxyglucose and rotenone, and then dialyzed with metabolic inhibitors in the presence of 2 mM ATP and 0 mM Mg2+ or 2 mM ATP and 2 mM Mg2+ (Fig. 2 A).
Figure 2.
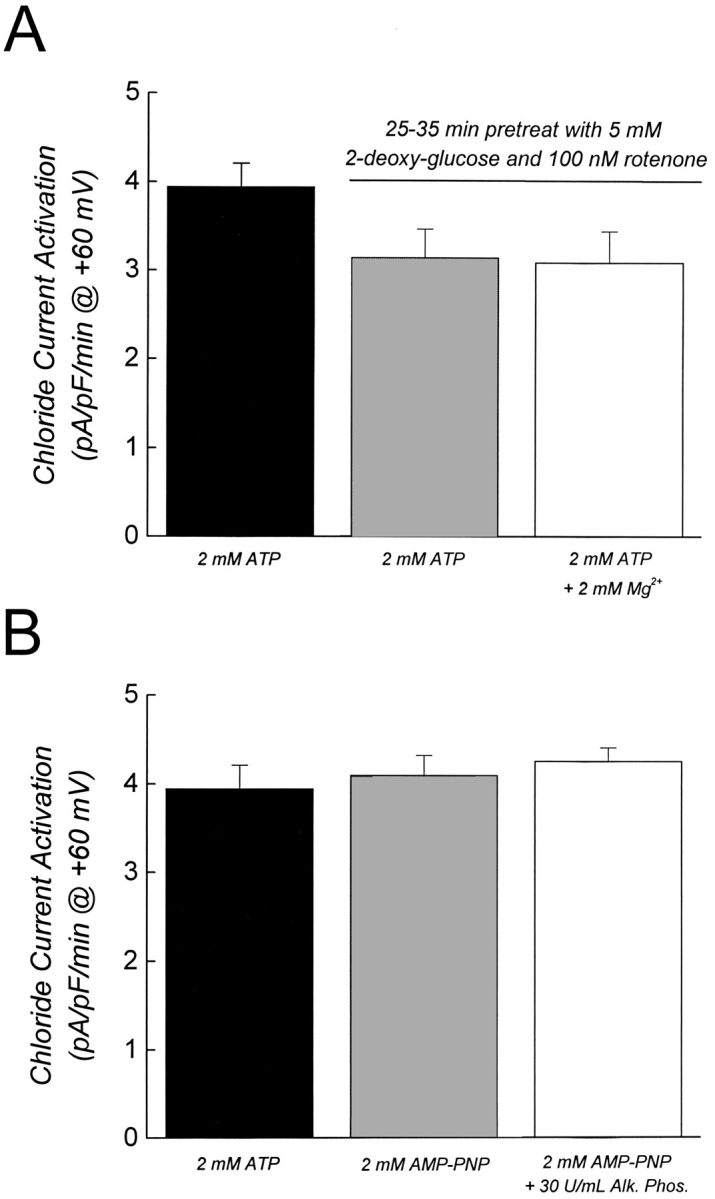
Activation of ICl, swell does not require phosphorylation and/or ATP hydrolysis. (A) Effect of prolonged metabolic inhibition on rate of ICl, swell activation. Cells were maintained in the standard bath solution or were pretreated with 5 mM 2-deoxyglucose and 100 nM rotenone for 25–35 min. Metabolic inhibitors were maintained in the bath throughout the experiment. The three groups of cells were dialyzed for 4–5 min with patch pipette solutions containing metabolic inhibitors before swelling was induced. Except where indicated (open bar), all experiments were done in the absence of intracellular Mg2+ using EDTA buffered solutions. In the Mg2+-containing pipette solution, 1 mM EDTA was replaced with 1 mM EGTA. Values are means ± SEM (n = 4–28). Results were not statistically different (P > 0.1). (B) Effect of intracellular dialysis with alkaline phosphatase. Cells were dialyzed with the patch pipette solution for 4–5 min before swelling was induced. Values are means ± SEM (n = 5–28). Results were not statistically different (P > 0.8). In both A and B, swelling was induced by a 100-mOsm reduction of bath. Rates of cell swelling were similar under all experimental conditions (data not shown).
As a further test for the involvement of phosphorylation in regulating ICl, swell, we patch clamped cells with a pipette solution containing alkaline phosphatase. As shown in Fig. 2 B, there was no significant (P > 0.8) difference in the rate of swelling-induced current activation in the presence of 2 mM ATP and 0 mM Mg2+, 2 mM AMP-PNP, and 0 mM Mg2+, or 2 mM AMP-PNP, 0 mM Mg2+, and 30 U/ml of alkaline phosphatase. We conclude from the results shown in Fig. 2 that swelling- induced activation of ICl, swell in N1E115 neuroblastoma cells requires only ATP binding, and not phosphorylation events and/or ATP hydrolysis.
