Abstract
Principles of olfactory coding can be clarified by studying the olfactory bulb activity patterns that are evoked by odorants differing systematically in chemical structure. In the present study, we used series of aliphatic esters, ketones, and alcohols (27 odorants total) to determine the effects of functional group position on glomerular-layer activity patterns. These patterns were measured as uptake of [14C]2-deoxyglucose and were mapped into standardized data matrices for statistical comparison across different rats. The magnitude of the effect of position differed greatly for the different functional groups. For ketones, there was little or no effect of position on evoked patterns. For esters, uptake in individual glomerular modules increased, while uptake in others decreased with changing group position, and yet the overall patterns remained similar. For alcohols, group position had a profound effect on evoked activity patterns. For example, moving the hydroxyl group in either heptanol or nonanol from the first to the fourth carbon changed the activity patterns so greatly that the major areas of response did not overlap. Within every functional group series, however, responses were globally chemotopic, such that pairs of odorants with the smallest difference in functional group position evoked the most similar patterns. These results help to define further the specificities of glomeruli within previously described glomerular modules, and they show that functional group position can be an important feature in encoding an odorant molecule.
Indexing terms: deoxyglucose, olfactory bulb, odors, imaging techniques
In an ongoing effort to elucidate principles underlying the olfactory code, we have been characterizing spatial patterns of odorant-evoked [14C]2-deoxyglucose (2-DG) uptake in the glomerular layer of the rat olfactory bulb using sets of odorants differing incrementally in chemical structure. Two basic coding strategies have emerged from these studies. First, certain differences in chemical structure apparently are recognized as distinct molecular features. For example, different odorants sharing a straight-chained hydrocarbon structure but differing in functional groups evoked activity that overlapped in certain parts of the bulb (Johnson and Leon, 2000a). When the functional group was changed, particular clusters of active glomeruli became silent, while new clusters of glomeruli became active (Johnson and Leon, 2000a). To describe these differences, we introduced the concept of functional modules that contained glomeruli sensitive to the odorant functional group as a molecular feature.
The usefulness of this concept of glomerular modules was further supported by studies showing that odorants differing in hydrocarbon structure, but possessing the same functional group, continued to activate glomeruli within the previously defined functional-group sensitive modules (Johnson and Leon, 2000b). The same set of odorants differed in the stimulation of the areas of the bulb that had overlapped for the odorants possessing a similar hydrocarbon structure (Johnson and Leon, 2000b). By comparing a total of 55 odorants, we ultimately hypothesized the existence of 26 glomerular modules (Johnson et al., 2002). These 26 modules are comprised by 13 pairs of lateral and medial modules that contain glomeruli of related specificity, probably corresponding to the paired lateral and medial projection areas of olfactory sensory neurons expressing the same odorant receptor gene (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996). We have identified individual modules by delineating spatially clustered responses to different odorants with the understanding that each identified module constitutes an ongoing hypothesis that can be modified by new data. At the same time, these clusters form a basis for comparison of new glomerular response profiles with a large number of previously described odorants. In addition to functional groups, other molecular features that appear to be represented using differences in the activity of glomeruli in distinct modules include different enantiomers (Linster et al., 2001) and large differences in carbon chain length (Johnson et al., 1999; 2004). We have anticipated that our ongoing research will continue to refine our concept of glomerular modules (Johnson et al., 2002), leading not only to a better understanding of the specificity of glomeruli within previously defined modules, but also to adjustments in the boundaries of these modules (Johnson et al., 2004), and possibly to the discovery of new modules containing sets of glomeruli with distinct, but related specificities.
A second coding strategy that appears to be used in the olfactory bulb involves chemotopic progressions of activity across nearby glomeruli within individual modules. Single straight-chained odorants tend to stimulate clusters of glomeruli within a given module (Johnson et al., 1999). The stimulation of a cluster of glomeruli suggests that these glomeruli within a module have similar specificity in order to respond to the same pure odorant chemical, especially since other individual odorants stimulate only one or a very few glomeruli (Johnson et al., 1998; 1999). Odorants with the same functional group but a slightly different carbon number or molecular length appear to stimulate overlapping glomeruli in the same module, but the center of activation shifts in a consistent direction (Johnson et al., 1999; 2004; Rubin and Katz, 1999; Johnson and Leon, 2000b; Uchida et al., 2000) These local chemotopic progressions may optimize the use of lateral inhibitory networks to impose a greater specificity on bulbar projection neurons, given that these closely related chemicals may always activate overlapping receptor proteins and sensory neurons (Yokoi et al., 1995; Johnson et al., 1999; Malnic et al., 1999; Araneda et al., 2004).
Odorant chemicals can differ in structure along many other dimensions (e.g., functional group position, hydrocarbon branching, presence of ring structures, bond saturation, and stereochemistry at double bonds), most of which have not been well explored in studies of olfactory coding. It therefore is unclear which changes in the structure of an odorant would be recognized as distinct molecular features and would involve separate glomerular modules, and which molecular characteristics would be recognized as being sufficiently related to involve nearest neighbor relationships within a glomerular response module.
To explore the range of possible ways that odorants of different chemistry might be represented in the olfactory bulb, it is desirable to use a technique that is capable of reliably measuring activity across the entire structure. It also is desirable to collect results in a standardized data format that allows statistical comparisons across different animals in order to insure that any finding applies to the entire population, rather than to only a single individual. Analysis of odorant-driven uptake of 2-DG satisfies these criteria (Stewart et al., 1979; Royet et al., 1987; Johnson et al., 1998; 1999; Leon and Johnson, 2003). In the present study, we have used the 2-DG method to investigate the effect of functional group position on spatial representations of aliphatic esters, ketones, and alcohols. A change in functional group position produces a structural isomer, which has the same molecular formula but different chemical properties. Given the subtle nature of their differences, it seemed possible that positional isomers would have greater similarities with respect to olfactory coding than would compounds of incrementally different carbon number within a homologous series.
