Abstract
pH is a potent modulator of gap junction (GJ) mediated cell–cell communication. Mechanisms proposed for closure of GJ channels by acidification include direct actions of H+ on GJ proteins and indirect actions mediated by soluble intermediates. Here we report on the effects of acidification on connexin (Cx)46 cell–cell channels expressed in Neuro-2a cells and Cx46 hemichannels expressed in Xenopus oocytes. Effects of acidification on hemichannels were examined macroscopically and in excised patches that permitted rapid (<1 ms) and uniform pH changes at the exposed hemichannel face. Both types of Cx46 channel were found to be sensitive to cytoplasmic pH, and two effects were evident. A rapid and reversible closure was reproducibly elicited with short exposures to low pH, and a poorly reversible or irreversible loss occurred with longer exposures. We attribute the former to pH gating and the latter to pH inactivation. Half-maximal reduction of open probability for pH gating in hemichannels occurs at pH 6.4. Hemichannels remained sensitive to cytoplasmic pH when excised and when cytoplasmic [Ca2+] was maintained near resting (∼10−7 M) levels. Thus, Cx46 hemichannel pH gating does not depend on cytoplasmic intermediates or a rise in [Ca2+]. Rapid application of low pH to the cytoplasmic face of open hemichannels resulted in a minimum latency to closure near zero, indicating that Cx46 hemichannels directly sense pH. Application to closed hemichannels extended their closed time, suggesting that the pH sensor is accessible from the cytoplasmic side of a closed hemichannel. Rapid closure with significantly reduced sensitivity was observed with low pH application to the extracellular face, but could be explained by H+ permeation through the pore to reach an internal site. Closure by pH is voltage dependent and has the same polarity with low pH applied to either side. These data suggest that the pH sensor is located directly on Cx46 near the pore entrance on the cytoplasmic side.
Keywords: connexin, intercellular communication, ion channel, pH, gating
introduction
Reduction in junctional conductance (gj)1 by intracellular acidification has been reported for both vertebrate and invertebrate gap junctions (GJs). Unlike the reduction in gj to a residual (plateau) value with increasing transjunctional voltage (Vj), strong acidification reduces gj to undetectable levels; i.e., cells become completely electrically uncoupled. While a physiological role for acidification-induced uncoupling has not been established, possible roles may involve pathologies where there is cell injury. A lowered intracellular pH (pHi) in response to injury may be of protective value in reducing GJ-mediated communication and limiting the spread of injury within a tissue.
The reduction in gj by cytoplasmic acidification, in some cases, has been described with a simple titration curve (Spray et al., 1981b). Wide differences in pH sensitivity have been reported for GJ-mediated cell communication, with apparent pK as ranging from ∼pH 6 to 7.5. Also, both direct and indirect actions of H+ have been proposed (for reviews, see Spray and Bennett, 1985; Bennett and Verselis, 1992). Direct action by H+ was suggested in early studies of cell pairs obtained from blastomeres of amphibian and teleost embryos (Spray et al., 1981b, 1982). Evidence for indirect action through soluble cytoplasmic intermediaries has come from studies in both invertebrate and vertebrate GJs, but GJs in insects and nematodes, and perhaps invertebrates in general, appear to be formed by a family of proteins unrelated in primary sequence to connexins (Phelan et al., 1998). In crayfish septate GJs and GJs between paired Xenopus oocytes, Ca2+/calmodulin was reported to be an essential intermediary (Arellano et al., 1986, 1988; Peracchia et al., 1983, 1996). A requirement for Ca2+ in acidification-induced uncoupling has been reported in rat ventricular myocytes and Novikoff hepatoma cells, cells that both predominantly express connexin (Cx)43 (Lazrak and Peracchia, 1993; White et al., 1990). In these cases, low pHi was reported to have no effect on gj without an accompanying increase in intracellular Ca2+.
Mutations involving removal, substitution, and change in the position of His residues in the cytoplasmic loop of Cx43 were shown to significantly affect pH sensitivity, suggesting that protonation of these residues may be important in the pH dependence of GJs (e.g., Ek et al., 1994). More recently proposed molecular mechanisms of pH sensitivity involve both cytoplasmic loop (CL) and carboxy terminal (CT) domains. In Cx43, CT is thought to behave like a gating particle that, when bound to a receptor domain putatively localized in CL, closes the Cx43 channel (Ek-Vitorin et al., 1996; Morley et al., 1996). In Cx32, charge interactions within CL, as well as between CL and the proximal portion of CT, have been suggested to be responsible for pH sensitivity (Wang et al., 1996; Wang and Peracchia, 1997).
Difficulties and differences in the methods of quantifying pHi together with multiple connexin expression in native cells may have contributed to wide differences in reported sensitivities (see Bennett and Verselis, 1992). Also, studies of pH sensitivity in tissue or cell-pair preparations have been confounded by an inability to rapidly change pHi to determine kinetics and avoid slower secondary effects by nonuniform changes in pHi. Here we report the use of Cx46 hemichannels to investigate the effects of pH on GJ communication. When expressed in Xenopus oocytes, Cx46 hemichannels are functional (Ebihara and Steiner, 1993; Paul et al., 1991) and can be readily recorded in cell-attached and excised patch configurations (Trexler et al., 1996). Single hemichannels in excised patches exposed to fast perfusion provide a means of examining the action of chemical modulators on connexins with millisecond time resolution.
materials and methods
Expression of Cx46 in Xenopus Oocytes
The coding region of Cx46 was cloned into the EcoR1 and Hind III sites of pGem-7Zf+ (Promega Corp.) from rat genomic DNA using PCR amplification with primers corresponding to amino- and carboxy-terminal sequences. Preparation of Xenopus oocytes and synthesis of RNA have been described previously (Rubin et al., 1992a,b). Each oocyte was injected with 50–100 nl of an aqueous solution of mRNA (2 mg/ml) together with DNA antisense to the endogenous XenCx38 (8 pmol/μl). We used the phosphorothioate antisense oligo 5′-GCT TTA GTA ATT CCC ATC CTG CCA TGT TTC-3′, which is complementary to XenCx38 commencing at NH2 terminus–5 with respect to the ATG initiation codon.
Expression of Cx46 in Neuro-2a Cells
The EcoR1 and HindIII fragment cut from the Cx46-pGem construct was blunt-end ligated into pCl-Neo (Promega Corp.) at the EcoR1 site. Communication-deficient Neuro-2a cells (CCL-131; American Type Culture Collection) were transfected with this construct using lipofectin (GIBCO BRL) according to the manufacturer's protocol. Transfected cells were selected and maintained with 300 μg/ml (active) G418 (GIBCO BRL). Positive clones were screened by dual whole-cell patch recordings. These cells are referred to as N2a-Cx46.
Bath and Recording Solutions
In macroscopic recordings of Cx46 hemichannel currents, Xenopus oocytes were bathed in a standard solution containing (mM): 88 NaCl, 1 KCl, 2 MgCl2, 1.8 CaCl2, 5 glucose, 5 HEPES, and 5 PIPES, pH 7.6. Both current-passing and voltage-recording pipettes contained 2 M KCl. A perspex recording chamber was designed for rapid solution exchange and consisted of a rectangular canal connecting inflow and outflow compartments. A suction tube was placed in the outflow compartment and a separate reservoir connected to the main chamber with an agar bridge was used for grounding. Bath volume was ∼0.5 ml, and total volume exchange was achieved in 1–2 s by application of test solutions to the inflow compartment. Flow rates in all experiments were consistent. Test solutions consisted of the standard bath solution pH adjusted over a range of 5.0–7.6 with HCl and NaOH. The pH of the solution in the inflow reservoir was monitored over the course of each experiment.
For patch clamp recordings of Cx46 hemichannel currents, Xenopus oocytes were manually devitellinized in a hypertonic solution consisting of (mM): 220 Na aspartate, 10 KCl, 2 MgCl2, and 10 HEPES. Typically, patch pipettes were filled with (mM): 100 KCl, 30 NaCl, 2 MgCl2, 10 HEPES, 10 PIPES, 10 EGTA, and 1 CaCl2, pH adjusted to 7.5 with KOH. We refer to this solution as IPS-A (free [Ca2+] ≈ 10−8 M at pH 7.5). In experiments where the effect of Ca2+ was examined, 2 mM BAPTA replaced EGTA. We refer to this pipette solution as IPS-B (free [Ca2+] ≈ 10−7 M with 0.7 mM added CaCl2 at pH 7.5). To achieve the desired level of free [Ca2+] at a given pH, an appropriate amount of CaCl2 was added as computed using CHELATOR (Schoenmakers et al., 1992). The test solutions used to examine pH sensitivity in excised patches consisted of either IPS-A or IPS-B, with pH adjusted to the desired value with KOH and HCl. These solutions were applied to the patches from a theta tube mounted on a piezoelectric actuator (see Fig. 1). Throughout the course of an experiment, the bath was continuously perfused with a solution containing (mM): 88 NaCl, 1 KCl, 2 MgCl2, 5 glucose, and 10 HEPES, pH adjusted to 7.6.
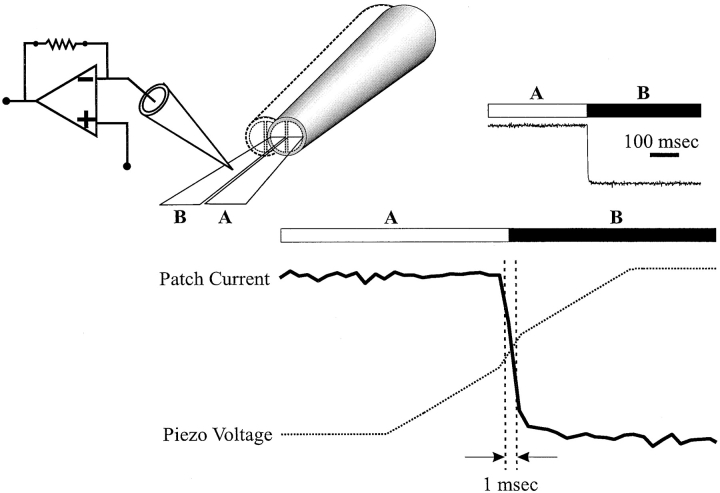
Figure 1.
Rapid solution switching at the exposed face of excised patches. The speed of switching a patch recording pipette between two solutions flowing through the barrels of a theta tube mounted on a piezo actuator was tested with pipettes dipped in melted parafilm (see materials and methods). Barrel A of the theta tube was filled with IPS-A. Barrel B contained IPS-A in which KCl was replaced with tetraethylammonium chloride (TEACl). Shown is the change in current in response to solution exchange. In the expanded view of the current trace during a transition from solution A to B, the voltage waveform applied to the piezo actuator is superimposed to illustrate the relationship between voltage and pipette movement. The entire waveform moved the patch pipette relative to the theta tube from the center of stream A to the edge of the interface, quickly through the interface, and then at the original speed to the center of stream B. The voltage waveform consisted of ramps from 0 to −4 V for 10 ms, −4 to −6 V for the next 2 ms, and −6 to −10 V for the last 10 ms. The distances moved during each of the three ramps were 28, 14, and 28 μm, respectively; the piezo actuator had a maximum range of 70 μm.
In whole cell patch experiments on N2a-Cx46 cells, the bath solution was a modified Krebs-Ringer consisting of (mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES, 5 glucose, and 2 pyruvate, pH adjusted to 7.4 with NaOH. Patch pipette solutions consisted of (mM): 130 KCl, 10 NaCl, 1 MgCl2, 5 HEPES, 5 EGTA, and 1.4 CaCl2, pH adjusted to 7.2 with KOH. Free [Ca2+] was calculated to be ∼5 × 10−8 M. In some experiments, we used lower amounts of Ca2+ with BAPTA in the patch pipette solutions to minimize the rise in Ca2+ upon acidification. With intracellular acidification to pH 6.0 (by perfusing 100% CO2-equilibrated bath solution), free [Ca2+] increased approximately fourfold (from 5 × 10−8 to 1.7 × 10−7 M) using 2 mM BAPTA with 0.38 mM added CaCl2. The test solutions used to acidify the cells were directly perfused onto patched cells.
