Abstract
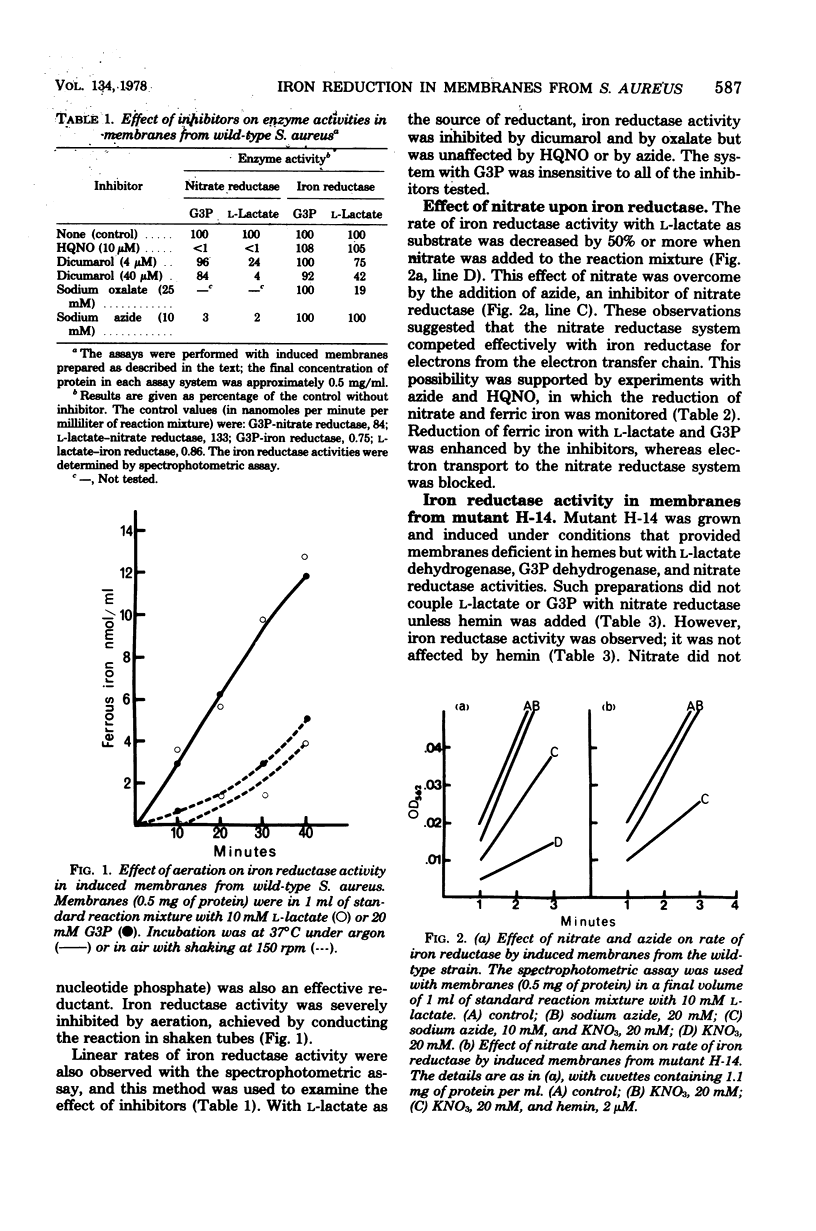
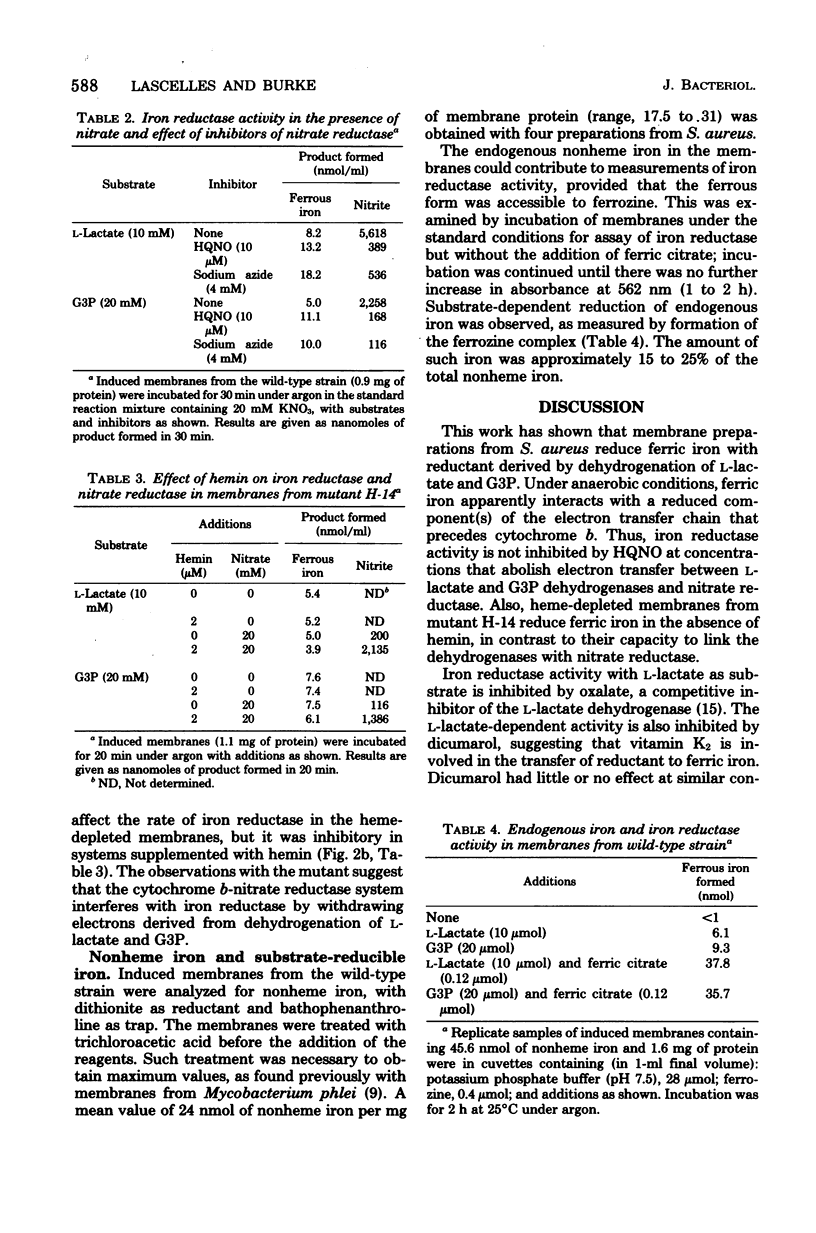
Membrane fractions with L-lactate dehydrogenase, sn-glycerol-3-phosphate (G3P) dehydrogenase, and nitrate reductase activities were prepared from Staphylococcus aureus wild-type and hem mutant strains. These preparations reduced ferric to ferrous iron with L-lactate or G3P as the source of reductant, using ferrozine to trap the ferrous iron. Reduction of ferric iron was insensitive to 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO) with either L-lactate or G3P as reductant, but oxalate and dicumarol inhibited reduction with L-lactate as substrate. The membranes had L-lactate- and G3P-nitrate reductase activities, which were inhibited by azide and by HQNO. Reduction of ferric iron under anaerobic conditions was inhibited by nitrate with preparations from the wild-type strain. This effect of nitrate was abolished by blocking electron transport to the nitrate reductase system with azide or HQNO. Nitrate did not inhibit reduction of ferric iron in heme-depleted membranes from the hem mutant unless hemin was added to restore L-lactate- and G3P-nitrate reductase activity. We conclude that reduced components of the electron transport chain that precede cytochrome b serve as the source of reductant for ferric iron and that these components are oxidized preferentially by a functional nitrate reductase system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill W. J. Regulation and genetics of bacterial nitrogen fixation. Annu Rev Microbiol. 1975;29:109–129. doi: 10.1146/annurev.mi.29.100175.000545. [DOI] [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase activity in heme-deficient mutants of Staphylococcus aureus. J Bacteriol. 1976 Apr;126(1):225–231. doi: 10.1128/jb.126.1.225-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol. 1975 Jul;123(1):308–316. doi: 10.1128/jb.123.1.308-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Properties of nonheme iron in a cell envelope fraction from Escherichia coli. J Bacteriol. 1971 Sep;107(3):664–670. doi: 10.1128/jb.107.3.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXIX. The involvement of nonheme iron in the respiratory pathways of Mycobacterium phlei. J Biol Chem. 1967 Dec 25;242(24):5830–5837. [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXVII. The nature of nonheme iron in Mycobacterium phlei. J Biol Chem. 1967 Jun 25;242(12):2909–2916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Stouthamer A. H. Biochemistry and genetics of nitrate reductase in bacteria. Adv Microb Physiol. 1976;14(11):315–375. doi: 10.1016/s0065-2911(08)60230-1. [DOI] [PubMed] [Google Scholar]