MATERIALS AND METHODS
Odorants
All ester odorants were purchased from Fisher Scientific (Tustin, CA: Acros brand), as were 1- and 2-heptanol, 1- and 2-nonanol, 3-hexanone, and 2-, 3-, and 4-heptanone. The ketone 2-hexanone and the alcohols 3- and 4-heptanol were purchased from TCI America (Portland, OR). The alcohols 3- and 5-nonanol were purchased from Sigma-Aldrich (St. Louis, MO: Fluka brand). The alcohol 4-nonanol was purchased from Alfa Aesar (Ward Hill, MA). For each exposure, 100 mL of undiluted odorant was placed into a 125-mL gas-washing bottle fitted with an extra-coarse porosity diffuser. Research-grade (high-purity) nitrogen gas was bubbled through the odorant at 250 mL/min. Part of the resulting vapor was mixed with ultra-zero grade air and directed toward the exposure chamber at a final flow rate of 2 L/min to achieve the desired dilution, while the remainder of the vapor was diverted to a vent. For each exposure, the system was equilibrated for at least fifteen minutes before an animal was introduced. Separate Teflon tubing was dedicated to each odorant.
Table 1 shows the dilution and final vapor phase concentration of each odorant in the study, together with each odorant’s unique Chemical Abstract Service registry number and the number of rats exposed to each odorant. Vapor phase concentration was calculated from the odorant’s vapor pressure. The value used for vapor pressure was the mode of unique experimental and estimated values obtained from two Internet databases (Interactive PhysProp Database from Syracuse Research Corporation: http://www.syrres.com/esc/physdemo.htm and the Chemical and Physical Properties Database from the Pennsylvania Department of Environmental Protection: http://www.dep.state.pa.us/physicalproperties/CPP_Search.htm) as well as from two chemistry software packages (Molecular Modeling Pro v. 3.14 from ChemSW, Fairfield, CA and ChemDraw Ultra v.6.0 from CambridgeSoft, Cambridge, MA). Some odorant chemicals have been better studied than others and have multiple experimental values as entries in the databases, whereas for others, the only available values come from estimates or extrapolations, which tend to be more variable and thereby less reliable. The latter situation applied particularly to heptanol and nonanol isomers in the present study. We accordingly decided to present these odorants at an equal vapor dilution, rather than basing any differential dilution on the estimated values for vapor pressure (Table 1).
Table 1.
Dilutions and vapor phase concentrations of odorants.
| Odorant | CAS# | Formula | Dilution factor | Vapor concentration (ppm) | Number of rats |
|---|---|---|---|---|---|
| 1-Heptanol | 111-70-6 | C7H16O | 8 | 36 | 4 |
| 2-Heptanol | 543-49-7 | C7H16O | 8 | 63 | 4 |
| 3-Heptanol | 589-82-2 | C7H16O | 8 | 34 | 4 |
| 4-Heptanol | 589-55-9 | C7H16O | 8 | 63 | 4 |
| 1-Nonanol | 143-08-8 | C9H20O | 8 | 7 | 4 |
| 2-Nonanol | 628-99-9 | C9H20O | 8 | 10 | 4 |
| 3-Nonanol | 624-51-1 | C9H20O | 8 | 10 | 4 |
| 4-Nonanol | 5932-79-6 | C9H20O | 8 | 10 | 4 |
| 5-Nonanol | 623-93-8 | C9H20O | 8 | 10 | 4 |
| Ethyl propionate | 105-37-3 | C5H10O2 | 630 | 75 | 5 |
| Propyl acetate | 109-60-4 | C5H10O2 | 605 | 75 | 5 |
| Ethyl caproate | 123-66-0 | C8H16O2 | 21 | 75 | 5 |
| Hexyl acetate | 142-92-7 | C8H16O2 | 19 | 75 | 5 |
| Ethyl butyrate | 105-54-4 | C6H12O2 | 224 | 75 | 5 |
| Propyl propionate | 106-36-5 | C6H12O2 | 244 | 75 | 5 |
| Butyl acetate | 123-86-4 | C6H12O2 | 206 | 75 | 5 |
| Isopropyl propionate | 637-78-5 | C6H12O2 | 395 | 75 | 5 |
| Methyl caproate | 106-70-7 | C7H14O2 | 53 | 75 | 5 |
| Ethyl valerate | 539-82-2 | C7H14O2 | 84 | 75 | 5 |
| Propyl butyrate | 105-66-8 | C7H14O2 | 104 | 75 | 5 |
| Butyl propionate | 590-01-2 | C7H14O2 | 59 | 75 | 5 |
| Amyl acetate | 628-63-7 | C7H14O2 | 72 | 75 | 5 |
| 2-Hexanone | 591-78-6 | C6H12O | 28 | 200 | 4 |
| 3-Hexanone | 589-38-8 | C6H12O | 31 | 200 | 3 |
| 2-Heptanone | 110-43-0 | C7H14O | 35 | 200 | 5 |
| 3-Heptanone | 106-35-4 | C7H14O | 78 | 200 | 5 |
| 4-Heptanone | 123-19-3 | C7H14O | 92 | 200 | 5 |
In previous studies, we found that the patterns of 2-DG uptake evoked by a limited number of odorants can be different at different odorant concentrations (Johnson and Leon, 2000a; Johnson et al., 2004). The odorants that showed these effects also change in their perceived odor with concentration (Johnson and Leon, 2000a). At least in some cases, the changed patterns at higher concentrations may be due to the influence of minor contaminants (Johnson et al., 2004). Although most odorants appear to give patterns that are consistent across different concentrations (Johnson et al., 1999; 2002; Johnson and Leon, 2000a), it is possible that we would have achieved somewhat different results in the present study if we had used a range of concentrations for each odorant.