Electrophysiological Recording and Analysis
Voltage clamp recordings of macroscopic Cx46 hemichannel currents from single Xenopus oocytes were obtained with a two-electrode voltage clamp (GeneClamp 500; Axon Instruments, Inc.). Data was acquired using pClamp 6.0 software and a Digidata 1200 interface (Axon Instruments, Inc.).
Single Cx46 hemichannel currents in Xenopus oocytes were recorded from cell-attached and excised patches using an Axopatch 200B (Axon Instruments, Inc.) with the headstage in capacitive feedback mode. Cx46 cell–cell channel currents in N2a-Cx46 cells were recorded from cell pairs using the dual whole-cell patch clamp technique (Neyton and Trautmann, 1985) and two Axopatch 1-D patch clamp amplifiers (Axon Instruments, Inc.). gj was evaluated in the following manner. Both cells of a pair were maintained at the same holding potential from which voltage steps, ΔVj, were applied to one cell. The voltage steps were small and brief so as not to invoke Vj- dependent changes in gj. Junctional current was measured directly as the change in current in the unstepped cell, ΔI. gj is thus given by ΔI/ΔVj. Data from both hemichannels and cell–cell channels were acquired with AT-MIO-16X D/A boards from National Instruments using our own acquisition software.
The titration curve for inside-out patches was obtained from mean current analysis of multiple applications of low pH solutions to the exposed (cytoplasmic) face of each patch. For each patch, the leak-subtracted mean current during low pH application (after the first closure) was normalized to the leak-subtracted mean current during exposure to pH 7.5 to obtain I/I7.5 for that application. This procedure allowed for comparison among patches with different numbers of hemichannels at different voltages. For analysis of closed durations in outside-out patches, single hemichannel currents were idealized with the Sublevel Hinkley Detector (SHD) (Draber and Schultze, 1994) to two levels, fully open and closed. When idealizing to two levels, the SHD acts as a half-height threshold algorithm. In Cx46 hemichannels, transitions between open and closed are characterized by transitions among multiple short-lived substates (see Trexler et al., 1996), leading to multiple crossings of the half height before entering a long lived open or closed state. The SHD was set at low sensitivity to avoid detection of these crossings. Dwell-time histograms were compiled and fitted in Origin (Microcal Software, Inc.). Ensemble currents were obtained by summing the current from each trace minus the leak current of the patch. Hence, 0 pA in the ensemble summations represents closure of all hemichannels in the patch. Fitted parameters to exponential functions were obtained in Origin.
Measurement of Intracellular pH
Single Neuro-2a cells were loaded with the indicator BCECF (Molecular Probes, Inc.) in one of two ways. In cells that were not patch clamped, loading was achieved by bathing cells in 10 μM BCECF-AM, the membrane permeable form. In cells that were patch clamped, loading was achieved by including the acidic form of BCECF in the patch pipette solution at a concentration of 10 μM. We found no significant differences with the two methods. Xenopus oocytes were injected with 50 nl of 2 mM BCECF (acidic form) to a achieve a final concentration of ∼10 μM. pH was determined from the ratio of emission values recorded with excitation wavelengths of 440 and 490 nm using a SPECTRAMASTER high speed monochromator and a MERLIN imaging system (Life Science Resources). Calibration was performed in Neuro-2a cells using 10 μM nigericin (Molecular Probes, Inc.) to equilibrate intracellular and extracellular pH. Calibration solutions ranged from pH 5.5 to 8.0. The 440/490 ratio for each pH was taken when the value had not changed for 3 min. A plot of the ratio value recorded for each tested pH was fitted to 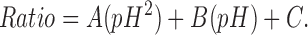 Ratios were converted to pH with the solution to the quadratic equation:
Ratios were converted to pH with the solution to the quadratic equation:
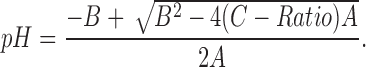 |
For intracellular pH measurements in oocytes with BCECF, we used the calibration curve obtained using Neuro-2a cells. Although calibration curves for pH-sensitive dyes can shift depending on the type of cell (e.g., Morley et al., 1996), we merely make comparisons of relative changes in intracellular pH for applications of solutions equilibrated with 100% CO2 and solutions acidified with HCl.
Rapid Solution Switching
Rapid solution switching in excised patches was achieved by using a theta tube mounted on a LSS 3100 piezoelectric driven actuator (Burleigh Instruments). The theta tube was pulled to an overall tip diameter of ∼150 μm. Flow rate through the theta tube was adjusted by changes in the heights of the reservoirs feeding each barrel to achieve a thin interface between the two streams. An excised patch was switched rapidly between the two streams by applying a voltage ramp waveform to the piezoelectric driven actuator. The waveform produced a 10-ms approach from the center of one stream towards the interface, followed by a 2-ms movement through the interface and again by a 10-ms approach to the center of the second stream. This waveform configuration minimized ringing and resonance of the piezo device and theta tube while achieving a rapid transition through the interface. The speed of solution switching was tested by measuring the change in the holding current due to a change in tip potential of a high-resistance patch pipette when switching between two solutions differing in salt composition (Fig. 1, solutions A and B). The 10– 90% difference in tip potential (measured in voltage clamp as shift in holding current) was typically traversed in ∼1 ms (Fig. 1).
results
Effects of Acidification on Junctional Coupling between N2a-Cx46 Cell Pairs
Bath application of a modified Krebs-Ringer solution equilibrated with 100% CO2 to pairs of Neuro-2a cells expressing Cx46 rapidly reduced gj to below detectable levels. Recovery depended on the time of exposure to CO2. Longer exposures caused recovery to be delayed and less complete. An example of a cell pair exposed to 100% CO2 for ∼45 s is shown in Fig. 2 A, I. gj rapidly fell to below detectable levels, and no recovery of gj was detected for nearly 3 min after washout. Thereafter, gj slowly increased, but reached only a small fraction of the original value. Recovery was incomplete even as long as 30–40 min after washing (data not shown). In a different cell pair, effects of two consecutive shorter exposures to 100% CO2 are illustrated. With an ∼25-s exposure (Fig. 2 A, II, top), gj rapidly fell to below detectable levels, but remained undetectable for a shorter time (∼60 s) after washout. Thereafter, recovery proceeded relatively rapidly, reaching 50% of its original value within a few minutes. With a subsequent briefer exposure to this same cell pair, ∼15 s (Fig. 2 A, II, middle), gj again decreased but not completely before washout elicited a relatively rapid (3–4 min) and nearly full recovery to the level before this exposure. The dependence of recovery on the duration of exposure to CO2 was the same in cell pairs that were exposed to CO2 for the first time and in those that had prior exposures to CO2.
Figure 2.
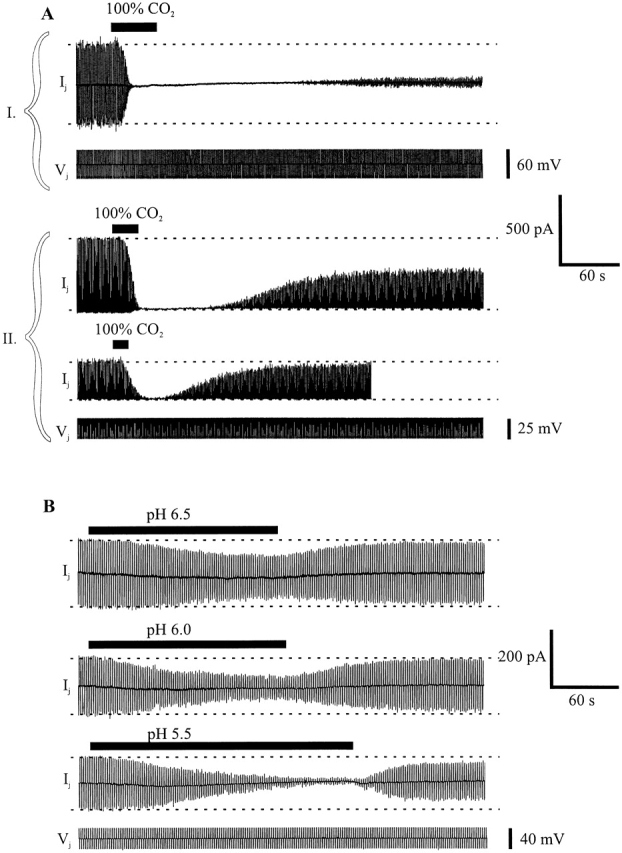
Acidification-induced uncoupling in pairs of Cx46-transfected Neuro-2a cells. A and B show whole-cell patch recordings of junctional current (Ij) in cell 1 in response to a series of brief Vj steps applied to cell 2. (A, I) A recording from a cell pair with Ij in response to alternating Vj steps of +30 and −30 mV. Application of 100% CO2-equilibrated medium for 45 s reduced Ij to undetectable levels. Recovery was delayed for ∼3 min, and only reached 10–15% of the original value in the subsequent 2 min. (A, II) Two recordings from a second cell pair with Ij in response to Vj steps of +25 mV. Application of 100% CO2-equilibrated medium for 25 s reduced Ij to undetectable levels. Wash solution was applied when Ij decreased to undetectable levels. Recovery was delayed for ∼60 s on washout, and Ij increased slowly to ∼50% of the original value. A shorter exposure to 100% CO2 (15 s) significantly but not completely reduced Ij. Washout began soon after the initial decline in Ij. Recovery was delayed less with this shorter exposure and was essentially complete (>90%) within 2 min. (B) Three recordings from a third cell pair with Ij in response to alternating Vj steps of +20 and −20 mV. Ij decreased steadily during application of medium acidified with HCl to pH 6.5 (∼3 min), pH 6.0 (∼3 min), and pH 5.5 (∼4 min). Recovery from low pH applications began soon after washout with pH 7.5 solution. Ij reached >90% of the original values for pH 6.5 and 6.0, but with the longer exposure to pH 5.5, Ij recovered only to ∼70% of the original value.
Although equilibrating the Krebs-Ringer bath solution with 100% CO2 reduced the extracellular pH from 7.2 to ∼5.5, CO2 is known to cross cell membranes readily and cause rapid intracellular acidification (see Thomas, 1984). Thus, we examined whether reductions in gj occur by perfusing solutions acidified with HCl and buffered with combinations of HEPES and PIPES, presumably membrane impermeant buffers. Shown in Fig. 2 B are recordings of gj with applications of bath solutions with pH values adjusted to 6.5, 6.0, and 5.5. With application of a pH 6.5 solution, a modest reduction in gj occurred and essentially recovered fully upon washing. Applications of pH 6.0 and 5.5 solutions reduced gj increasingly, but not completely. Recovery of gj after washout of HCl acidified solutions was relatively faster than after washout of 100% CO2. For example, after perfusion of a pH 5.5 bath solution (Fig. 2 B, third trace), gj recovered to ∼70% of control in <1 min, almost three times as fast as the decrease in gj. Recovery from prolonged exposure to pH 5.5 solutions was incomplete as with prolonged exposure to 100% CO2.
In general, the reductions in gj with applications of HCl acidified solutions differed from those caused by 100% CO2 by being smaller in magnitude and slower to develop even when the pH of the HCl acidified solution was ∼5.5, comparable with that of the 100% CO2-equilibrated solution. To examine the extent and time course of the changes in pHi that occurred with application of 100% CO2- and HCl-acidified solutions, we measured pHi using the pH indicator BCECF in separate N2A-Cx46 cells. Data from a representative N2A-Cx46 cell is shown in Fig. 3. Changes in pHi occurred with both treatments, but differed in degree and time course. Typically with 100% CO2, pHi dropped rapidly from ∼7.25 to ∼6.0 within 25 s of application (∼0.05 pH U/s) (Fig. 3 A). Upon washing, pHi recovered slowly, taking ∼5 min to reach control values (∼0.004 pH U/s). With HCl acidified solutions ranging from 5.5 to 6.5, pHi declined much more slowly, but recovered more rapidly. With an application of a pH 5.5 solution, pHi decreased from ∼7.25 to ∼6.0 over a period of 5 min (∼0.004 pH U/s) (Fig. 3 B). pHi recovered to control values in ∼100 s (∼0.013 pH U/s). We presume that the faster rate of intracellular acidification achieved with 100% CO2 is due to its high membrane permeability. Although we have no explanation for the difference in recovery rates, the recovery from bathing in HCl-acidified solutions may be faster because there is less acidification of intracellular compartments.