The Rate of ICl, swell Activation Is a Saturable Function of Intracellular ATP Concentration
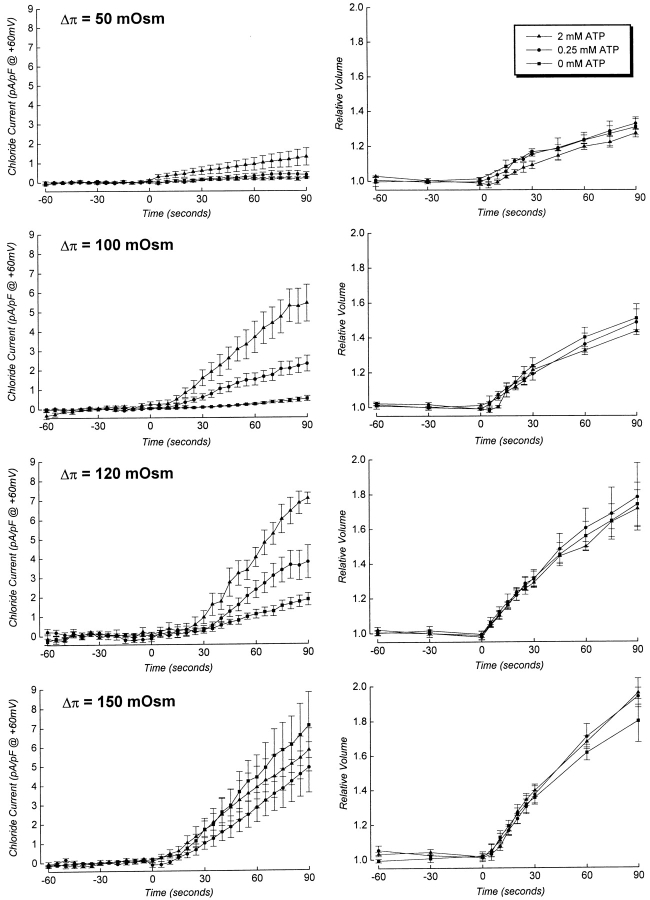
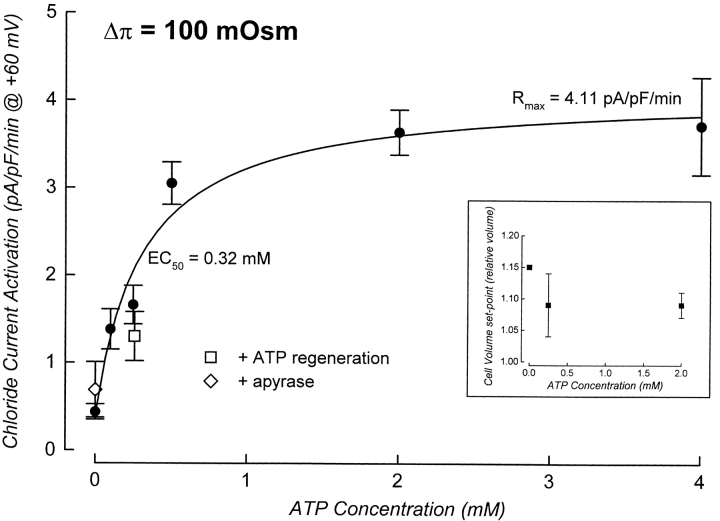
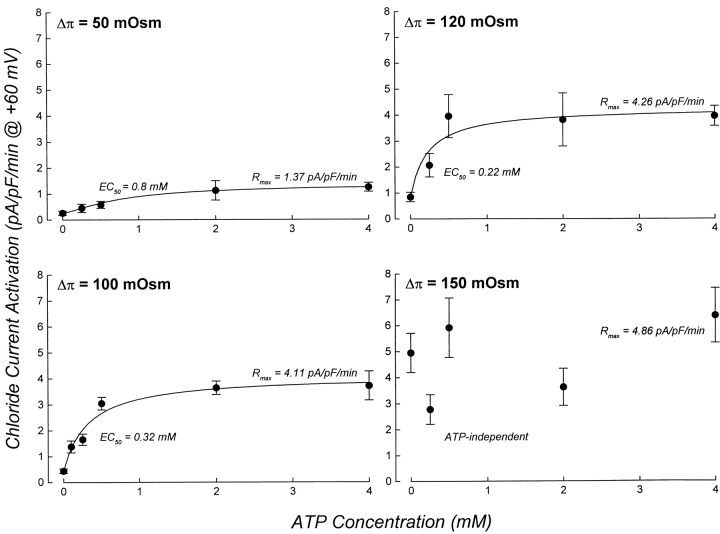
To further characterize the dependence of ICl, swell activation on ATP binding, we quantified the effect of ATP concentration on the rate of current activation, the rate of cell swelling, and the cell volume set-point of the channel simultaneously in single patch-clamped cells. We define cell volume set-point as the relative cell volume at which current activation is initiated (see Fig. 1). In initial experiments, cells were swollen at a rate of 35–40%/min via reduction of bath osmolality by 100 mOsm (see Fig. 3 and Table I). As shown in Fig. 3 (right), intracellular ATP concentration had no effect on the rate of cell swelling. The rate of current activation, however, was increased by increasing ATP levels in the patch pipette solution (Fig. 3, left). The rate of current activation was a saturable function of intracellular ATP concentration (Fig. 4). The maximal rate of current activation (R max) was 4.11 pA/pF per min and the EC50 for ATP was 0.32 mM. The cell volume set-point of the channel was 1.09–1.15 and was unaffected by ATP concentration (Fig. 4, inset, and Table II).
Figure 3.
Effects of intracellular ATP concentration on rate of current activation and rate of cell swelling. Cells were swollen at different rates by a 50–150-mOsm reduction in bath at 0 min. Osmotic shock used is shown in bold in the upper left corner of the current plots. Data are plotted on the same scale to facilitate comparison. Current and volume measurements were made simultaneously in patch clamped cells (e.g., see Fig. 1). Values are means ± SEM (n = 3–9).
Table I.
Rates of Swelling in Patch-Clamped Cells Exposed to Hypotonic Solutions
| Reduction in bath osmolality | Rate of cell swelling | |||
|---|---|---|---|---|
| mOsm | percent/min | n | ||
| 50 | 14.9 ± 2.0 | 14 | ||
| 75 | 25.6 ± 4.0 | 12 | ||
| 100 | 38.1 ± 2.8 | 25 | ||
| 120 | 54.0 ± 3.7 | 17 | ||
| 150 | 68.1 ± 3.7 | 16 | ||
| 200 | 98.7 ± 16 | 4 |
Rates of cell swelling were obtained by averaging data obtained from cells dialyzed with ATP concentrations of 0–4 mM.2 Concentration of ATP had no effect on the rate of volume increase (see Fig. 3). Values are means ± SEM (n).
Figure 4.
 |
Table II.
Cell Volume Set-Points of the ICl, swell Channel Measured in the Presence of Different Osmotic Gradients and Different Intracellular ATP Concentrations
| Reduction in bath osmolality | ATP concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 2 | 4 | |||||
| mOsm | mM | mM | mM | mM | ||||
| 50 | 1.09 (n = 1) | 1.14 ± 0.03 (n = 5) | 1.09 ± 0.06 (n = 3) | |||||
| 75 | 1.28 ± 0.08 (n = 4) | |||||||
| 100 | 1.15 (n = 1) | 1.09 ± 0.05 (n = 3) | 1.09 ± 0.02 (n = 7) | |||||
| 120 | 1.11 ± 0.06 (n = 5) | 1.16 (n = 2) | 1.11 ± 0.03 (n = 4) | |||||
| 150 | 1.14 ± 0.02 (n = 5) | 1.17 ± 0.04 (n = 5) | 1.15 ± 0.03 (n = 4) | |||||
| 200 | 1.21 ± 0.09 (n = 4) | |||||||
The cell volume set-point of the channel is defined as the relative cell volume at which ICl, swell activation is initiated (see Fig. 1). Values are single measurements or means of two or more measurements. Standard errors are presented when n > 2. There were no significant (P > 0.29) differences between any of the measured set-points.