Odorant exposures
The UC Irvine Institutional Animal Care and Use Committee approved all procedures involving rats. Litters culled to 8 pups on the day after birth (postnatal day 1) were moved to clean cages along with the dam one hour before odorant exposure to reduce any odors carried into the exposure from soiled cages. Individual rats ranging in age from postnatal day 18 to 21 were given subcutaneous injections of [14C]2-DG (16 μL/g, 0.1 mCi/mL, 52 mCi/mmol, Sigma Chemical Company, St. Louis) and were placed into a clean, 1-L glass jar. The odorant, or gas vehicle, entered the lid of the jar, which was closed immediately after the rat was introduced. The odorant concentration steadily increased over the course of the exposure. The lid of the jar also was fitted with a vent. The exposure extended for 45 minutes, after which the rat was removed and decapitated.
For each litter, the first rat was exposed to the gas vehicle, and the rest of the rats were exposed to odorants. No two rats from the same litter were exposed to the same odorant, and the order of odorant presentation was varied across different litters, such that systematic relationships between odorants were avoided. The study was composed of several independently conducted experiments possessing their own vehicle blanks. The ester odorants were all presented in a single, large experiment, which also included homologous series of ethyl esters and acetates (Johnson et al., 2004). The positional isomers of hexanone and heptanone were presented in a single experiment, while separate experiments were conducted for each of the heptanol and nonanol series.
Data analysis
Sectioning, imaging, and mapping were carried out as described previously (Johnson et al., 1999; 2004). Briefly, uptake of 2-DG was measured in coronal sections taken at regular intervals throughout the entire olfactory bulb. Measurements of 2-DG uptake were taken from autoradiographic images at the intersection of a traced glomerular layer (as seen in an overlaid image of an adjacent Nissl-stained section) and polar gridlines that were chosen on the basis of the rostral-caudal position of the sections relative to anatomical landmarks (Johnson et al., 1999). Film densities in units of grayscale were converted first to units of nCi/g by using radioactivity standards, and then into units of glomerular layer uptake divided by uptake measured in the subependymal zone of sections taken at standardized rostral-caudal positions within the bulb. Data files corresponding to individual sections were merged into data matrices, which then were standardized in size relative to anatomical landmarks. The left and right bulbs of each brain were averaged. Upon completion of all brains in a given study, average data matrices of the vehicle-exposed rats from that study were subtracted from each matrix corresponding to an odorant-exposed rat. These difference matrices then were transformed further into units of z scores relative to the mean and standard deviation of uptake across the glomerular layer in that brain.
To illustrate the odorant-evoked activity patterns, we averaged the z-score data matrices across each odorant condition and plotted them as color-coded contour charts in our typical, two-dimensional, ventral-centered format generated using Microsoft Excel. We prefer the ventral-centered format because it minimizes the influence of occasional missing dorsal values caused by loss of tissue during sectioning (Johnson et al., 1999). To facilitate comparison between our data and data from other labs that favor a dorsal-centered format, we provide both dorsal-centered and ventral-centered charts of each of the present patterns on our website (http://leonlab.bio.uci.edu/), which contains an archive of all of our published activity patterns. Our website also displays each pattern on a rotatable, three-dimensional model of the glomerular surface, which avoids the distortion that inevitably accompanies any attempt to map a rounded three-dimensional surface into two dimensions.
RESULTS
Alcohol positional series
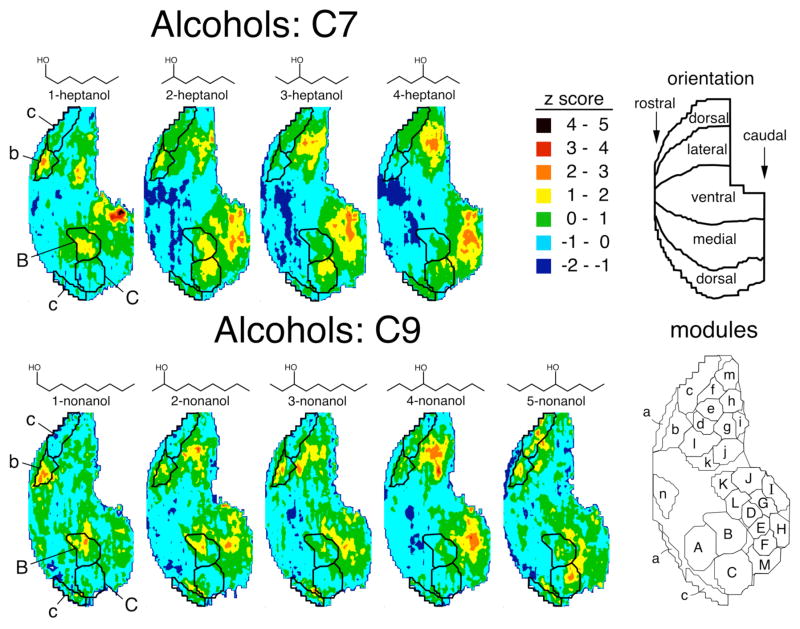
To investigate the effects of hydroxyl group position on the spatial representations of alcohol odorants, we studied isomers of heptanol (7 carbons) and nonanol (9 carbons). The position of the functional group had a profound effect on the evoked activity patterns (Fig. 1). The average z score patterns evoked by 1-heptanol and 4-heptanol had no apparent overlap in their largest responses (Fig. 1).
Fig. 1.