Figure 3.
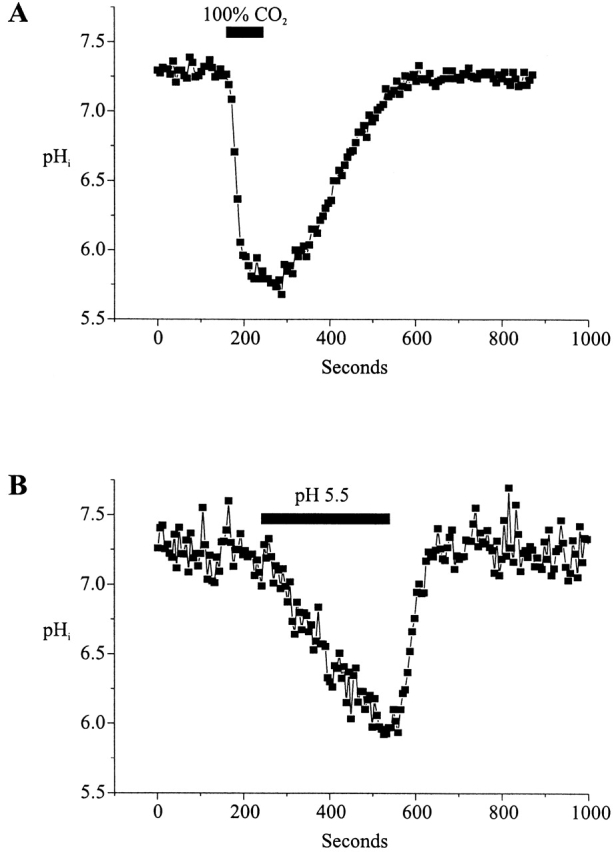
Measurement of intracellular pH in Cx46-transfected Neuro-2a cells. pHi was measured using the acidic form of BCECF. Single cells were whole-cell patch clamped with BCECF added to the patch pipette solution. (A) Application of CO2-equilibrated medium for 90 s decreased pHi to ∼5.8 within 50 s. However, recovery was much slower and lasted for almost 300 s. (B) pHi steadily decreased from 7.25 to ∼6.0 with a 300-s application of a medium acidified to pH 5.5 with HCl. Recovery after washout was complete within ∼100 s.
The general agreement between the degree and time course of the reversible changes in gj and in pHi are consistent with the accepted view that GJ channels are closed by intracellular, but not extracellular acidification. The rapidity and reversibility of uncoupling with modest and/or brief acidification suggests that a component of the pHi effect may be a form of gating, perhaps by means of a direct action of H+ on connexins. Delayed recovery and apparently irreversible loss of Cx46-mediated coupling with prolonged acidification indicates that there are additional long-lasting effects produced by acidification that are slower to develop.
Cx46 Hemichannel Currents Are also Reduced by Acidification
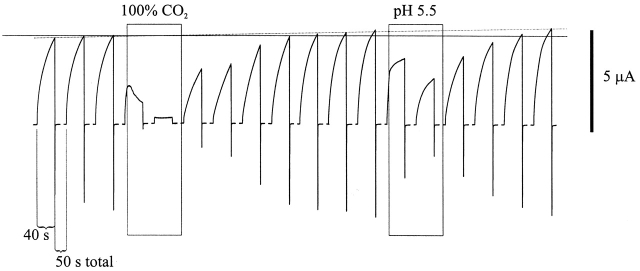
As a precursor to studies of single Cx46 hemichannels, we examined the effects of acidification on macroscopic currents of Cx46 hemichannels expressed in Xenopus oocytes. Cx46 hemichannels are typically activated by steps to positive voltages and mediate large, slowly rising outward currents (see also Ebihara and Steiner, 1993). Fig. 4 illustrates the effects of applications of solutions acidified with 100% CO2 and those acidified to pH 5.5 with HCl on the activation of Cx46 hemichannel currents. Currents were activated by 40-s depolarizations to +30 mV from a holding potential of −70 mV, spaced 50-s apart. During the fourth depolarization, application of 100% CO2 equilibrated bath solution immediately reduced hemichannel currents. By the next depolarizing step, the slowly activating outward currents characteristic of Cx46 hemichannels were completely abolished, leaving a small endogenous current that is also seen in oocytes injected only with DNA antisense to XenCx38. With application of a pH 5.5 bath solution, currents decreased but, unlike with 100% CO2, were not completely abolished by the next depolarization. Washing with pH 7.5 bath solution led to full recovery for both treatments in these oocytes. Longer exposures to pH 5.5 solutions caused greater reductions in the Cx46 currents and prolonged exposure to either solution delayed recovery more (data not shown). However, unlike recovery of N2a-Cx46 junctional currents, the degree of recovery of hemichannel currents in Xenopus oocytes was erratic, sometimes recovering partially and other times recovering fully or even exceeding control values. Some of the erratic recovery was likely due to different rates and degrees of expression of new hemichannels. Electrophysiological monitoring of Cx46 expression in oocytes without acidification showed increases in hemichannel currents that were irregular over time and varied considerably among oocytes.
Figure 4.
Cx46 hemichannels in Xenopus oocytes are sensitive to both internal and external pH. Shown are consecutive depolarizing voltage steps to +30 mV applied to a Xenopus oocyte expressing Cx46. The cell was held at −70 mV between voltage steps. The duration of each voltage step was 40 s and the interval between voltage steps was 50 s. The periods the oocyte was exposed to either 100% CO2-equilibrated medium or HCl-acidified pH 5.5 medium are bounded by rectangles. Hemichannel currents responded rapidly to application of 100% CO2 solution in the fourth depolarization and were abolished within ∼80 s of the application. Washing with pH 7.5 bathing media led to full recovery within four depolarizations. Hemichannel currents were also decreased with application of pH 5.5 HCl acidified medium during the 13th depolarization. By the next depolarization, Cx46 currents were reduced more. Hemichannel currents recovered by the third depolarization after solution exchange with original pH 7.5 bathing medium. The solid horizontal line marks the level of the activation of Cx46 hemichannels before any treatment. The dashed line approximates the increase in peak current with time, which is probably due to continued expression of new Cx46 hemichannels.
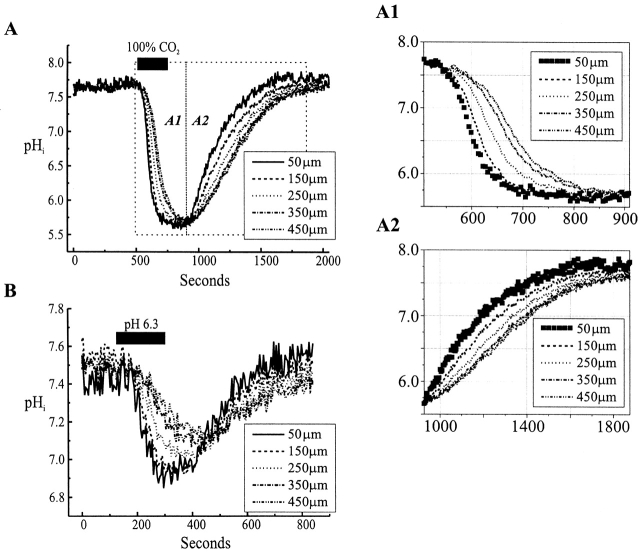
When examining the time course of the changes in pHi using BCECF in single Xenopus oocytes, we observed a substantial and rapid acidification with application of 100% CO2 (Fig. 5 A). However, in the large oocytes, pHi did not change uniformly. Close to the cell periphery, pH dropped at an average rate of −0.02 pH U/s and thus could rapidly affect hemichannels from the inside. At increasing distances from the periphery, acidification was considerably slower, but eventually reached the same steady state value (Fig. 5 A1). An application of an HCl-acidified solution caused intracellular acidification that was considerably slower than that with CO2, falling at an average rate of −0.005 pH U/s at the cell periphery (Fig. 5 B). As with 100% CO2, the rate of acidification slowed with increasing distance from the surface. With HCl-acidified solutions, equilibration of internal and external pH was not achieved during a 3-min application.
Figure 5.
Measurement of pHi in Cx46 expressing Xenopus oocytes during application of medium (A) equilibrated with 100% CO2 and (B) adjusted to pH 6.3 with HCl. pHi was monitored with BCECF and recorded at five distances from the edge of the oocyte, ranging from 50 to 450 μm. Experimentally, these values were obtained by placing 100-μm circular “regions of interest” (ROI) centered at the specified distances on the acquired image of the oocyte and replaying the saved acquisition. During image acquisition, the objective was focused on the oocyte's perimeter. With a large, round oocyte, this focal plane would pass through the center of the oocyte, although with considerable light scatter. The ratiometric determination of pH in this plane will include some signal from the periphery above and below the center of the oocyte, but the image from the perimeter of the oocyte will include entirely peripheral cytoplasm. Thus, there is a significant difference in the amount of signal contributed from deep within the oocyte cytoplasm as the ROI is moved away from the perimeter. The slower change in pH towards the center of the oocyte was observed consistently, but eventually the pH reached the same value in the center as at the periphery. (A) A 4-min application of 100% CO2-equilibrated medium lowered pHi from ∼7.6 to ∼5.8. An enlarged view (A1) shows how the kinetics of intracellular acidification slows with increasing distance from the oocyte periphery. The same steady state value of pHi was reached in all cases before recovery was initiated by washing. Similar kinetics was seen with recovery (A2). (B) An ∼3-min application of HCl-acidified pH 6.3 medium also lowered pHi. Again, the effects were faster at the edge of the oocyte. With the duration of the application shown, pHi decreased less at the center of the oocyte.
Similar to the results in Neuro-2a cells, applications of 100% CO2 solutions and HCl-acidified solutions to Xenopus oocytes induced intracellular acidification to different degrees that correlated with the degree to which Cx46 hemichannel currents were reduced. These results are consistent with the sensitivity exhibited by Cx46 cell–cell channels in Neuro-2a cells to intracellular acidification. The more rapid effect on hemichannel currents seen with application of HCl-acidified pH 5.5 solution contrasts the slow decrease in gj seen with cell–cell channels (Fig. 2 B). These data suggest that either there is an extracellular site of Cx46 pH sensitivity exposed in the unapposed hemichannel configuration or that changes in external pH can rapidly act internally by permeation of H+ through the open hemichannel pore.