With prolonged exposure to hypotonic medium, N1E115 cells underwent dramatic volume increases (see Figs. 3 and 9). The ability to withstand such large degrees of swelling most likely reflects the presence of extensive membrane microvilli (Walum and Marchner, 1983) and possibly membrane infolding. Similar large volume increases have been observed in patch-clamped lymphocytes (Ross et al., 1994) and have been attributed to the presence of numerous microvilli that amplify the total membrane surface area.
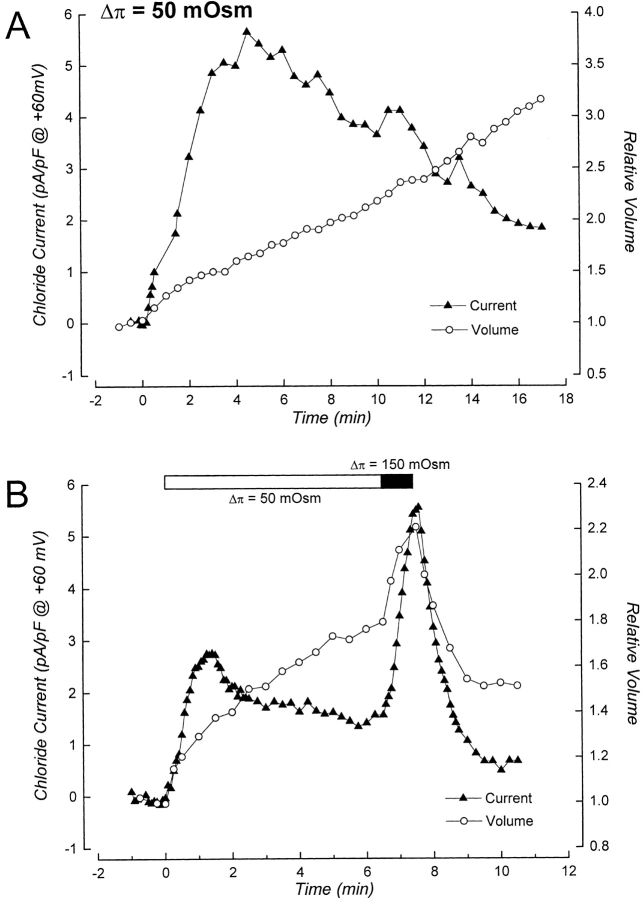
Figure 9.
Properties of swelling-induced anion current during washout of intracellular ATP and under ATP-free conditions. (A) Current rundown occurs during washout of intracellular ATP when the rate of cell swelling is slow. Cell was patch clamped with an ATP-free pipette solution containing metabolic inhibitors. Swelling was induced by exposure to a 50-mOsm reduction in bath 60 s after obtaining the whole cell configuration. Current rundown occurs despite continued cell swelling. Rundown is presumably due to loss of intracellular ATP (Lewis et al., 1993; Jackson et al., 1994; Oike et al., 1994). Data shown in graph are from a single cell. Similar results were obtained in experiments on four other cells (data not shown). (B) Current rundown is reversed by increasing the rate of cell swelling. Cell was patch clamped with an ATP-free pipette solution containing metabolic inhibitors. Swelling was induced by exposure to a 50-mOsm hypotonic shock (open bar) 90 s after obtaining the whole cell configuration. The rate of cell swelling was 12%/ min. Rundown began ∼95 s after current activation was observed. Bath osmolality was reduced by 150 mOsm (closed bar) when relative cell volume was 1.8, which increased the rate of cell swelling to 63%/min and induced a rapid activation of current. Data shown in graph are from a single cell. Similar results were obtained in experiments on five other cells (data not shown).
It is likely that ATP-consuming reactions are active in patch-clamped cells. These reactions may occur at a rate that approaches the rate of ATP diffusion from the patch pipette solution. Thus, it was conceivable that ATP-consuming reactions close to the channel and/or its activation machinery may have reduced local ATP concentrations, resulting in an overestimation of the concentration of the nucleotide required for any given rate of current activation. To address this important issue, we dialyzed cells with an ATP regeneration system consisting of 10 mM creatine phosphate and 80 U/ml of creatine kinase. The solution also contained 0.25 mM ATP. This concentration was close to the EC50, and was chosen so that small increases in ATP concentration potentially brought about by the regeneration system would be detected as relatively large increases in the rate of current activation. The rate of current activation in the presence of the ATP regeneration system (Fig. 4, □) was not significantly (P > 0.15) different from that observed with 0.25 mM ATP alone. We conclude that the ATP concentration sensed by the channel and/or its regulatory machinery is similar to that in the bulk pipette solution.
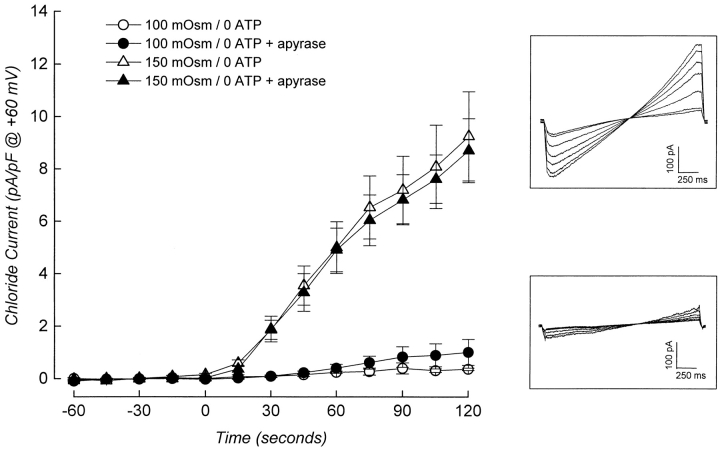
Surprisingly, swelling-induced activation of an outwardly rectifying anion current was also detected in metabolically poisoned cells dialyzed with an ATP-free pipette solution, albeit at a greatly reduced rate (Figs. 3–5). The mean ± SEM rate of current activation observed was 0.44 ± 0.09 pA/pF per min (n = 10). This suggested that the cells may contain trace amounts of ATP. To test for this possibility, we dialyzed cells for 5 min with an ATP-free pipette solution containing metabolic inhibitors and the ATP-consuming enzyme apyrase. Apyrase was present at a concentration of 2 U/ ml. Concentrations of apyrase of 0.5–1 U/ml have been shown previously to be effective in depleting both intracellular (Wang et al., 1996) and extracellular (Schwiebert et al., 1995) ATP. The presence of apyrase had no significant (P > 0.25) effect on the rate of current activation (Figs. 4, ⋄, and 5, bottom two traces plotted with ○ and •).
Figure 5.