Color-coded contour charts illustrating patterns of glomerular layer 2-DG uptake evoked by alcohol odorants. These charts represent data matrices averaged across multiple rats and are presented as rolled-out maps of the glomerular layer as if a cut had been made dorsally running anterior to posterior. Anterior is located on the left and lateral is in the top half of the charts. The ventral surface is positioned horizontally at the center of each chart (see “orientation” panel). The amount of uptake in units of z scores is indicated using colors as shown in the key. The “modules” inset shows locations of glomerular modules derived from responses to 54 previously studied odorants (Johnson et al., 2002). Modules discussed in depth regarding the representations of the odorants are superimposed on the contour charts. Dorsal-centered and simulated 3-dimensional versions of the same patterns can be found on our website (http://leonlab.bio.uci.edu).
To describe the activity patterns in the present study, we have superimposed on contour charts the modular boundaries that we proposed in a prior study (Johnson et al., 2002). We have averaged z score values of 2 DG uptake across these modules to simplify the patterns for statistical analysis and qualitative description (Fig. 2). Because the modular boundaries were derived in previous experiments involving a different set of odorants, it is likely that the precise boundaries used for these modules will not represent coding units that are applicable to all odorants, and they may not in all cases correspond to boundaries suggested by the activity patterns evoked by particular odorants in the present study. Nevertheless, analysis of the patterns in terms of these modules allows effective comparisons to previous experiments involving odorants overlapping systematically with the present odorants in both chemical structure and evoked activity patterns. The primary alcohol 1-heptanol stimulated glomeruli in the region of the bulb corresponding to our previously defined paired modules “b” and “B”, as well as in modules “g” and “G” (Fig. 2). Similar regions of modules “b” and “B” appeared to be activated by 2-heptanol. The secondary alcohols 2- and 3-heptanol evoked 2-DG uptake within modules “c/C” and “h/H” (Fig. 1), and 4-heptanol stimulated glomeruli in modules “h” and “H” with even greater efficacy (Fig. 2).
Fig. 2.
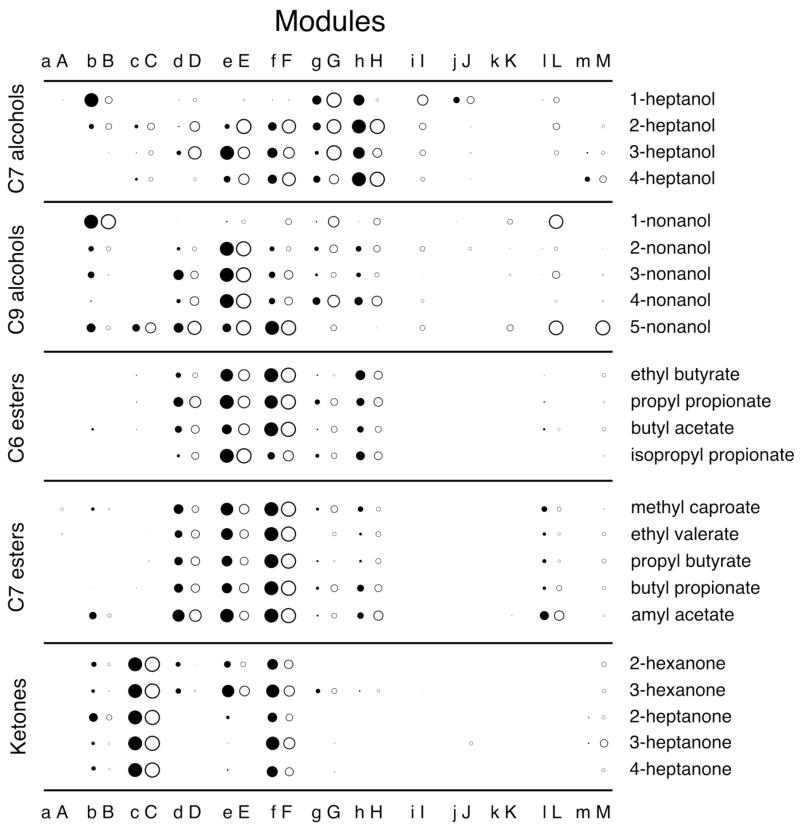
Modular responses. Uptake of 2-DG across each module (see Figure 1) was averaged for each odorant-evoked z score pattern. Each modular value then was expressed as a fraction of the maximum modular value evoked by the same odorant in the same bulbar aspect (lateral or medial). These ratios were used to adjust the diameters of circles to represent the relative stimulation of each module. Only positive values are shown. Lateral modules are labeled with lower case letters and are symbolized using solid circles, while paired medial modules are labeled with the corresponding upper case letter and are symbolized by open circles. The similarity in the average activation of paired modules likely represents the paired lateral and medial projection of sensory neurons expressing the same odorant receptor gene (Vassar et al., 1999; Ressler et al., 1999). Because uptake is averaged across entire modules, small foci of elevated 2-DG uptake, such as those occurring in module “A” for ethyl butyrate, propyl propionate, methyl caproate, and ethyl valerate (see Figure 1) are diminished in impact or even obscured in this figure, which nevertheless captures and simplifies the principal differences in the representations of both different functional groups (e.g., esters versus ketones) and different isomers (e.g., 1-heptanol versus 4-heptanol or 1-nonanol versus 4-nonanol).
Similarly, 1-nonanol and 4-nonanol evoked activity patterns that were almost non-overlapping, and the additional shift in the location of the hydroxyl group from 4-nonanol to 5-nonanol resulted in an apparent third pattern which did not overlap much with the other two (Fig. 1). The primary alcohol 1-nonanol primarily stimulated glomeruli in modules “b” and “B” (Fig. 1, Fig. 2), which previously have been associated with the alcohol functional group (Johnson and Leon, 2000a; Uchida et al., 2000; Johnson et al., 2002, 2004). Uptake within modules “b” and “B” contributed less to the patterns evoked by the nonanol isomers having a more central hydroxyl group position. Instead, glomeruli in modules “e” and “E” were major contributors to the patterns evoked by 2-, 3-, and 4-nonanol (Fig. 2). Uniquely among the nonanol isomers, 5-nonanol evoked 2-DG uptake in modules “c” and “C” (Fig. 1).