Excised Patches Containing Cx46 Hemichannels Remain Sensitive to Acidification on the Cytoplasmic Side
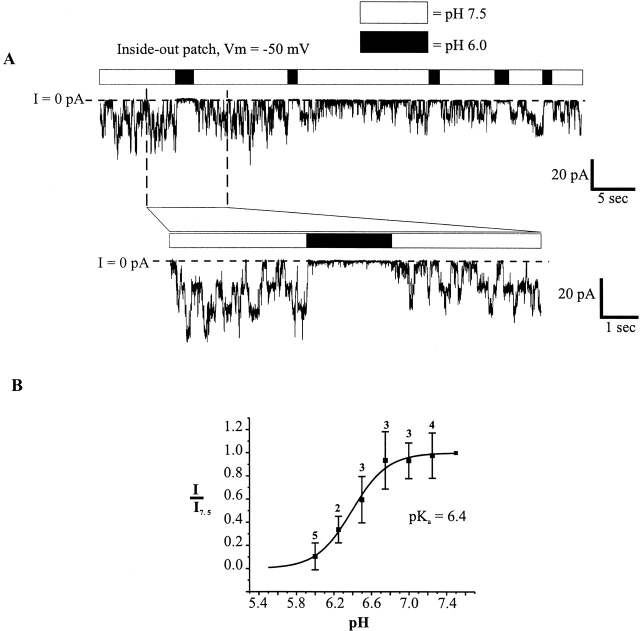
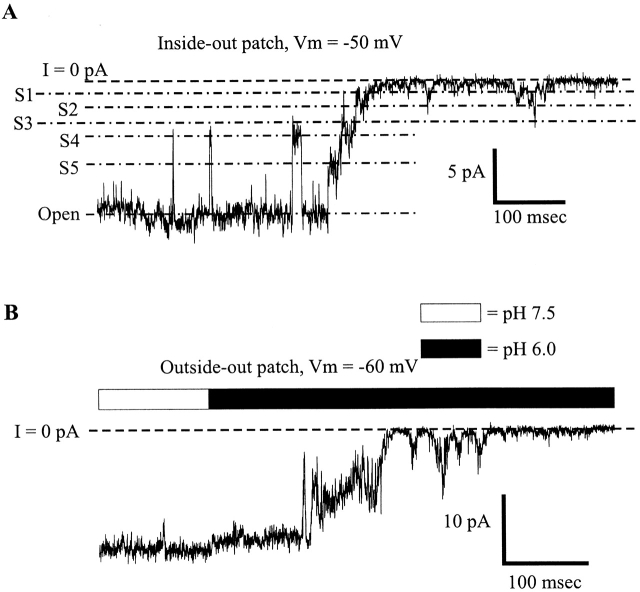
To determine whether soluble cytoplasmic factors mediate pH sensitivity and to permit more rapid changes in pH, patches containing Cx46 hemichannels were excised from Xenopus oocytes in inside-out configurations. Patches were switched between high and low pH solutions by means of a piezoelectric actuator (see materials and methods). Fig. 6 A shows a recording from a patch containing four active hemichannels placed in a stream composed of IPS-A at pH 7.5. Switching to IPS-A at pH 6.0 led to rapid and complete closure of all the hemichannels. Upon returning to pH 7.5, the hemichannels reopened promptly. Multiple applications to the same patch show the robustness of the low pH effect; rapid closure occurred each time the pH 6.0 solution was applied. In an expanded view of one of these applications, all the hemichannels closed rapidly. Although there were occasional brief, low amplitude bursts (perhaps due to partial openings), no full openings occurred for the duration of this pH 6.0 application. Upon switching back to pH 7.5, the hemichannels returned to normal gating behavior. This result clearly demonstrates that no soluble cytoplasmic factors are required for pH sensitivity in Cx46 hemichannels. Furthermore, the effect of pH 6.0 application was essentially the same in solutions buffered with concentrations of HEPES and/or PIPES ranging from 0.25 to 25 mM (data not shown), suggesting no dependence on buffer composition or strength, (see Harris and Bevans, 1998). The titration curve of the reduction in mean current by acidification is fit by the Hill equation with an apparent pKa of 6.4 and an n of 2.3 (Fig. 6 B).
Figure 6.
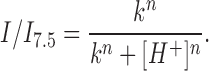 |
Excised Patches Containing Cx46 Hemichannels Remain Sensitive to pHi in Low Ca2+
H+ and Ca2+ have been shown to act synergistically to modulate coupling between neonatal (Burt, 1987) and adult (White et al., 1990) rat myocytes. To examine pH sensitivity from the cytoplasmic side with Ca2+ concentrations fixed at high and low levels, we used inside-out patches and exposed them to solutions containing 2 mM BAPTA (IPS-B) with appropriate amounts of added Ca2+ at pH 7.5 and 6.0 according to Schoenmakers et al. (1992). Fig. 7 shows applications of pH 6.0 solutions with the Ca2+ concentration fixed at (A) 10−7 and (B) 10−5 M to inside-out patches each containing three Cx46 hemichannels. At either Ca2+ concentration, the mean current rapidly decreased ∼90% upon switching to pH 6.0. Furthermore, changing Ca2+ from 10−7 to 10−5 M while maintaining pH fixed at 7.5 did not alter hemichannel open probability (data not shown). These data indicate that Ca2+ up to 10−5 M does not appreciably affect Cx46 hemichannel open probability and that Ca2+ concentrations over a physiologically significant range (10−7 to 10−5 M) have little or no qualitative effect on pH sensitivity. These results are in agreement with those we obtained for Cx46 cell–cell channels in pairs of N2a-Cx46 cells in which application of 100% CO2 uncoupled cell pairs equally well when the patch pipettes were filled with IPS containing BAPTA or EGTA (data not shown). We did not examine the effects of elevated extracellular Ca2+ on pH sensitivity since increases in extracellular Ca2+ by themselves close Cx46 hemichannels and substantially shift voltage-dependent activation (Ebihara and Steiner, 1993).
Figure 7.
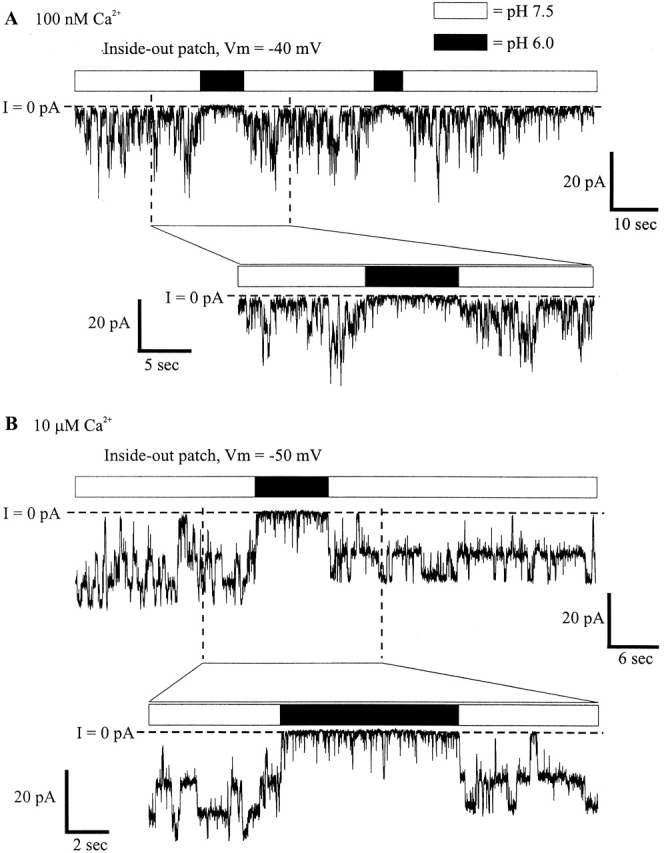
Cx46 hemichannels are equally sensitive to pH with [Ca2+] at 100 nM and 10 μM. IPS-B (2 mM BAPTA) was used for all solutions, with added CaCl2 adjusted to give 100 nM and 10 μM [Ca2+]. IPS-B with 100 nM [Ca2+] was used as the pipette filling solution in both cases. (A) Rapidly switching from pH 7.5 to 6.0 with 100 nM [Ca2+] significantly lowered the open probability of the three active channels in the patch. Few full reopenings are seen during the low pH application. I/I 7.5 = 0.1. (B) With 10 μM [Ca2+] solutions, Cx46 hemichannel open probability was similarly reduced with pH 6.0 applications. I/I 7.5 = 0.08. I = 0 pA denotes the leak conductance of the patch; channel openings are downward deflections in current.
The Rapidity of the Response to Acidification from the Cytoplasmic Side Suggests a Direct Effect of H+ on Cx46 Hemichannels
To better examine the time course of hemichannel closure with acidification, we performed an ensemble analysis of currents from inside-out patches. In Fig. 8 A, 20 sequential recordings from a representative patch containing a single Cx46 hemichannel demonstrate the reproducibility of the effect of a 2-s pH 6.0 application to the cytoplasmic side; the pH on the extracellular side (i.e., the pipette solution) was maintained at 7.5. On switching to pH 6.0, unitary conductance was lowered by ∼15% and open probability was markedly reduced. Few full reopenings occurred during the pH 6.0 applications, but those that did occur had reduced conductance. The sum of these 20 recordings and 112 more from four other patches containing multiple hemichannels is shown in Fig. 8 B. The decay in current was fit well by two exponentials (time constants of 110 and 935 ms), as shown in Fig. 8 C. The steady state value obtained from the fit was −282 pA. The average total current before pH 6.0 application was −4,032 pA. Thus, ∼7% of the original current remained in pH 6.0. This value is in good agreement with the average value of 10% obtained from analysis of mean currents through individual patches (see Fig. 6 B). As shown in Fig. 8 D, on switching to pH 6.0 (dashed line), the onset of the ensemble current decrease had no measurable delay. The rapidity of the reduction in current with acidification suggests that protons are acting directly on Cx46 hemichannels.
Figure 8.
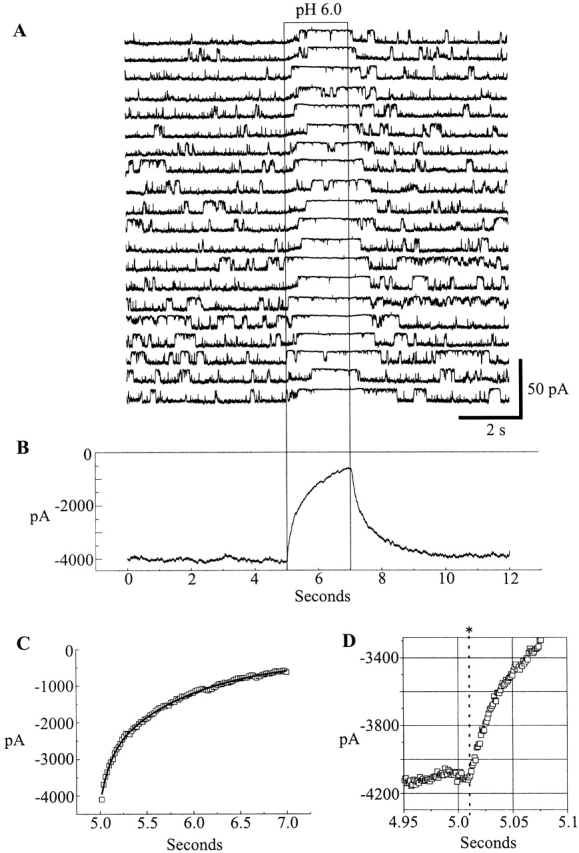
Ensemble currents show the time course of pH- induced closure. (A) An inside-out patch held at −30 mV and containing a single Cx46 hemichannel was subjected to repeated 2-s applications of pH 6.0 IPS-A (region within the rectangle). Hemichannel openings are downward deflections in current. The beginning of each 12-s trace immediately followed the end of the previous trace. (B) Summation of the currents from the patch shown in A, together with 112 more traces from four other patches at −30 mV containing multiple channels. (C) A sum of two exponentials fitted to the pH 6.0 induced decay of ensemble currents. The time constants of the decay were 110 and 935 ms, and the constant parameter of the fit was −232 pA. (D) An enlarged view showing the time of solution switching (denoted by the dashed line) in comparison with the onset of ensemble current decay. There was no delay between pH 6.0 application and current decrease.