Activation of ICl, swell can occur in the absence of intracellular ATP. Cells were patch clamped and dialyzed for 4–5 min with ATP-free pipette solutions containing metabolic inhibitors. Swelling was induced at time 0 by reducing bath osmolality by either 100 or 150 mOsm. The presence of apyrase (2 U/ml plus 0.5 mM CaCl2) in the patch pipette had no effect on the rate of current activation. Values are means ± SEM (n = 4–17). (Insets) Examples of voltage ramps (−80 to +80 mV at 80 mV/s) recorded during current activation in cells swollen by exposure to a 150- (top) or 100- (bottom) mOsm reduction in bath.
The observation that a small amount of current is activated in the absence of intracellular ATP is consistent with findings of Volk et al. (1996). These investigators noted that swelling-induced anion current activation required intracellular ATP when inner medullary collecting duct cells were swollen by a 50-mOsm reduction in bath. In contrast, when swelling was induced by 100-mOsm reduction in bath osmolality, an ATP-independent anion current was activated.
Given these results, we examined the effect of increasing the rate of swelling on the ATP dependence of ICl, swell. N1E115 cells were dialyzed for 4–5 min with an ATP-free pipette solution containing metabolic inhibitors, and then exposed to a 150-mOsm reduction in bath. Under these conditions, the cells swelled at a rate of 65–70%/min (Fig. 3, Table I). As shown in Figs. 3 and 5, cell swelling induced a rapid increase in whole cell current. The mean ± SEM Erev for the current was 12.8 ± 1.2 mV (n = 17), indicating that it is carried by Cl−. The mean ± SEM rate of current activation was 5.0 ± 0.8 pA/pF per min (n = 17). Addition of 2 U/ml of apyrase to the patch pipette solution had no significant (P > 0.4) effect on the rate of current activation (Fig. 5, top two traces plotted with ▵ and ▴). Activation of the ATP-independent current was reversed by cell shrinkage. Exposure of swollen cells to a 300-mOsm bath solution induced cell shrinkage and a rapid reduction in whole cell current (Fig. 6; see also Fig. 9 B).
Figure 6.
Activation of ATP-independent current is reversed by cell shrinkage. Cells were patch clamped with an ATP-free pipette solution containing metabolic inhibitors. After dialysis for 4–5 min, cells were swollen by exposure to a 150-mOsm reduction in bath (open bar). Swelling was reversed by returning cells to control (300 mOsm) bath. Values are means ± SEM (n = 6).
To determine whether the ICl, swell channel was responsible for this ATP-independent current, we compared its biophysical and pharmacological characteristics to the current activated by a 100-mOsm reduction in bath in metabolically poisoned cells dialyzed with 2 mM ATP. As shown in Table III, both currents had similar reversal potentials, rectification and voltage sensitivity, and were inhibited in a voltage-dependent manner and to the same extent by 100 μM DIDS (4,4′-diisothiocyanostilbene-2,2′-disulfonate). In addition, the channels responsible for the currents had similar relative anion permeabilities. We conclude from these results that the ICl, swell channel gives rise to both currents.
Table III.
Characteristics of Cl− Currents Activated by Different Rates of Swelling in Cells Dialyzed with an ATP-free Solution or a Solution Containing 2 mM ATP
| 2 mM ATP, 100 mOsm reduction in bath osmolality | 0 mM ATP, 150 mOsm reduction in bath osmolality | |||
|---|---|---|---|---|
| Reversal potential | 11.8 ± 1.2 mV (n = 14) | 12.8 ± 0.6 mV (n = 17) | ||
| Rectification ratio* | 1.18 ± 0.09 (n = 14) | 1.27 ± 0.11 (n = 18) | ||
| Inactivation at +120 mV (τ) | 341 ± 80 ms (n = 6) | 323 ± 29 ms (n = 4) | ||
| Inhibition at +60 mV by 100 μM | 67 ± 5% (n = 4) | 61 ± 8% (n = 4) | ||
| DIDS | ||||
| Anion permeability‡ | P I/P Cl , 1.5 ± 0.2 (n = 4) | P I/P Cl , 1.22 ± 0.11 (n = 5) | ||
| P NO3/P Cl , 1.27 ± 0.2 (n = 5) | P NO3/P Cl , 1.11 ± 0.32 (n = 5) | |||
| P F/P Cl , 0.64 ± 0.08 (n = 5) | P F/P Cl , 0.45 ± 0.06 (n = 4) | |||
| P gluconate/P Cl , 0.17 ± 0.04 (n = 5) | P gluconate/P Cl , 0.12 ± 0.03 (n = 5) |
Values are means ± SEM (n).
Rectification ratio is the ratio of currents measured at +60 and −60 mV.
Relative anion permeabilities (P x/P Cl) were calculated using the Goldman-Hodgkin-Katz equation, and measured changes in reversal potential induced by complete replacement of bath Cl− with the test anion.
The effect of ATP concentration on ICl, swell activation in cells swollen by a 150-mOsm hypotonic shock is shown in Fig. 7. There was no significant difference (P > 0.2) between rates of current activation measured in the presence of different intracellular concentrations of ATP. Therefore, at high rates of cell swelling, current activation is insensitive to cytoplasmic ATP levels. The cell volume set-point of the channel was also unaffected by ATP concentration and was similar to that observed in cells swollen more slowly with smaller hypotonic shocks (Table II).3
Figure 7.
ATP dependence of ICl, swell activation varies with rate of cell swelling. Cells were swollen at different rates (see Table I) by exposure to 50–150-mOsm reductions in bath. Osmotic shock used is shown in the top left corner of each graph. Data for the 100-mOsm reduction in bath are reproduced from Fig. 4. To facilitate comparison, data are plotted on the same scale. For the 50–120-mOsm reductions in bath, curves were fit to the data as described in Fig. 4 legend. Rates of current activation measured with various concentrations of ATP for cells swollen by a 150-mOsm reduction in bath were not significantly different (P > 0.2). R max value shown was determined by averaging rates of current activation measured in the presence of 0–4 mM ATP. Values are means ± SEM (n = 3–23).
The effect of high rates of swelling on the ATP sensitivity of current activation suggested that it would also be altered by slower rates of cell swelling. Fig. 3 shows the effects of intracellular ATP concentrations and different osmotic perturbations on whole cell current activation and the rate of cell swelling. When cells were swollen at a rate of 13–17%/min by a 50-mOsm hypotonic shock, current activation was again a saturable function of ATP concentration (Fig. 7), but both the EC50 (0.8 mM) and R max (1.37 pA/pF per min) were dramatically altered compared with cells swollen by a 100-mOsm reduction in bath (Fig. 7). The cell volume set-point of the channel was unaffected by ATP concentration and was similar to that observed at higher rates of swelling (Table II).