To determine if the differences in patterns across the alcohol isomers were statistically significant, we determined the maximal z score value within each of 27 previously defined glomerular modules (Fig. 1) for each rat. Then, we used an ANOVA to compare across individual odorants for each module. Probability criteria were adjusted for the multiple ANOVA comparisons by using false discovery rate corrections (Curran-Everett, 2000), which is a method we have used previously (Linster et al., 2001; Johnson et al., 2004). The patterns evoked by isomers of both heptanol and nonanol were found to be significantly different in several modules (Table 2). In the case of the seven-carbon alcohols, the significant differences were spread out throughout the entire layer, while for the nine-carbon alcohols only modules “e” and “M” reached significance in this rigorous statistical test. This ANOVA-based analysis indicates that there are significant differences in the overall pattern of activity across the odorants within a given series, but it does not indicate which particular odorant is different from which other odorant(s). The use of 27 modules for purposes of analysis reduces the complexity of the data matrices in a rational manner that is based on a priori partitions of the bulb (i.e., the boundaries chosen for the analysis were not selected based on the current patterns in a way that would increase the likelihood of a particular result). The analysis does not in its own right validate the modules as processing units in olfaction, and it is not sensitive to the possibility that different odorants might activate different areas within the same modules. The reduction in data complexity afforded by the use of the 27 modules also may have been accomplished by the use of arbitrary small subdivisions of the data matrices, and it is possible that such arbitrary divisions also would have resulted in statistically significant results.
Table 2.
Modules showing significant differences across positional series1.
| Odorant series | Modules2 |
|---|---|
| C7 alcohols | j,B,E,b,A,k,e,C,H,L,f,i,J |
| C9 alcohols | e,M |
| C6 esters | b,E,A,B,I,h |
| C7 esters | A,B,a,b |
| C6 ketones | --3 |
| C7 ketones | --3 |
Statistical significance was tested using ANOVA followed by false discovery rate (FDR) correction (Curran-Everett, 2000), except for in the case of the C6 ketones, which were evaluated by t-tests followed by FDR correction.
Significantly different modules are listed in order of increasing P value.
No module was significantly different across the ketone series.
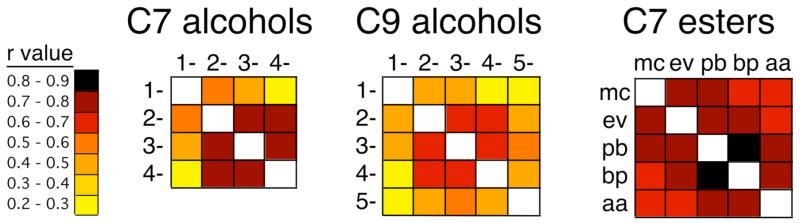
The large impact of hydroxyl group position was further evidenced by the relatively low correlation coefficients obtained when the average z-score data matrices were subjected to pair-wise Pearson correlations (Fig. 3). In this analysis, corresponding pairs of cells in the data matrices serve as the X-Y pairs, so that a higher correlation coefficient reflects a greater similarity in the entire glomerular pattern. The coefficients for the alcohol isomers fell to below 0.3 when the odorants differed by three or more carbons in hydroxyl group position (Fig. 3). Despite the differences, the overall patterns displayed a global chemotopic organization in that correlation coefficients were greatest for alcohols having the hydroxyl groups in the most similar positions (Fig. 3).
Fig. 3.
Patchworks of correlation coefficients reveal global chemotopy with respect to functional group position for esters and alcohols. Each patchwork represents Pearson’s correlation coefficients obtained by correlating every pair of averaged data matrices within an odorant series. Individual isomers of heptanol (C7 alcohols) and nonanol (C9 alcohols) are identified by their substitution position. Individual esters are indicated by abbreviations: mc, methyl caproate; ev, ethyl valerate; pb, propyl butyrate; bp, butyl propionate; aa, amyl acetate. Global chemotopy is evident from the higher correlation coefficients for odorants substituted at adjacent positions (i.e., along the diagonal of the patchwork).