Recovery of Hemichannel Currents in Excised Patches Depends on the Duration of Acidification
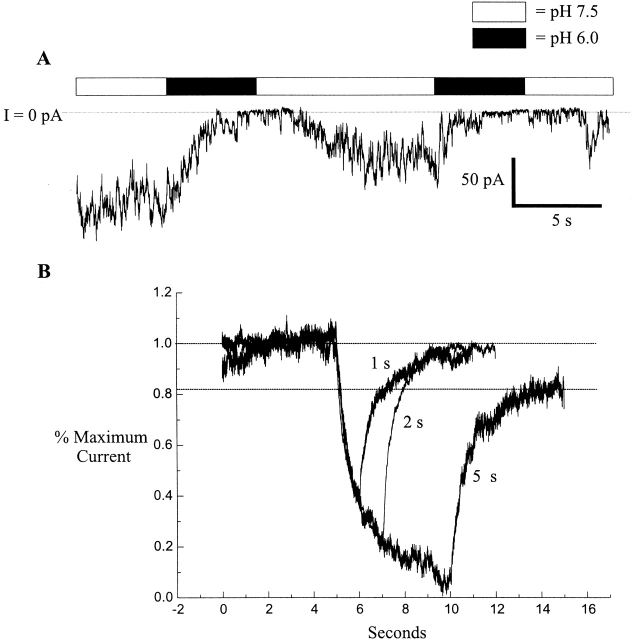
As evident in the ensemble analysis (Fig. 8 B), Cx46 hemichannel currents fully recovered from each successive 2-s acidification. However, less recovery was seen in patches exposed to pH 6.0 for longer times. With applications of 5–10 s in duration, the degree of hemichannel loss in multichannel patches ranged from 0 to 50%. In the example shown in Fig. 9 A, two successive 5-s applications of pH 6.0 were applied to an inside-out patch containing multiple hemichannels. After the first application, recovery was delayed, and the number of active hemichannels was reduced by roughly 50%. The second application reduced the number of active hemichannels even more. In Fig. 9 B, ensemble currents normalized to the mean current at pH 7.5 for 1-, 2-, and 5-s applications of pH 6.0 solutions are superimposed. The initial current decay of all three followed nearly the same time course, but the degree of recovery from 5-s applications was substantially reduced; ∼20% of the channels did not recover. These data show that the degree of recovery is dependent on the duration of acidification, qualitatively similar to the observations with macroscopic recordings of gj in Neuro-2a cells expressing Cx46 (Fig. 2).
Figure 9.
Ensemble currents show the effect of the duration of acidification on the extent of recovery. (A) An inside out patch held at −30 mV and containing at least 20 Cx46 hemichannels was subjected to two applications of pH 6.0 for 5 s each. After each application, the number of active channels was markedly reduced. (B) Ensemble currents from multiple patches were normalized to the average of the currents over the 5 s before acidification. The 1-s ensemble is the sum of 96 pH 6.0 applications from three patches, the 2-s ensemble is the sum of 132 applications from five patches (the same as in Fig. 8), and the 5-s ensemble is the sum of 31 applications from a total of five patches. Recovery from 1- and 2-s applications is nearly 100%, whereas recovery from 5-s applications is only ∼80%.
The Site of Cx46 Sensitivity to pHi
At voltages at which hemichannel open probability is high and closed durations are relatively short (e.g., −30 mV) application of a pH 6.0 solution to the cytoplasmic face of an open hemichannel induced closure that usually lasted for the duration of the application, although occasional reopenings occurred. These closed durations at pH 6.0 were substantially longer than those associated with gating at pH 7.5. This is evident in the 20 sequential pH 6.0 applications shown in Fig. 8 A. Likewise, if a pH 6.0 solution were applied to a closed hemichannel, the hemichannel also often remained closed for the duration of the application. In the example shown in Fig. 10 A, a pH 6.0 application occurred >300 ms after both hemichannels in the patch had fully closed. No full reopenings were seen for the duration of the application. These results indicate that the site of pHi sensitivity is accessible from the cytoplasm when the pH or voltage gate fully closes a Cx46 hemichannel.
Figure 10.
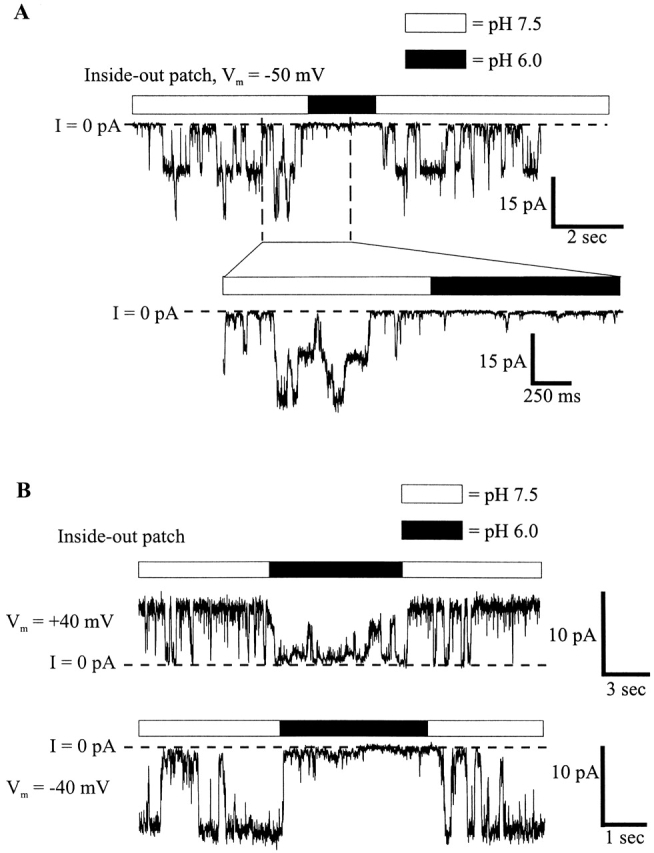
(A) Application of low pH to the cytoplasmic face of Cx46 hemichannels in inside-out patches decreases channel open probability whether the application occurs when the Cx46 hemichannel is open or closed. In this example, taken from the same patch depicted in Fig. 6, rapid solution switching to pH 6.0 followed a spontaneous closure. During the remainder of the application, channel open probability was reduced, and no full reopenings were evident. Opening is downward and I = 0 pA is the leak conductance of the patch. (B) The open probability of Cx46 hemichannels during pH 6.0 applications exhibits moderate voltage dependence. An inside-out patch held at +40 mV was exposed to pH 6.0 for a brief time. Closure occurred soon after application, but there were several brief reopenings to a reduced conductance during the low pH exposure. At −40 mV, there were no complete openings while the patch was exposed to pH 6.0. I = 0 pA is the leak conductance of the patch.
To test whether the cytoplasmic site of pH sensitivity is in the electric field induced by an applied voltage, we examined voltage dependence of pHi sensitivity. If H+ interacts with a site in the voltage-induced field, positive voltages would be expected to increase the effectiveness of low pH applied to the cytoplasmic face by driving H+ into the membrane and pore. Fig. 10 B is an example of an application of a pH 6.0 solution to the cytoplasmic face of a single Cx46 hemichannel in an inside-out patch. At +40 mV, H+ is less effective at causing closure than at −40 mV, opposite that expected for an increased entry of H+ into the membrane or pore. These data suggest that H+ acts on a cytoplasmic site whose accessibility and/or efficacy in inducing closure is voltage dependent. At negative voltages, the affinity of the hemichannel for H+ is increased or the open/ closed equilibrium for H+-induced gating is shifted towards closed.
The polarity of voltage dependence together with no need for an open hemichannel to effect acidification-induced closure from the inside indicate that the H+ binding site (pH sensor) for cytoplasmic pH sensitivity is exposed in both closed and open conformations of the hemichannel and that block by H+ or some H+- induced factor within the pore is unlikely.
Sensitivity of Excised Patches Containing Cx46 Hemichannels to Extracellular Acidification Can Be Explained by H+ Acting Intracellularly
To examine the effects of extracellular acidification at the single hemichannel level, we excised patches containing Cx46 hemichannels in outside-out configurations and rapidly switched between pH 7.5 and 6.0 solutions. The pH on the intracellular side (i.e., the pipette solution) was maintained at 7.5. 20 sequential recordings of 2-s pH 6.0 applications from a patch containing a single active hemichannel are shown in Fig. 11. During the pH 6.0 application to the extracellular side, open probability was reduced, but not nearly as much as with pH 6.0 applications to the cytoplasmic side. The sum of the leak-subtracted currents from a total of 50 applications to this patch is displayed in Fig. 11 B. The ensemble current decayed with a single exponential time course to a steady state value of −336 pA (Fig. 11 C) from an initial average of −502 pA, a 33% decrease. This is in contrast to the >90% reduction for pH 6.0 applications to the cytoplasmic face of the hemichannel. As for low pH applications to the cytoplasmic side, the effect is rapid.
Figure 11.
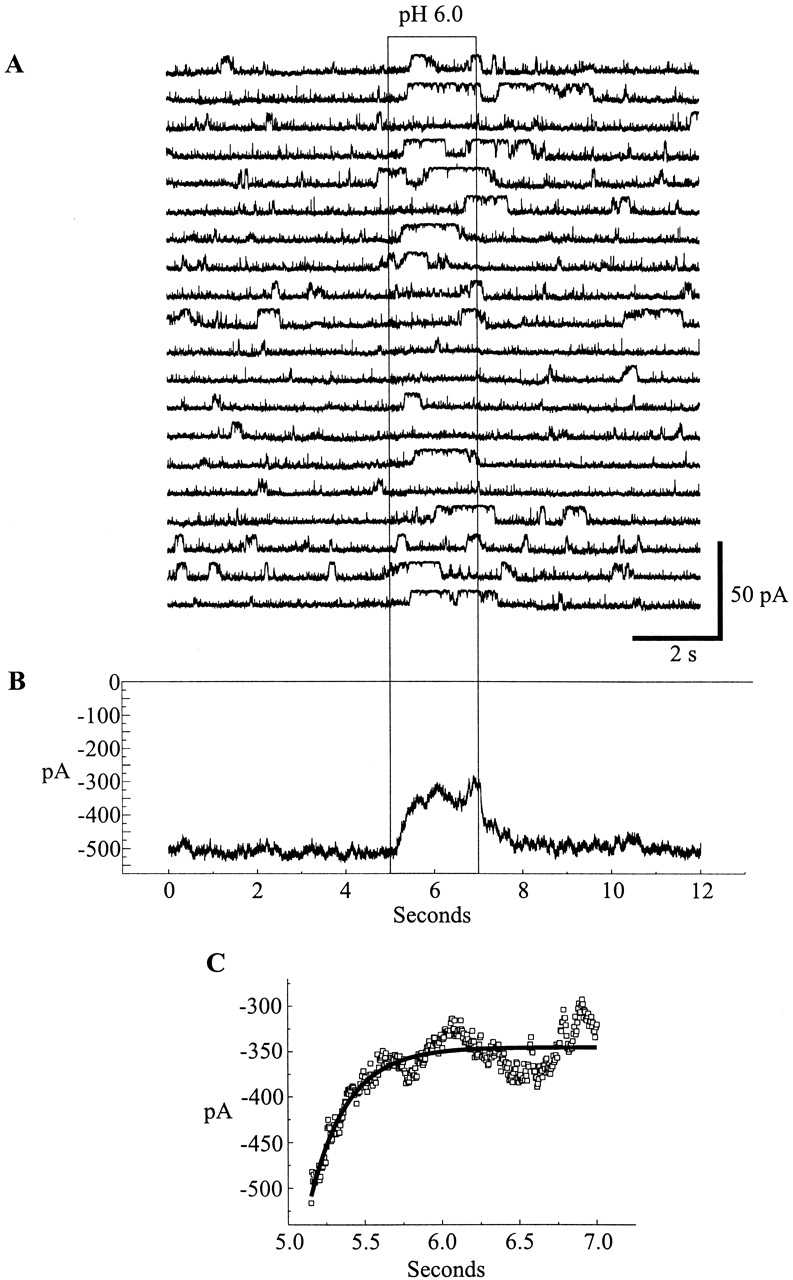
Ensemble currents from an outside-out patch reveal a much lower sensitivity to extracellular pH 6.0 applications. (A) An outside-out patch containing a single Cx46 hemichannel was repeatedly exposed to pH 6.0 IPS-A. Hemichannel open probability is not as reduced as with pH 6.0 applications to the cytoplasmic face of the channel. (B) Summation of the 20 traces displayed in A with 30 additional traces from the same patch (not shown). (C) An exponential fitted to the pH 6.0 induced ensemble current decrease. The time constant of decay was 241 ms, and the constant parameter of the fit was −336 pA.