The effects of varying the rate of cell swelling on ATP dependence are shown in Figs. 3 and 7 and summarized in Fig. 8. Fig. 8 A shows the rate of current activation in metabolically poisoned cells dialyzed with an ATP-free pipette solution (i.e., ATP-independent current activation). ICl, swell activation was relatively slow (0.26–0.44 pA/pF per min) when the rate of cell swelling was 15–40%/min (50–100-mOsm reductions in bath). The rates of ATP-independent current activation observed under these conditions were not significantly different (P > 0.3), indicating that they were relatively insensitive to the rate of cell volume increase. In only 1 (swollen by a 50-mOsm reduction in bath) of the 20 cells used in these experiments, were we unable to detect swelling-induced, ATP-independent current activation.
Figure 8.
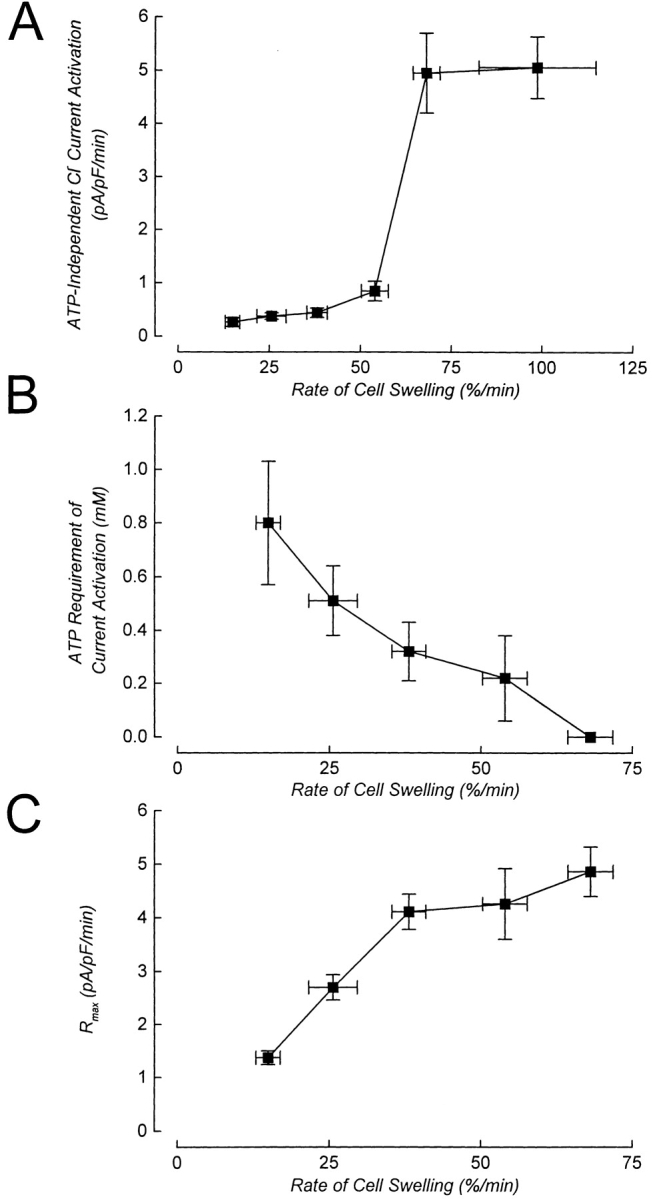
Summary of the effect of rate of cell swelling on the ATP dependence of ICl, swell activation. (A) Rate of current activation in metabolically poisoned cells dialyzed with an ATP-free pipette solution and swollen at different rates. Values are means ± SEM (n = 4–17). (B) ATP requirement of current activation decreases with increasing rates of cell swelling. At rates of swelling between 15 and 55%/min (i.e., 50–120-mOsm reductions in bath), current activation is a saturable function of intracellular ATP concentration (see Figs. 4 and 7). For swelling within this range, the ATP requirement is defined as the EC50 value obtained from dose– response relationships shown in Fig. 7. Current activation at rates of swelling ≥65%/min (i.e., 150-mOsm reduction in bath) is ATP independent (Fig. 7), and the ATP requirement is plotted as 0 mM. (C) Maximal rate of current activation (R max) is a direct function of rate of cell swelling. EC50 and R max values were determined by nonlinear regression analysis as described in the Fig. 4 legend. Vertical error bars for the points in B and C are standard errors, which were generated as part of the curve fitting routine. Rate of cell swelling values are means ± SEM (obtained from Table I).
When cells were swollen at 55%/min (120-mOsm reduction in bath), the rate of ATP-independent current activation increased significantly (P < 0.05) to 0.85 pA/pF per min (Fig. 8 A). Importantly, current activation under these conditions was still a saturable function of intracellular ATP concentration (Figs. 3 and 7).
ATP-independent current activation increased dramatically to ∼5 pA/pF per min when the rate of cell swelling was increased to 65–70%/min (150-mOsm reduction in bath). Increasing the rate of cell swelling to 100%/min (200-mOsm reduction in bath)4 had no further effect on the rate of ATP-independent current activation (Fig. 8 A).
The EC50 for ATP varied inversely with the rate of cell swelling (Fig. 8 B). At low rates of swelling, half maximal rates of current activation required higher concentrations of ATP compared with that observed in cells swollen at higher rates. R max was a direct function of the rate of cell swelling (Fig. 8 C).
Current Rundown
Transient activation of ICl, swell has been observed in several cell types when they are patch clamped with ATP-free solutions and activated shortly after obtaining the whole cell configuration (Lewis et al., 1993; Jackson et al., 1994; Oike et al., 1994). This “rundown” of the current is prevented when the pipette solution contains ATP or ATP analogues. The simplest interpretation of these findings is that current activation is initially supported by the presence of endogenous ATP. As this ATP is dialyzed away, the ICl, swell channels spontaneously inactivate (e.g., Lewis et al., 1993; Jackson et al., 1994; Oike et al., 1994).
To assess whether N1E115 neuroblastoma cells also exhibited such rundown behavior, we patch clamped cells with an ATP-free pipette solution containing metabolic inhibitors. Cells were swollen by exposure to a 50-mOsm reduction in bath within 60 s after obtaining the whole cell configuration. As shown in Fig. 9 A, ICl, swell activated in response to cell swelling. The activation was transient, however, and the current slowly inactivated despite continued volume increase.