Ester positional series
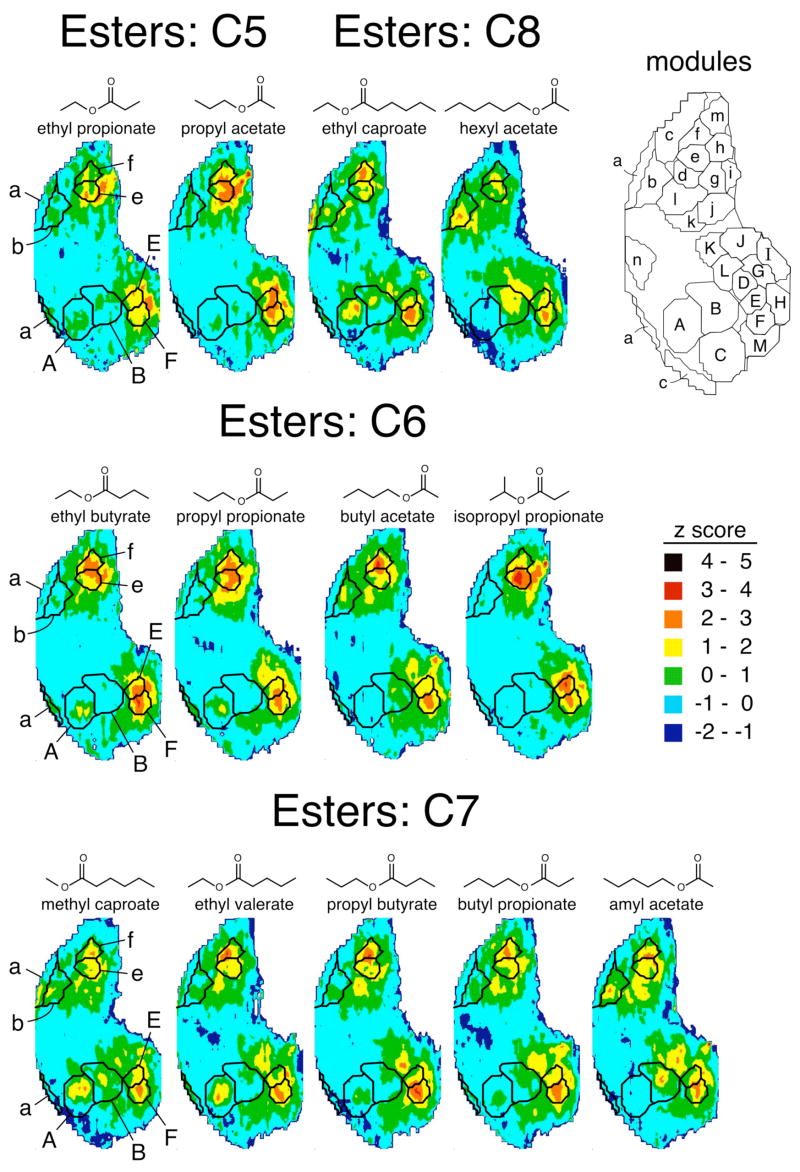
To investigate the effects of ester bond position on the spatial representations of aliphatic ester odorants, we studied four sets of isomers, including two odorants each of five and eight carbons, four odorants of six carbons, and five of seven carbons (Fig. 4). All four sets of isomers involved one ethyl ester and one acetate, thus allowing comparisons of odorants with the ester group on opposite sides of the molecules. The five-carbon ethyl ester and acetate appeared to evoke rather similar patterns, whereas the eight-carbon isomers appeared to evoke more different patterns (Fig. 4). To compare the ethyl acetate and ethyl ester patterns quantitatively, we performed cell-by-cell Pearson correlations, which showed that the patterns evoked by the isomers became more distinct with increasing carbon number (Table 3). Another way of viewing these results is that the patterns were more similar for odorants that had the ester bond in more similar locations. For the isomers possessing six or more carbons (and not for the five-carbon molecules), the most obvious difference in pattern between the ethyl esters and the acetates was that the ethyl esters stimulated glomeruli in modules “A”, whereas the acetates stimulated glomeruli within modules “b” and “B” (Fig. 4), a difference that we have reported previously (Johnson et al., 2004). In contrast, responses in modules “e/E” and “f/F” were relatively unaffected by the difference in ester bond position between ethyl esters and acetates (Fig. 4).
Fig. 4.
Contour charts illustrating patterns of glomerular layer uptake evoked by ester odorants. The charts are oriented as in Figure 1. The boundaries of modules containing foci of 2-DG uptake that are discussed in greater detail in the text are superimposed on the charts.
Table 3.
Pattern similarity between ethyl esters and acetates declines with increasing carbon number.
| Carbon number | Ethyl ester | Acetate | R value |
|---|---|---|---|
| 5 | Ethyl propionate | Propyl acetate | 0.78 |
| 6 | Ethyl butyrate | Butyl acetate | 0.79 |
| 7 | Ethyl valerate | Amyl acetate | 0.67 |
| 8 | Ethyl caproate | Hexyl acetate | 0.59 |
To investigate in more detail the changes in activity patterns associated with ester bond position, we used additional isomers of six- and seven-carbon esters (Fig. 4). Using multiple ANOVAs and false discovery rate correction on modular z score values, we found that there were significant differences across the odorant-evoked activity patterns in both the six-carbon and the seven-carbon ester series. Table 2 shows the modules that were determined to be significantly different in this analysis. In agreement with the impressions from the average charts (Fig. 4), modules “a/A” and “b/B” were among those showing significant differences in 2-DG uptake across the isomers.