The extracellular pH sensitivity in excised patches suggests that a lower-affinity extracellular site may exist that closes Cx46 hemichannels. However, it is possible that low pH, applied extracellularly, acts rapidly at the cytoplasmic site by permeation of H+ and/or buffer. We examined this possibility by comparing the closed time distribution of outside-out patches exposed to extracellular pH 6.0 (bulk cytoplasmic pH maintained at 7.5) to the distribution of opening latencies upon rapidly switching cytoplasmic pH in inside-out patches from 6.0 to 7.5. Assuming that protonation/deprotonation is rapid compared with channel gating kinetics and that closed hemichannels do not conduct H+ and/ or buffer, hemichannel closure with extracellular pH at 6.0 should prevent the entry of H+/buffer, thereby causing the cytoplasmic site to reequilibrate with the bulk cytoplasmic pH of 7.5. Hemichannel closure under this condition would lead to reopening and is analogous to opening of closed hemichannels exposed to pH 6.0 on the cytoplasmic side upon rapid switching to pH 7.5. As seen in Fig. 12, B and C, the mean closed time of 600 ms of hemichannels in outside-out patches at pH 6.0 is essentially the same as the mean latency to opening of hemichannels in inside-out patches when switched from 6.0 to 7.5 (636 ms). These values are more than three times longer than the mean closed time at pH 7.5 (170 ms; Fig. 12 D), indicating that low cytoplasmic pH favors the hemichannel to adopt a closed state with a mean dwell time of ∼600 ms. Dwell time analysis of single hemichannels at pH 7.5 indicate that this closed state is a small but visible component of a complex set of closed states (data not shown).
Figure 12.
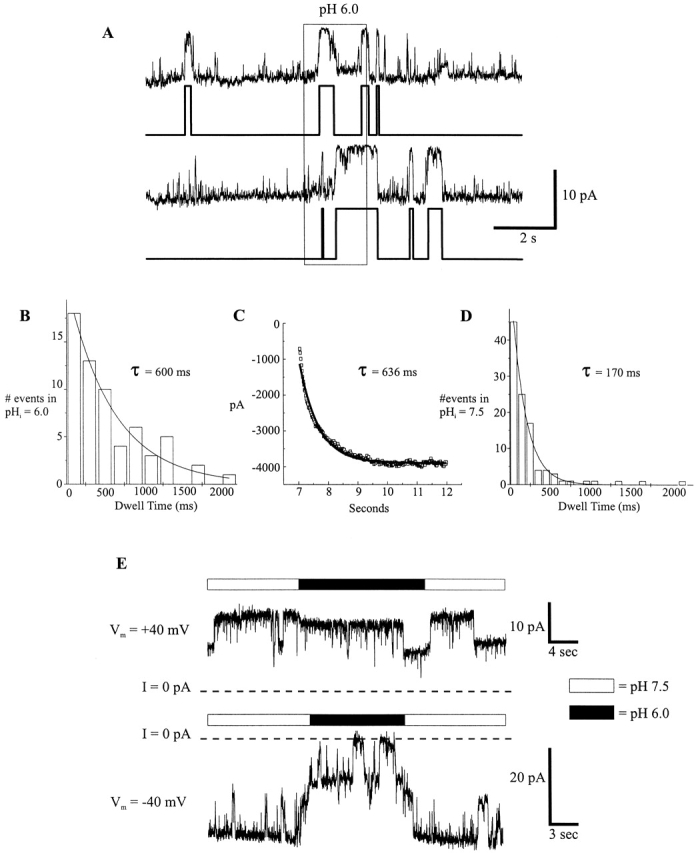
Comparison of Cx46 hemichannel closed times during application of pH 7.5 and 6.0 to an outside-out patch. (A) Example of two current idealizations obtained using the Sublevel Hinkley detector set to low sensitivity. Channel openings are downward deflections in current. Traces are from the same patch as in Fig. 11. Closed times were categorized as pH 7.5 closed times or pH 6.0 dwell times according to the condition in which the event began. (B) Dwell-time histogram of the closed times at pH 6.0 compiled for all 50 traces of the same patch. The histogram was fitted to a single exponential to give a mean closed time of 600 ms. (C) The recovery of the ensemble currents shown in Fig. 8 B after rapidly switching to pH 7.5 from a 2-s application of pH 6.0 was fitted by a single exponential with a time constant of 636 ms, which represents the mean latency to first opening. (D) Dwell-time histogram of the closed times at pH 7.5. A single exponential fit to the distribution gave a mean closed time of 170 ms. Due to the smaller number of events at pH 6.0, binning was increased to 200-ms intervals, compared with 100-ms intervals for pH 7.5 events. (E) Comparison of pH 6.0 applications to an outside-out patch held at +40 and −40 mV. There was an ∼10–15% single channel conductance decrease at +40 mV, but very little change in the open probability of the two channels in the patch (I/I 7.5 ≅ 0.91). The pH 6.0 application at −40 mV occurred after one of the channels had already closed. The open probability of the two channels was markedly reduced (I/I 7.5 ≅ 0.4). The opening events during the pH 6.0 application may be attributable to both channels. I = 0 pA denotes the leak conductance of the patch.
These data argue that closure of hemichannels by low extracellular pH can be explained by H+ acting intracellularly. If there is no extracellular site for H+, and H+ action requires entry through the pore to access the cytoplasmic site, then the expectation would be that extracellular acidification would also have the same polarity of voltage dependence as internal acidification due to the conformational sensitivity. As evidenced in Fig. 12 E with applications of pH 6.0 solutions to the extracellular face of a hemichannel, the voltage dependence indeed has the same polarity. There is a tendency of H+ to close hemichannels more effectively at inside negative voltages, consistent both with the conformational dependence of the cytoplasmic site and with an increased flux of H+ through the hemichannel to access the cytoplasmic site. The combination of reduced H+ influx and conformational sensitivity makes Cx46 hemichannels appear nearly insensitive to low extracellular pH at large positive voltages.
H+-induced Closing Transitions in Cx46 Hemichannels Are Complex and Take Tens of Milliseconds to Complete
At inside negative voltages, gating transitions of Cx46 hemichannels between fully open and fully closed states are relatively slow, and appear to involve transitions among numerous, short-lived subconductance levels (Fig. 13 A, and Trexler et al., 1996, 1998). We have termed these slow transitions “loop gating” transitions (Trexler et al., 1996). Acidification-induced closures are similarly slow for Cx46 hemichannels, as demonstrated in Fig. 13 B. Approximately 100 ms after rapidly switching to pH 6.0 IPS, a widely fluctuating closing transition began and took ∼100 ms to complete. After closing, there were additional small fluctuations that may represent partial reopenings; similar fluctuations are seen with voltage-dependent loop gating at pH 7.5 as well. Although the time course of the acidification-induced transitions varied from ∼10 to 100 ms, all hemichannel closures at low pH in both inside-out and outside-out configurations exhibited this slow, sloppy gating between fully open and closed states.
Figure 13.
Transitions from fully open to fully closed states are slow for both pH-induced and hyperpolarization-induced closures. (A) A single Cx46 hemichannel in an inside-out patch held at −50 mV transited from fully open to fully closed over the course of ∼50 ms. Numerous transitions among discrete substates are evident. Dash-dot lines indicate putative substate levels. (B) Rapidly switching from pH 7.5 to 6.0 IPS-A caused full closure of a single Cx46 hemichannel in an outside-out patch. Closure did not initiate for >100-ms after the solution exchange, and required >100 ms to complete. The channel reopened fully during this application (not shown). The full closing transition is very noisy with no clearly resolvable substates.
An Intact CT Domain and the Highly Conserved H95 Residue in the CL Domain Are Not Required for pH Gating in Cx46 Hemichannels
Removal of a large portion of the CT domain of Cx43 was shown to render Cx43 cell–cell channels nearly insensitive to acidification (Morley et al., 1996). In the same connexin, H95, which is highly conserved among connexins, was found to be important in pH gating, possibly as a site of protonation with intracellular acidification (Ek et al., 1994). We tested whether truncation of CT or mutation of H95 affected pH gating in Cx46 hemichannels expressed in Xenopus oocytes.
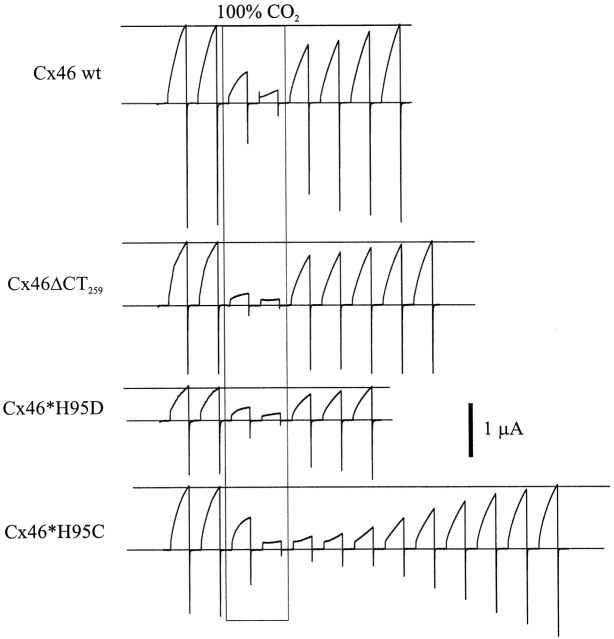
Fig. 14 shows currents in Xenopus oocytes expressing wild-type and mutant Cx46 hemichannels elicited by depolarization to various potentials (+5 to +15 mV). Oocytes were exposed to 100% CO2-equilibrated bath solutions for ∼140 s. BCECF monitoring of an oocyte exposed to 100% CO2 for the same amount of time revealed that pHi would have dropped to 6.0 uniformly within the oocyte by the end of the application and would have recovered to pH 7.5 by the fourth depolarization after washout (data not shown). For wild-type Cx46 (Fig. 14, top), currents decreased rapidly with CO2 and recovered quickly upon washing (the current is near control amplitude by the next trace). Cx46 hemichannels truncated at residue 259, Cx46ΔCT259, responded with no noticeable difference compared with wild type (Fig. 14). This mutant lacks 82% of the CT. These data indicate that, unlike in Cx43, most of CT is not necessary for pH gating in Cx46.
Figure 14.
Mutations shown to affect the pH sensitivity of Cx43 have little effect on Cx46. Hemichannel currents were induced by a series of 20-s depolarizing voltage steps separated by 70 s; the displayed interval between steps is truncated to 10 s for clarity. Voltage was held at −60 mV between depolarizing steps. In all cases, medium equilibrated with 100% CO2 was applied between the second and third depolarizations, and was washed out after the fourth depolarization. Wild-type Cx46 currents were nearly abolished with medium equilibrated with 100% CO2. Cx46ΔCT259, a mutant lacking 82% of the COOH terminus, and the mutant Cx46*H95D hemichannel behaved similarly to wild-type Cx46. Full recovery for these three hemichannel types occurred, on average, by the fourth episode after washout. The small variation in the time to full recovery seen among these three different hemichannel types was similar to that seen for oocytes expressing the same hemichannel type (n = 4, data not shown). In contrast, the mutant Cx46*H95C hemichannel, while similarly sensitive to CO2 application, recovered considerably more slowly upon washout, requiring twice as long as the three previous hemichannel types. Hemichannel currents were elicited by voltage steps to +5 mV for wild-type Cx46 and Cx46*H95D, +10 mV for Cx46ΔCT259, and +15 mV for Cx46*H95C.