Fig. 9 B shows the effect of increasing the rate of swelling on current rundown. The cell shown in the figure was initially swollen at a rate of 12%/min by exposure to a 50-mOsm reduction in bath. Current activation was initiated at a relative cell volume of 1.04 and spontaneous rundown was observed when the relative cell volume was 1.38. Approximately 5 min after rundown began, relative cell volume was 1.8, and the cell was then swollen at a rate of 63%/min by exposure to a 150-mOsm reduction in bath. Rapid current reactivation occurred immediately upon switching the solution. Similar experiments were carried out in five additional cells. In all cases, exposure to a 150-mOsm reduction in bath induced a rapid reactivation of current (data not shown). The amount of additional swelling that occurred before current reactivation was initiated was between 6 and 12% in three cells. In three other cells, current reactivation was initiated immediately after the solution switch and it was not possible to accurately measure the cell volume change at which it occurred.
discussion
Role of Phosphorylation in ICl, swell Activation
Most studies of ICl, swell activation that have examined channel ATP dependence have concluded that nonhydrolyzable ATP analogues substitute normally for ATP (reviewed by Strange et al., 1996; Nilius et al., 1997; Okada, 1997). However, studies in cultured mouse cortical collecting duct (Meyer and Korbmacher, 1996) and carotid body type I cells (Carpenter and Peers, 1997) have shown that activation only occurs with ATP (Meyer and Korbmacher, 1996; Carpenter and Peers, 1997) or ATPγS (Meyer and Korbmacher, 1996), that it requires the presence of intracellular Mg2+ (Meyer and Korbmacher, 1996; Carpenter and Peers, 1997), which is needed for kinase activity, and that it can be inhibited by the protein kinase inhibitor staurosporine (Meyer and Korbmacher, 1996). These results have been interpreted as indicating that phosphorylation of the ICl, swell channel and/or accessory proteins is required for swelling-induced activation.
The reasons for the contradictory findings on the role of intracellular ATP are unclear. One possibility is that methodological differences may unmask constitutive phosphorylation of the channel and/or accessory proteins. Meyer and Korbmacher (1996) exposed collecting duct cells to metabolic inhibitors for 20–45 min before they were patch clamped. Such prolonged metabolic inhibition is likely to lead to dephosphorylation of the channel and/or important regulatory proteins. If phosphorylation were crucial to the function of these proteins, rephosphorylation by ATP would have to occur before the channel could be activated by swelling and ATP binding. Our findings do not support this hypothesis, however. Channel activation is normal in cells exposed to prolonged metabolic inhibition after they were dialyzed with Mg-free pipette solutions containing 2 mM ATP (Fig. 1 A). Furthermore, channel activation is unaffected by dialyzing metabolically poisoned cells with Mg-free solutions containing 2 mM AMP-PNP and 30 U/ml of alkaline phosphatase (Fig. 2 B) to dephosphorylate proteins. We conclude therefore that in N1E115 neuroblastoma cells, activation of ICl, swell requires ATP binding rather than hydrolysis and/or phosphorylation reactions. The requirement for phosphorylation observed in other cell types (Meyer and Korbmacher, 1996; Carpenter and Peers, 1997) may reflect the existence of distinct channel types. Alternatively, it may reflect the existence of multiple signaling/regulatory pathways involved in swelling-induced channel activation. These pathways could be cell-specific, they may reflect the physiological status of the cell, and/or they may be sensitive to experimental parameters such as solution composition3 and the mechanism (e.g., Strange, 1994) or rate of cell swelling.
Characteristics of ICl, swell Activation
There are several aspects of ICl, swell channel activation that bear on the discussion of the modulatory role of intracellular ATP. First, it is important to note that graded increases in cell volume do not activate ICl, swell by inducing graded changes in the P o of a single, homogeneous population of channels. Instead, channels exist in either an inactive state where P o = 0, or an active state where P o ∼ 1 (Jackson and Strange, 1995; Boese et al., 1996; Meyer and Korbmacher, 1996). Swelling increases the number, n, of active channels in the cell membrane (Jackson and Strange, 1995; Meyer and Korbmacher, 1996), and n is therefore a direct function of the magnitude of the volume increase.
ICl, swell can be activated by forcing fluid into a cell via the patch pipette (Cannon et al., 1998). This indicates that activation does not require transmembrane water flow or the presence of an osmotic gradient. Spontaneous channel activation does not occur with prolonged dialysis of the cell, suggesting that dilution of a freely diffusible factor is not involved. Activation only requires expansion of the cell volume. Thus, activation must ultimately involve mechanical events. These mechanical events may be detected directly by the channel and/or associated regulatory proteins. Putative regulatory proteins may include components of the cytoskeleton that are altered by cell swelling. Alternatively, cell swelling may alter the activity of signal transduction pathways that ultimately are responsible for activating the channel.
When using whole cell patch clamp to study ICl, swell, current activation can be characterized in terms of a cell volume set-point of the channel and a rate of current activation. Cell volume set-point is defined as the relative cell volume at which current activation is detected and is a measure of the sensitivity of the channels to swelling. The set-point is regulated by intracellular ionic strength (Emma et al., 1997; Cannon et al., 1998) and G-proteins (Voets et al., 1998). The fact that n increases as a direct function of the amount of cell swelling suggests that individual channels or groups of channels have different cell volume set-points. Alternatively, macroscopic cell swelling may have different effects on the mechanical properties of microdomains within the plasma membrane or in submembrane structures.
Modulation of ICl, swell Activation by Intracellular ATP
It has been concluded previously that ATP is an essential cofactor for swelling-induced activation of ICl, swell (reviewed by Strange et al., 1996; Nilius et al., 1997; Okada, 1997). However, as shown in Figs. 3–9, Table III, and by Volk et al. (1996), current activation can be observed under ATP-free conditions. With rates of swelling between 15 and 55%/min, increases in intracellular ATP increase the rate of ICl, swell activation without changing the cell volume set-point of the channel (Fig. 7, Table II). The rate of ICl, swell activation is a saturable function of ATP concentration, which can be defined as an EC50 for ATP and a maximal rate of current activation (R max) at saturating ATP levels (Figs. 4, 7, and 8). Two conclusions can be drawn from these observations. First, ATP interacts with a saturable binding site(s) that selectively recognizes the nucleotide and certain analogues. Second, ATP binding induces a conformational change(s) in the channel and/or regulatory proteins that is required for channel activation. The rate at which this conformational change, and hence current activation, occurs is a saturable function of ATP concentration.