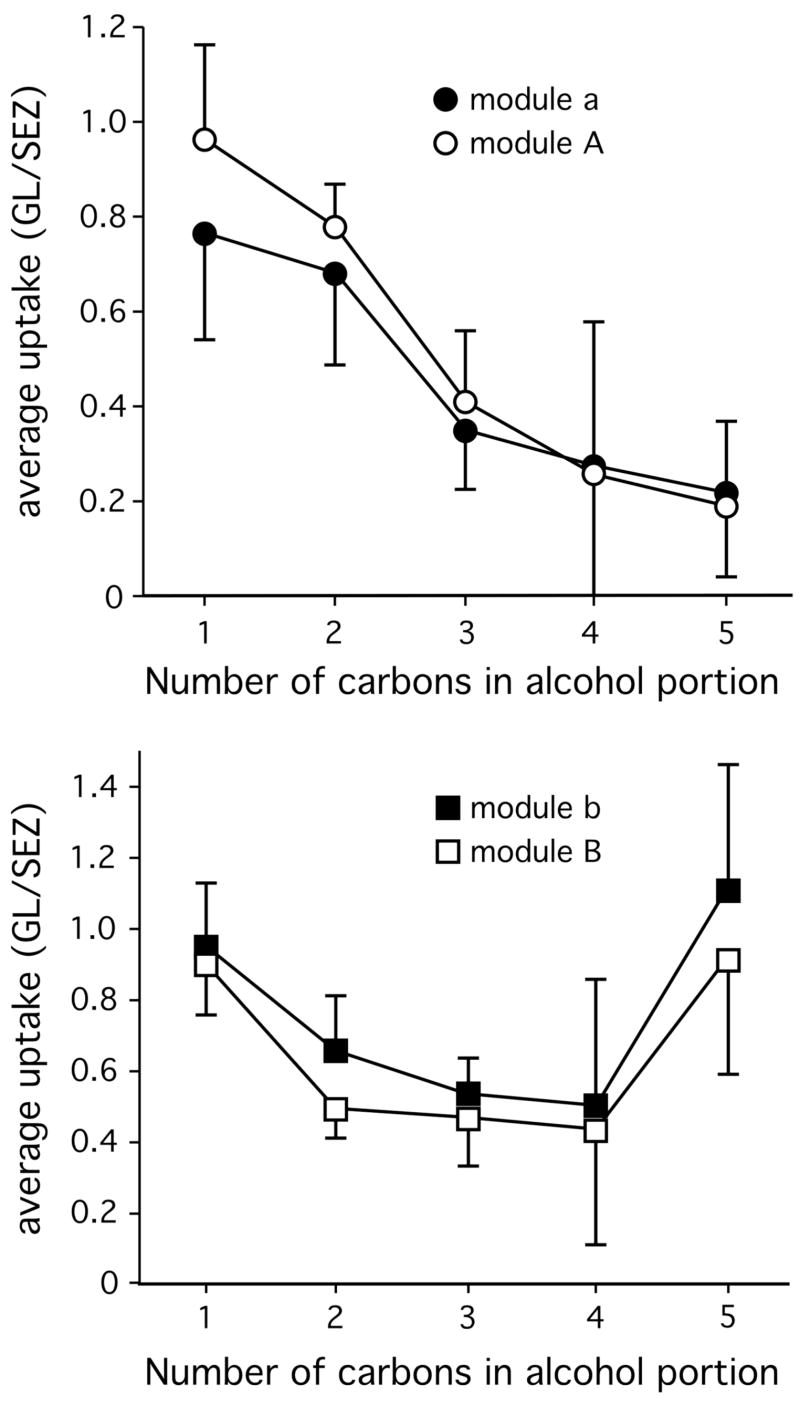
Differences in activity within modules “a/A” and “b/B” across the seven-carbon positional isomer series were explored further by averaging 2-DG uptake across the modules in individual rats using units of glomerular layer uptake/subependymal zone uptake. Standardizing uptake with respect to the subependymal zone (which contains immature neurons migrating into the structure) corrects for differences either in amount of injected radiolabel or in circulating glucose levels (Johnson et al., 2004). Unlike z scores, these units give an indication of changes in the amount of local activity independently of uptake occurring in other regions of the bulb. Activity in modules “a” and “A” decreased progressively as the ester bond was moved incrementally from its position in methyl caproate to its position in amyl acetate, that is, with increasing length of the alcohol portion of the ester molecule (Fig. 5). In contrast, the average uptake in modules “b” and “B” was high for methyl caproate, decreased with increasing length of the alcohol portion up to butyl propionate, and then increased again for amyl acetate (Fig. 5). Thus, glomeruli within modules “b” and “B” responded best when the ester bond was on one end or the other of the molecule, and least well when the bond was in the middle of the odorant molecule. It should be noted that, although similar areas of modules “b” and “B” were activated by methyl caproate and amyl acetate, it is possible that these responses involved different glomeruli.
Fig. 5.
Modules “a”/“A” and “b”/“B” display different effects of ester group position on amounts of modular 2-DG uptake. Shown here are responses to isomers of seven-carbon esters differing in their alcohol portion from one carbon (methyl caproate) to five carbons (amyl acetate). For each rat, uptake across modules was averaged in units of nCi/g wet weight of tissue and was divided by the average uptake measured in the same units in the subependymal zone of a restricted range of coronal sections from the same rat (Johnson et al., 1999). Error bars denote the standard error of the mean across the different animals. Modules “a” and “A” show a progressive decrease in 2-DG uptake as the alcohol portion lengthens (and the acid portion shortens), while modules “b” and “B” show a U-shaped curve indicating greater uptake when the ester bond is on either side of the molecule.
In addition to positional isomers of six-carbon esters, we also tested responses to the branched isomer, isopropyl propionate. Whereas stimulation of module “A” by ethyl butyrate and propyl propionate was clearly evident in the average z score charts (Fig. 4), isopropyl propionate did not appear to stimulate that module (Fig. 4). The difference in activation of modules “a” and “A” between isopropyl propionate and propyl propionate was even more remarkable when responses were analyzed in units of glomerular layer uptake/subependymal zone uptake, where the isopropyl ester was found to be entirely ineffective in stimulating the modules. For propyl propionate, the value for module “a” was 0.56 ± 0.30 (mean ± standard error) and the value for module “A” was 0.50 ± 0.28, whereas for isopropyl propionate the values were 0.00 ± 0.04 and 0.12 ± 0.12, respectively. Thus, both alkyl branching and length in the alcohol portion of an ester odorant appear to interfere with the activation of glomeruli in modules “a” and “A”. Isopropyl propionate stimulated 2-DG uptake in modules “e” and “E” more strongly than did the other six-carbon esters, which preferentially stimulated glomeruli in modules “f” and “F” (Fig. 4, Fig. 2). Thus, branching also appears to affect activity in glomeruli located in these modules.