We substituted Asp and Cys for His95. The side chain pKa should be significantly shifted in Asp (∼3.9 for Asp, compared with 6.1 for His), decreasing the likelihood that position 95 is protonated with acidification to pH 6.0. The side chain pKa of Cys is more basic, 8.1, increasing the likelihood that position 95 is protonated even at neutral pH. Both Cx46*H95D and Cx46*H95C functionally expressed as hemichannels. With 100% CO2 application, both Cx46*H95D and Cx46*H95C hemichannel currents were nearly abolished (Fig. 14), as with wild-type currents. The recovery of Cx46*H95C currents from acidification was markedly slower than for the other hemichannels, requiring more than twice the time to recover. The similarity of the closing kinetics and degree of current reduction for both mutants compared with wild type suggests that the apparent pKa of Cx46 pH sensitivity does not reflect the pKa of the side chain at position 95. The delayed recovery of Cx46*H95C currents after acidification with 100% CO2 is not likely due to a shift in the pH sensitivity to more basic values, based on the BCECF data indicating that pHi would have recovered to control values by the fourth trace after washout. Inspection of single H95 mutant hemichannels was difficult, as their expression was consistently lower than that of wild type. In one successful cell-attached patch of Cx46*H95D hemichannels, unitary conductance was reduced by ∼60% and voltage gating appeared flickery (data not shown).
These data suggest that H95 is not required for pH gating in Cx46, but moderate shifts in pH sensitivity with H95 substitutions could have gone undetected by our procedure. Also, H95 substitutions can affect gating and conductance, and possibly structure, which confounds interpretations of their effects on pH gating. That H95 (H94 in Group I connexins) is highly conserved in connexins and that a number of H95 substitutions in Cx43 are nonfunctional suggest that this residue may play an important structural role (Ek et al., 1994). The importance of this residue in GJ channel function is also indicated by the naturally occurring H94N and H94Y substitutions in humCx32 that causes CMTX (X-linked form of Charcot-Marie-Tooth disease), a peripheral neuropathy that leads to axonopathy and demyelination (Bergoffen et al., 1993; Bone et al., 1997).
discussion
The Effect of Acidification on Cx46 Hemichannels and Cell–Cell Channels Is Complex
Cx46 hemichannels and cell–cell channels share a high sensitivity to cytoplasmic pH. They also both exhibit an incomplete recovery from prolonged acidification. The lack of complete recovery was particularly evident for electrical coupling between Neuro-2a cell pairs exposed to 100% CO2 for ∼45 s (Fig. 2). Junctional conductance was completely abolished and remained undetectable for several minutes after washout, long after pHi would have returned to normal values. The recovery that did occur was much slower than the relatively rapid recovery with shorter CO2 exposures (e.g., 15 s) and never reached the original conductance. We did not quantify the degree of recovery because it slowly increased with time and took so long that maintenance of the whole-cell recording was compromised. Recovery of macroscopic Cx46 hemichannel currents from prolonged acidification in single Xenopus oocytes was faster and often more complete. However, we often observed increases in expression over time and did not try to control temperature or adjust the amount of mRNA injected in the oocytes to stabilize expression. Excised patches containing hemichannels also showed incomplete recovery from prolonged acidification on the cytoplasmic side, indicating that soluble cytoplasmic factors are not necessary. When hemichannel activity in excised patches was lost during prolonged acidification, we did not see recovery even after times as long as 10 min. Reports of recovery of gj from acidification in Xenopus oocyte cell pairs expressing other connexins and mutants range from poor to good (e.g., Wang and Peracchia, 1997). It is possible that there are differences among connexins and/or cells' abilities to recover from prolonged acidification.
From the hemichannel data obtained from patches, it appears that prolonged acidification causes a population of Cx46 channels and hemichannels to enter a nonconducting state from which there is slow or no recovery. We provisionally term the process of entering this state pH inactivation. The fraction of channels and hemichannels entering the inactivated state increases with the duration of acidification. The remaining fraction recovers rapidly upon washing. We consider this reversible form of pH regulation to be pH gating. We cannot distinguish whether pH gating and pH inactivation are mediated by the same or separate sites of H+ action. We have not determined whether recovery from the pH-inactivated state is absolutely prohibited. The very slow recovery of Cx46-mediated coupling in Neuro-2a cells after prolonged acidification may represent exit from the pH-inactivated state or may represent another process such as formation of new channels.
Although we have no mechanistic explanation for the long-term pH inactivation of connexin channels and hemichannels, this form of regulation may be important in delaying the recoupling of cells that are recovering from acidosis, such as that which can accompany ischemia (see Cotrina et al., 1998). We have observed incomplete recovery of gj with prolonged exposure to CO2 in other connexins expressed in HeLa cells, such as Cx32 and Cx43 (Bukauskas, F.F., unpublished results), suggesting that pH inactivation may be widespread among connexins. If this were the case, the titration curves obtained during experiments in which cells are slowly acidified over a period of 30–60 min would reflect both the pH gating and inactivation processes (see Liu et al., 1993; Ek et al., 1994; Ek-Vitorin et al., 1996; Morley et al., 1996, 1997). Inactivation by pH could decrease the number of active channels with time, thus shifting the apparent pKa of pH gating to more alkaline values.
pH Effects in Cx46 Hemichannels Do Not Require Cytoplasmic Intermediates
An obvious and simple conclusion than can be made from our excised patch experiments is that pH gating of Cx46 hemichannels does not require a cytoplasmic intermediate. The possibility that Ca2+ acts as an intermediary in Cx46, at least over a physiologically relevant concentration range of 10−7 to 10−5 M, was also ruled out, as were the buffers we used, HEPES and PIPES. These data contrast the key role of Ca2+ proposed by Peracchia et al. (1996) and Cotrina et al. (1998). Lowering of intracellular pH without a concomitant rise in intracellular Ca2+ was reported to have no effect on gj in Xenopus oocytes expressing the endogenous Cx38 and in Novikoff hepatoma cells, which express Cx43 (Lazrak and Peracchia, 1993; Peracchia et al., 1996). Thus, acidification was proposed to have no role except to raise intracellular Ca2+, which in turn closed GJ channels by a Ca2+/calmodulin-mediated mechanism. While we did not quantitatively assess the effects of Ca2+ on pH sensitivity over the full range of pH in Cx46 hemichannels, closure induced by application of pH 6.0 to excised patches was as robust in 10−7 M [Ca2+] as in 100-fold higher concentrations. Furthermore, the use of BAPTA-containing pipettes in Neuro-2a cell pairs expressing Cx46 did not prevent uncoupling with application of CO2 or acidified solutions. At least for Cx46, a rise in Ca2+ does not appear to mediate or be necessary for acidification-induced closure. We did not examine pH sensitivity in Ca2+ concentrations <10−7 M and, therefore, cannot rule out the possibility that some amount of Ca2+ is necessary to express sensitivity to pH.
H+ Acts Directly on Cx46 Hemichannels
With no need for a soluble intermediate for Cx46, one is left with either a direct action of H+ on the connexin itself or an indirect action via a membrane-associated protein. Direct effects of H+ on Cx46 may be distinguished from indirect effects via membrane-associated intermediates by examining the speed of H+ action in excised patches subjected to rapid solution switching. Although modulation by means of soluble cytoplasmic second messengers is impossible in excised patches, membrane-delimited pathways, in which indirect effects on channels are mediated by close proximity to a membrane-bound intermediate, can be quite rapid. For example, muscarinic responses of IK(ACh) channels via G-proteins can develop with average latencies of ∼100 ms; effects could be seen in individual channels as early as ∼30–40 ms (Yatani and Brown, 1989; see also Hille, 1994). We observed a minimal latency to the onset of closure near zero in ensemble currents with fast application of pH 6.0 to the cytoplasmic side of an open hemichannel. Thus, the effects of H+ on Cx46 hemichannels appears to be too fast to result from a membrane-delimited process that would involve activation, lateral diffusion, and contact of an intermediate with the connexin. Recently, Cx32 has been shown to contain two cytoplasmic calmodulin-binding domains (Torok et al., 1997). However, bound calmodulin is unlikely to have a role as mediator of the rapid and reversible form of H+-induced closure in Cx46 as we have shown this effect to be quite Ca2+ insensitive.
Mutagenesis, in principle, should also provide a means of distinguishing direct from indirect actions by identifying titratable residues. However, mutational studies of connexins performed thus far indicate that this approach is not likely to be straightforward. Mutations of the putative titratable His residue at position 95 in Cx43 have not clearly established the role of H95 in pH gating (Ek et al., 1994). We qualitatively examined the pH sensitivity of mutants of Cx46 similar to those examined in Cx43. We replaced H95 with an acidic residue, aspartate, and a more basic residue, cysteine. The lack of an obvious effect of His95 substitutions on susceptibility to acidification suggests that this residue may not be responsible for pH gating in Cx46. Moreover, removal of 82% of CT did not appear to severely impair acidification-induced closure in Cx46; a similar degree of truncation of CT in Cx43 did reduce pH sensitivity (Liu et al., 1993). These data suggest that the mechanisms of pH dependence in Cx46 and Cx43 differ.
The inside-out excised patch permitted rapid, uniform acidification, and recovery was complete with brief exposures; thus, it was reasonable to determine a titration curve, which is an equilibrium measure that assumes complete reversibility. We could have obtained a titration curve when acidifying outside-out patches, but we established that H+ applied extracellularly acts intracellularly at the same site as intracellular acidification. Given that the same site is acted on in both excised patch configurations and that we could not control intracellular pH during extracellular application, it made little sense to obtain a titration curve for extracellular H+ in outside-out patches. We could not obtain accurate titration curves in intact cells because during the relatively slow cytoplasmic acidification and recovery, what we term pH inactivation makes hemichannel closure incompletely reversible. Thus, a change in pH sensitivity upon excision of patches remains a possibility. However, comparison of the effect of acidification with CO2 and with HCl on oocytes (Figs. 4 and 5) and the titration curve for acidification of the intracellular side of inside-out patches (Fig. 6 B) suggests that patch excision does not reduce pH sensitivity.
Possible Locations of the pH Sensor; i.e., Titratable Residue(s) in Cx46
By applying solutions with similar pH values that induced different rates of intracellular acidification (i.e., media equilibrated with 100% CO2 and media adjusted to pH 5.5 with HCl), we demonstrated that the decrease in gj is similar in time course to the decrease in pHi. This result suggests that Cx46 cell–cell channels are sensitive only to intracellular acidification, which would place the pH sensor on the cytoplasmic side. We were surprised that acidified HEPES/PIPES buffered solutions, lacking CO2 or a membrane permeable weak acid, were still able to substantially lower intracellular pH. For the case of a whole-cell patch of a Neuro-2a cell, application of pH 5.5 medium decreased pHi from 7.25 to 6.0 in 300 s (see Fig. 3 B). For calculation purposes, we considered a 20-μm diameter cell filled with a solution buffered with 10 mM HEPES (pKa of 7.55). At pH 7.25, the ratio of deprotonated to protonated buffer is 0.5. At pH 6.0, that ratio drops to 0.028. Thus, the concentration of protonated buffer increases from 6.67 mM at pH 7.25 to 9.73 mM at pH 6.0. To produce this change, ∼7.7 × 109 H+ ions need to cross the membrane at an average rate of ∼2.6 × 107/s. Perhaps protons are diffusing through ion channels in the Neuro-2a cell membrane. Zhou and Jones (1996) were able to block intracellular acidification in bullfrog sympathetic neurons with 1 μM tetrodotoxin, suggesting that Na+ channels are the pathway for H+ entry. We did not attempt to block intracellular acidification by blocking ion channels in Neuro-2a cells or Xenopus oocytes.