Rundown of ICl, swell occurs when endogenous ATP is dialyzed out of the cytoplasm (Fig. 9). This indicates that ATP is required to maintain the channel and/or regulatory proteins in the activated conformation. Thus, ATP must be bound to the activated channel and/or regulatory proteins.
One of the more novel findings of our studies is that the rate of cell swelling alters channel ATP sensitivity (Figs. 3, 7, and 8). As the rate of cell swelling is increased, the EC50 for ATP falls (Figs. 7 and 8). At high rates of volume increase, ICl, swell activation is insensitive to intracellular ATP concentration (Figs. 3, 5, and 7–9).
How does the rate of cell swelling modulate the ATP dependence of ICl, swell activation? There are two obvious models to consider. First, the rate of cell swelling could somehow change the affinity of the ATP binding site(s). Alternatively, channel activation may occur via distinct ATP-dependent and -independent pathways.
Changes in ATP affinity cannot explain all of the experimental observations adequately. As shown in Figs. 4, 5, 7, and 8, ATP-independent current activation occurs, albeit slowly, at rates of cell swelling (15–55%/ min) where there is a strong dependence of current activation on intracellular ATP concentration. Furthermore, if ATP affinity were a function of the rate of volume expansion, the K d of the binding site will fall as the rate of swelling is increased. However, this effect would have to be reversed at high rates of swelling where current activation is independent of intracellular ATP concentration (Figs. 7 and 8). Indeed, it would be necessary to postulate that the K d for ATP rises to infinity (i.e., the site is no longer capable of binding ATP) when cells are swollen at ≥65%/min.
The alternative and, in our opinion, simpler explanation for the observed changes in ATP sensitivity is that the ICl, swell channel can be activated via distinct ATP- dependent and -independent mechanisms. Swelling- induced changes in the EC50 for ATP can be explained if increases in the rate of cell swelling increase the proportion of channels activating in the ATP-independent mode. As the rate of swelling is increased, less ATP would be needed (i.e., the EC50 for ATP would fall) to drive a given rate of current activation.
The results of our studies have important implications for understanding the regulation of ICl, swell. It is entirely possible that channel activation is modulated by multiple factors and signal transduction pathways. The physiological status of the cell, as well as experimental variables such as the rate and mechanism of swelling, and the magnitude of the volume increase may determine which factors and signaling pathways control channel activation. This possibility needs to be taken into account in the design and interpretation of future studies of ICl, swell regulation.
Clearly, extensive additional biophysical and cellular studies are required to test the models described above. A complete understanding of the ATP dependence of the channel ultimately requires molecular identification of the pore-forming and associated regulatory proteins.
How Is the Rate of Cell Swelling Sensed?
An important finding of these studies is the demonstration that the rate of cell swelling alters the ATP requirement of the ICl, swell channel (Figs. 3, 5, 7, and 8). How does a cell sense the rate at which its volume is changing? One possibility is that rate per se is not sensed, but instead there is some critical volume that is reached more rapidly at higher rates of swelling. This critical volume could in turn determine whether the channel activates via the ATP-independent pathway. The data in Table II argue strongly against this possibility. When cells were swollen at 65–70%/min (150-mOsm reduction in bath) in the absence of intracellular ATP, the cell volume set-point of the channel was 1.14. This value is not significantly different from those observed under all other experimental conditions (Table II). Furthermore, in cells that are exhibiting current rundown, current can be reactivated rapidly at various times after rundown begins simply by increasing the rate of swelling to 65–70%/min (Fig. 9 B). Taken together, these results indicate that the cells are sensing the rate of volume increase and not some absolute volume change.
The molecular mechanisms responsible for sensing the rate of cell swelling are completely unknown. However, it is interesting to speculate that the cytoskeleton may play an important role. Numerous studies have demonstrated that the cytoskeleton undergoes structural reorganization in response to mechanical forces. These changes can occur on a time scale of seconds to minutes and appear to play important roles in fundamental processes such as maintenance of cell shape, cell locomotion, mechanosensitivity, and signal transduction (Choquet et al., 1997; Hamill and McBride, 1997; Ingber, 1997; Chicurel et al., 1998).
It is reasonable to postulate that the mechanical stress associated with cell swelling may also induce changes in cytoskeletal organization. These putative changes could be important for maintaining plasma membrane–cytoskeleton interactions, the spatial organization of membrane and submembrane proteins, and the overall mechanical properties of the cell. If the rate of volume perturbation was more rapid than the rate at which cytoskeletal reorganization could take place, it is easy to envision that protein–protein and protein–membrane interactions might be altered. Changes in protein–protein interactions could in turn modulate the ATP dependence, as well as other aspects of ICl, swell activation. Assessment of the role of the cytoskeleton in regulation of ICl, swell will require extensive additional investigation using electrophysiological, pharmacological, molecular, and biophysical approaches.
Physiological Significance of ATP-dependent Channel Regulation
Volume regulatory efflux of structurally diverse organic osmolytes appears to occur primarily via the ICl, swell channel (Kirk and Strange, 1998). In addition, the channel is highly permeable to important metabolic intermediates such as pyruvate, the short-chain fatty acids acetate, and butyrate, the ketone body β-hydroxybutyrate, and several amino acids (Jackson et al., 1994). These metabolites are major inputs into the tricarboxylic acid cycle. Because of its high organic solute permeability, we have suggested previously that modulation of ICl, swell channel activation by ATP levels may function as an important feedback regulatory mechanism (Jackson et al., 1994; Strange et al., 1996). Unregulated loss of cellular metabolites would likely disrupt cellular energy production. Regulation of ICl, swell by cellular ATP levels could therefore prevent depletion of energy-producing carbon sources when cellular energy production is reduced.
ICl, swell activation has been invoked as an important component of cellular volume regulation when cells are swollen by anoxic and ischemic insults (Kimelberg, 1995; Roy, 1995). We have argued previously that the requirement for intracellular ATP would rule out or diminish such a role (Jackson et al., 1994; see also Patel et al., 1998). However, as we have demonstrated here, it is clearly possible to activate the channel in the absence of ATP.
In patch-clamped cells, channel activation via the ATP-independent pathway is controlled by the rate of volume increase. Other factors may control activation by this pathway as well. Whether the ATP dependence of ICl, swell is modulated in intact cells is uncertain and deserves further study. If such modulation occurs, it is interesting to speculate on its physiological significance. As noted above, activation of ICl, swell in a metabolically compromised cell may further compromise metabolism and lead to additional disruption of cellular functions. Thus, if swelling is not too severe, it may be beneficial to the cell to protect its metabolism at the expense of volume homeostasis. On the other hand, if swelling is extreme and membrane lysis is imminent, volume control may take precedence over energy metabolism. We suggest that the modulation of channel ATP dependence may be an adaptation that allows cells to cope with the potentially competing demands of preservation of energy metabolism and volume regulation.
Acknowledgments
We thank Dr. Fred Quant for the generous gift of the N1E115 mouse neuroblastoma cells.
This work was supported by National Institutes of Health grants NS-30591 and DK-51610. Dr. Tamara Bond was supported by a Stroke Investigator Award from the Heart and Stroke Foundation of Ontario.
Abbreviation used in this paper
- NMDG
N-methyl-d-glucamine
Footnotes
Dr. Basavappa's current address is Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06520.
A plot of the data in Table I revealed that there was a linear relationship between the rate of cell swelling and reductions in bath osmolality of 50–200 mOsm. However, the line intersected the x axis at ∼20 mOsm, instead of 0 mOsm as expected. This suggests that there is a resistance to cell swelling with osmotic gradients <20 mOsm. The reason for this resistance to swelling with small hypotonic shocks is uncertain. It should be emphasized, however, that it does not effect the interpretation of results presented in this paper.
It is noteworthy that the effect of a high rate of cell swelling on ATP sensitivity was altered by intracellular solution composition. During the course of these studies, the cells developed a nonselective cation conductance that interfered with measurements of ICl, swell. This conductance was eliminated by replacing CsCl in either the bath alone, or in both the bath and patch pipette solution with NMDG-Cl. However, in the presence of intracellular NMDG-Cl, ATP-independent activation of ICl, swell did not occur when cells were swollen by exposure to a 150-mOsm reduction in bath osmolality (data not shown). The nature of this effect of intracellular NMDG-Cl is unknown.
It was necessary to reduce bath NMDG-Cl concentration from 70 to 40 mM to reduce the osmolality by 200 mOsm. The mean ± SEM Erev of the current observed in cells exposed to this solution was 22.3 ± 0.8 mV (n = 7). The expected Erev calculated from the Goldman-Hodgkin-Katz equation is 25.1 mV. In experiments where bath NMDG-Cl concentration was 70 mM, whole cell currents were quantified at +60 mV, which is 47 mV above the calculated Erev. Therefore, whole cell currents were quantified at +72 mV in cells bathed with 40 mM NMDG-Cl.
Control experiments were performed to determine if the reduction in bath salt concentration altered current activation. Cells were exposed to a bath solution containing 40 mM NMDG-Cl and osmolality was reduced by 150 mOsm. The mean ± SEM rate of current activation in these cells was 4.8 ± 1.4 pA/pF per min (n = 3). This value was not significantly different (P > 0.1) from that observed in cells swollen in the presence of 70 mM NMDG-Cl.
references
- Boese SH, Kinne RHK, Wehner F. Single-channel properties of swelling-activated anion conductance in rat inner medullary collecting duct cells. Am J Physiol. 1996;271:F1224–F1233. doi: 10.1152/ajprenal.1996.271.6.F1224. [DOI] [PubMed] [Google Scholar]
- Bryan J, Aguilar-Bryan L. The ABCs of ATP-sensitive potassium channels: more pieces of the puzzle. Curr Opin Cell Biol. 1997;9:553–559. doi: 10.1016/s0955-0674(97)80033-6. [DOI] [PubMed] [Google Scholar]
- Cannon CL, Basavappa S, Strange K. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. Am J Physiol. 1998;275:C416–C422. doi: 10.1152/ajpcell.1998.275.2.C416. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Peers C. Swelling- and cAMP-activated Cl−currents in isolated rat carotid body type I cells. J Physiol (Lond) 1997;503:497–511. doi: 10.1111/j.1469-7793.1997.497bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- De Lean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Emma F, McManus M, Strange K. Intracellular electrolytes regulate the volume set point of the organic osmolyte/anion channel VSOAC. Am J Physiol. 1997;272:C1766–C1775. doi: 10.1152/ajpcell.1997.272.6.C1766. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr Induced membrane hypo/hyper-mechanosensitivity: a limitation of patch clamp recording. Annu Rev Physiol. 1997;59:621–631. doi: 10.1146/annurev.physiol.59.1.621. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu Rev Physiol. 1997;59:193–220. doi: 10.1146/annurev.physiol.59.1.193. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Single channel properties of a swelling-activated anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J Gen Physiol. 1995;105:643–660. doi: 10.1085/jgp.105.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Kirk K, Strange K. Functional properties and physiological roles of organic solute channels. Annu Rev Physiol. 1998;60:719–739. doi: 10.1146/annurev.physiol.60.1.719. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Korbmacher C. Cell swelling activates ATP-dependent voltage-gated Cl−channels in M-1 mouse cortical collecting duct cells. J Gen Physiol. 1996;108:177–193. doi: 10.1085/jgp.108.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Voets T, Eggermont J, Droogmans G. Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J Physiol (Lond) 1998;506:353–361. doi: 10.1111/j.1469-7793.1998.353bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M, Droogmans G, Nilius B. The volume-activated chloride current in human endothelial cells depends on intracellular ATP. Pflügers Arch. 1994;427:184–186. doi: 10.1007/BF00585960. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion–sensing outward-rectifier Cl−channel: fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lauritzen I, Lazdunski M, Honore E. Disruption of mitochondrial respiration inhibits volume-regulated anion channels and provokes neuronal cell swelling. J Neurosci. 1998;18:3117–3123. doi: 10.1523/JNEUROSCI.18-09-03117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P, Garber S, Cahalan M. Membrane chloride conductance and capacitance in Jurkat T lymphocytes during osmotic swelling. Biophys J. 1994;66:169–178. doi: 10.1016/S0006-3495(94)80754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy G. Amino acid current through anion channels in cultured human glial cells. J Membr Biol. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Strange K. Are all cell volume changes the same? . News Physiol Sci. 1994;9:223–228. [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Strange K, Spring KR. Methods for imaging renal tubule cells. Kidney Int. 1986;30:192–200. doi: 10.1038/ki.1986.171. [DOI] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol (Lond) 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk KA, Zhang C, Husted RF, Stokes JB. Cl−current in IMCD cells activated by hypotonicity: time course, ATP dependence, and inhibitors. Am J Physiol. 1996;271:F552–F559. doi: 10.1152/ajprenal.1996.271.3.F552. [DOI] [PubMed] [Google Scholar]
- Walum E, Marchner H. Effects of mercuric chloride on the membrane integrity of cultured cell lines. Toxicol Lett (Amst) 1983;18:89–95. doi: 10.1016/0378-4274(83)90076-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]