Unlike Cx46 junctional currents in Neuro-2a cells, Cx46 hemichannel currents recorded macroscopically from Xenopus oocytes or from excised patches are rapidly affected by both intracellular and extracellular acidification. However, the sensitivity to extracellular acidification appears to be explainable by H+ entry through the pore to reach the cytoplasmic site. The primary evidence that extracellular H+ acts intracellularly is the similarity of the mean closed time in extracellular pH 6.0 and the mean latency to opening of hemichannels rapidly switched from pH 6.0 to 7.5 on the cytoplasmic side. These two time intervals should be equal if extracellular and intracellular H+ ions act at the same intracellular site, and the following reasonable assumptions hold: closed hemichannels do not conduct H+ and pH at the sensor after hemichannel closure rapidly reaches the value in the solution bathing the inner face. These analyses used multiple low-pH applications 2 s in duration so as not to evoke pH inactivation. The agreement between closed time and latency to open also argues against the possibility that protons or buffer permeate through the seal between the membrane and the patch pipette glass to decrease intrapipette pH enough to affect channel open probability. Also in support of extracellular H+ acting intracellularly is the conservation of the polarity of voltage dependence of low pH action from either side. The polarity of voltage dependence with H+ added to the cytoplasmic side is opposite that expected for H+ movement to a pH sensor or binding site located deep in the pore, and thus the voltage dependence is likely to result from a charge movement associated with the conformational change of gating. The polarity of voltage dependence is the same with H+ applied externally, when the field would increase flux to an intracellular site. Since the pH gradient at the cytoplasmic mouth of an open hemichannel is unlikely to extend very far, the H+ binding site is likely to be near the entrance to the pore. The apparent lack of extracellular pH sensitivity of Cx46 cell–cell channels in Neuro-2a cells is readily explained by the removal of the critical pathway for H+ entry from the outside, namely through the open hemichannel. Cell–cell channels, once docked, establish a tight electrical seal as evidenced by the absence of a leakage current during de novo channel opening (Bukauskas and Weingart, 1994). Thus, for Cx46 cell– cell channels exposed to low extracellular pH, titration of the pH sensor would require sufficient acidification of the cytoplasm, presumably through other pathways not in such close proximity to the pH sensor as that through the hemichannel. Such a mechanism would take time and is consistent with the slower time course of uncoupling in response to application of solutions acidified with HCl. Likewise, the faster time course of uncoupling in response to application of 100% CO2 equilibrated solution is consistent with the faster time course of cytoplasmic acidification by means of CO2 permeating through the membrane.
With a minimal latency to the onset of closure near zero, as demonstrated by the ensemble currents, we conclude that the pH sensor is located on Cx46 itself. The same pH sensor is likely to mediate pH sensitivity both in Cx46 cell–cell channels and Cx46 hemichannels. It is certainly possible that the conformations of docked and undocked Cx46 hemichannels differ so that the pK(s) of titratable residue(s) differ or even that different titratable residue(s) are exposed. While we have not identified a critical residue for pH sensitivity, we would expect that a substitution that greatly altered pH sensitivity would do so both in cell–cell channels and hemichannels.
Gating Mechanisms that Fully Close Cell–Cell Channels and Hemichannels
At the single channel level, cell–cell channels both in vertebrate and invertebrate tissues show two types of gating transitions: (a) fast transitions between the fully open state and a long-lived substate termed the residual conductance state, and (b) “slow” transitions (tens of milliseconds) between the fully open state and a fully closed state (Bukauskas and Weingart, 1994). The fast transitions to and from the residual subconductance state are evoked by transjunctional voltages of either polarity. Vj gating generally does not close the GJ channel completely, which can largely explain the macroscopic minimum (residual) conductance characteristic of Vj dependence (Spray et al., 1981a; Moreno et al., 1994; Bukauskas and Weingart, 1993, 1994). In contrast, the slow transitions close the channel completely. These transitions are infrequent in most types of cell– cell channels, although their frequency may be increased at large Vjs, but are readily observed in response to a variety of chemical agents known to markedly decrease gj; e.g., CO2 (Bukauskas and Peracchia, 1997), volatile anesthetics, alkanols, and arachidonic acid (Weingart and Bukauskas, 1998). Bukauskas and Peracchia (1997) proposed that the fast Vj gating transitions and the chemically induced slow transitions represent two distinct forms of gating. Interestingly, de novo channel formation also involves slow transitions, which were described as formation currents (Bukauskas and Weingart, 1994). We demonstrated similar slow gating transitions to the fully closed state and fast transitions to a substate in unapposed Cx46 hemichannels and proposed the existence of two distinct voltage gates, a Vj gate and a “loop gate” (Trexler et al., 1996). The name “loop gate” was assigned because of the resemblance of the slow hemichannel gating transitions to the formation currents, which presumably involve the extracellular loops. Recent studies of Cx46 hemichannels using substituted cysteine accessibility measurement indicate that the loop gates are located extracellular to the 35 position in the first transmembrane domain (Pfahnl and Dahl, 1998).
In this study, we demonstrate that acidification closes Cx46 hemichannels fully with transitions that resemble those of loop gating and CO2-induced gating in cell– cell channels. This similarity suggests that the same mechanism may operate to close cell–cell channels and hemichannels in response to intracellular acidification. While we have not quantitatively analyzed the complex transitions induced by acidification in Cx46 hemichannels, they appear to differ from voltage induced loop gating transitions in that they are more noisy and less resolvable into discrete sub-conductance levels (compare Fig. 13, A and B). This difference suggests that loop and pH gating, while sharing common elements, may also use discrete elements. Nevertheless, the voltage dependence of pH gating suggests that both voltage and pH act on the loop gate.
Hemichannels and the Study of Connexin pH Sensitivity
The use of functional, unapposed hemichannels represents an approach complementary to the study of cell– cell channels. A major advantage of hemichannels demonstrated here and in an earlier paper (Trexler et al., 1996) is the possibility of obtaining cell-attached and excised membrane patches that allow for direct, stable, and low capacitance recordings at single and multiple channel levels. Recording of cell–cell channels requires double whole-cell patch clamping, which introduces whole cell capacitance and a limited prospect of recording from a single channel. While direct “attached patches” on junctional membranes have been reported in earthworm septate axons (Brink and Fan, 1989), this technique has not proved widely applicable to vertebrate cell pairs. The slow rate and lack of uniform solution exchange in cell pairs has also hindered studies of modulation of cell–cell channels. Excision of patches in inside-out or outside-out configurations allowed us to change pH rapidly and uniformly at either face of Cx46 hemichannels. It also allowed us to examine the effects of H+ in a cell-free environment and directly address the issue of cytoplasmic intermediates.
While hemichannels in isolation facilitate study of connexin modulation by H+ and other factors, they are not cell–cell channels and their properties as components in cell–cell channels may differ. However, knowledge of the properties of hemichannels in isolation is likely to reduce the measurements required to characterize cell–cell channels satisfactorily. Moreover, comparison of hemichannels and cell–cell channels should clarify the role of hemichannel interactions in channel function.
Acknowledgments
We thank Dr. Alan Finkelstein and Dr. Charles Abrams for helpful discussions. We also thank Angele Bukauskiene and Joseph Zavilowitz for technical assistance.
This work was supported by National Institutes of Health grants GM54179, NS07512, and GM46889.
Abbreviations used in this paper
- CL
cytoplasmic loop
- CT
COOH terminus
- Cx
connexin
- GJ
gap junction
- gj
junctional conductance
- Vj
transjunctional voltage
references
- Arellano RO, Ramon F, Rivera A, Zampighi GA. Lowering of pH does not directly affect the junctional resistance of crayfish lateral axons. J Membr Biol. 1986;94:293–299. doi: 10.1007/BF01869725. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Ramon F, Rivera A, Zampighi GA. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J Membr Biol. 1988;101:119–131. doi: 10.1007/BF01872827. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Verselis VK. Biophysics of gap junctions. Semin Cell Biol. 1992;3:29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Bone LJ, Deschenes SM, Balice-Gordon RJ, Fischbeck KH, Scherer SS. Connexin32 and X-linked Charcot-Marie-Tooth disease. Neurobiol Dis. 1997;4:221–230. doi: 10.1006/nbdi.1997.0152. [DOI] [PubMed] [Google Scholar]
- Brink PR, Fan SF. Patch clamp recordings from membranes which contain gap junction channels. Biophys J. 1989;56:579–593. doi: 10.1016/S0006-3495(89)82705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Weingart R. Multiple conductance states of newly formed single gap junction channels between insect cells. Pflügers Arch. 1993;423:152–154. doi: 10.1007/BF00374973. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994;67:613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Peracchia C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys J. 1997;72:2137–2142. doi: 10.1016/S0006-3495(97)78856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM. Block of intercellular communication: interaction of intracellular H+ and Ca2+ . Am J Physiol. 1987;253:C607–C612. doi: 10.1152/ajpcell.1987.253.4.C607. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S, Schultze R. Detection of jumps in single-channel data containing subconductance levels. Biophys J. 1994;67:1404–1413. doi: 10.1016/S0006-3495(94)80614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopusoocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek JF, Delmar M, Perzova R, Taffet SM. Role of histidine 95 on pH gating of the cardiac gap junction protein connexin43. Circ Res. 1994;74:1058–1064. doi: 10.1161/01.res.74.6.1058. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. pH regulation of connexin43: molecular analysis of the gating particle. Biophys J. 1996;71:1273–1284. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A.L., and C.G. Bevans. 1998. Direct modulation of connexin channels. Abstract at the Gap Junction Conference, Rio de Janeiro, Brazil.
- Hille, B. 1992. Ionic Channels in Excitable Membranes. 2nd ed. Sinauer Associates, Inc., Sunderland, MA. 607 pp.
- Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J. 1993;65:2002–2012. doi: 10.1016/S0006-3495(93)81242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Taffet S, Stoner L, Delmar M, Vallano ML, Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: the carboxyl tail length. Biophys J. 1993;64:1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AP, Rook MB, Fishman GI, Spray DC. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys J. 1994;67:113–119. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M. Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol. 1997;8:939–951. doi: 10.1111/j.1540-8167.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopusoocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C, Bernardini G, Peracchia LL. Is calmodulin involved in the regulation of gap junction permeability? . Pflügers Arch. 1983;399:152–154. doi: 10.1007/BF00663912. [DOI] [PubMed] [Google Scholar]
- Peracchia C, Wang X, Li L, Peracchia LL. Inhibition of calmodulin expression prevents low-pH–induced gap junction uncoupling in Xenopusoocytes. Pflügers Arch. 1996;431:379–387. doi: 10.1007/BF02207275. [DOI] [PubMed] [Google Scholar]
- Pfahnl A, Dahl G. Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys J. 1998;75:2323–2331. doi: 10.1016/S0006-3495(98)77676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, Barnes TM, Ford C, Hekimi S, et al. Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 1998;14:348–349. doi: 10.1016/s0168-9525(98)01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JB, Verselis VK, Bennett MV, Bargiello TA. A domain substitution procedure and its use to analyze voltage dependence of homotypic gap junctions formed by connexins 26 and 32. Proc Natl Acad Sci USA. 1992a;89:3820–3824. doi: 10.1073/pnas.89.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JB, Verselis VK, Bennett MV, Bargiello TA. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992b;62:183–193. doi: 10.1016/S0006-3495(92)81804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Spray DC, Bennett MV. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MVL. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981a;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981b;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spray DC, Stern JH, Harris AL, Bennett MV. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci USA. 1982;79:441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok K, Stauffer K, Evans WH. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem J. 1997;326:479–483. doi: 10.1042/bj3260479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler, E.B., M.V.L. Bennett, T.A. Bargiello, and V.K. Verselis. 1998. Studies of gating in Cx46 hemichannels. In Gap Junctions. R. Werner, editor. IOS press, Amsterdam, Netherlands.
- Wang X, Li L, Peracchia LL, Peracchia C. Chimeric evidence for a role of the connexin cytoplasmic loop in gap junction channel gating. Pflügers Arch. 1996;431:844–852. doi: 10.1007/s004240050076. [DOI] [PubMed] [Google Scholar]
- Wang XG, Peracchia C. Positive charges of the initial C-terminus domain of Cx32 inhibit gap junction gating sensitivity to CO2 . Biophys J. 1997;73:798–806. doi: 10.1016/S0006-3495(97)78112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R, Bukauskas FF. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflügers Arch. 1998;435:310–319. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- White RL, Doeller JE, Verselis VK, Wittenberg BA. Gap junctional conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca++ . J Gen Physiol. 1990;95:1061–1075. doi: 10.1085/jgp.95.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A, Brown AM. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989;245:71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- Zhou W, Jones SW. The effects of external pH on calcium channel currents in bullfrog sympathetic neurons. Biophys J. 1996;70:1326–1334. doi: 10.1016/S0006-3495(96)79689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]