Despite the differences in activation of glomeruli in modules “a/A” and “b/B” associated with differences in ester bond position, the stimulation of 2-DG uptake in more posterior and ventral modules by the different positional isomers of six- and seven-carbon esters was remarkably similar (Fig. 4, Fig. 2). These consistent posterior responses included modules “e/E” and “f/F”, which are commonly stimulated by ester odorants regardless of hydrocarbon structure (Johnson et al., 1998; 2002; 2004; Johnson and Leon, 2000a). Indeed, the overall patterns evoked by esters within the seven-carbon positional series were very similar, giving high Pearson correlation coefficients in analyses where pairs of average z score data matrices were correlated with one another on a cell-by-cell basis (Fig. 3). These correlation coefficients exceeded those found for the alcohol isomers (Fig. 3), and they also were higher than coefficients between ester odorants differing only slightly in carbon number (Johnson et al., 2004). Analyses of pair-wise correlation coefficients also showed that activity patterns were globally chemotopic with respect to ester bond position, so that esters with the most similar bond position gave the highest correlation coefficients (Fig. 3).
It should be noted that particular esters stimulated only portions of particular modules (e.g., methyl and ethyl esters stimulated only small foci of 2-DG uptake in modules “a”, “A”, “b”, and “B”). Particular alcohols also stimulated only portions of modules “b” and “B” (Fig. 4). Thus, modules do not respond as all-or-none functional units. Rather, they represent regions that contain glomeruli of related, but distinct specificities. Also, certain esters were characterized by average regions of 2-DG uptake that were not necessarily confined within the borders of previously defined modules. For example, propyl acetate, hexyl acetate, propyl propionate, and amyl acetate yielded areas of response in the lateral aspect of the bulb that were centered on the border between modules “e” and “f”. Although the precise location of average response foci depends on many variables, including slight variations in section plane and differences between individual animals, these data may indicate that modules “e” and “f” should be considered to be subdivisions of a larger module associated with ester odorants. Nevertheless, many of the ester odorants evoked uptake largely obeying the previously defined boundaries between modules “e” and “f” (e.g., butyl acetate activated a region corresponding fairly well to module “f”, whereas isopropyl propionate activated a region nearly corresponding to module “e”, as was mentioned above).
Ketone positional series
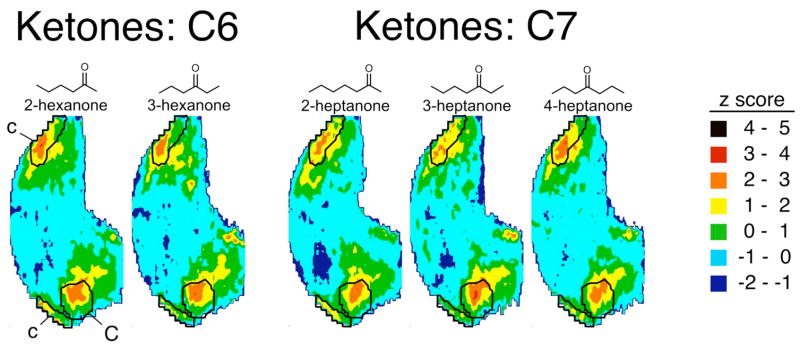
Functional group position had much less of an effect on activity patterns evoked by ketone odorants than it did for the esters of the same carbon number (Fig. 6). When subjected to a similar statistical analysis as was used for the alcohol and ester series, no module was found to be significantly different in 2-DG uptake in response to isomers of hexanone or heptanone (Table 2). When subjected to pair-wise correlations analysis (Table 4), the correlation coefficients were among the highest in our database, which at the time of writing includes coefficients from 233 distinct odorants. The high values of the correlation coefficients reflect a similar stimulation of glomeruli within various modules distributed across the glomerular layer (Fig. 2).
Fig. 6.
Contour charts illustrating patterns of glomerular layer uptake evoked by ketone odorants. The charts are oriented as in Figures 1 and 4. The boundaries of modules “c” and “C”, which encompass areas of 2-DG uptake previously associated with ketones, are superimposed on the charts.
Table 4.
Positional isomers of ketones evoke very similar patterns.
| Carbon number | Odorant 1 | Odorant 2 | R value |
|---|---|---|---|
| 6 | 2-hexanone | 3-hexanone | 0.87 |
| 7 | 2-heptanone | 3-heptanone | 0.86 |
| 7 | 3-heptanone | 4-heptanone | 0.89 |
| 7 | 2-heptanone | 4-heptanone | 0.89 |
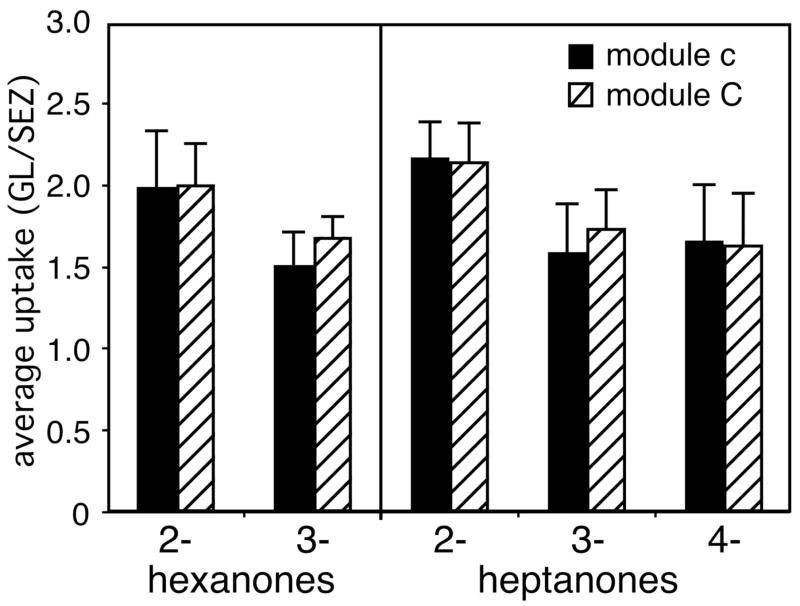
The most activity evoked by the ketone isomers occurred in paired regions located dorsally in the bulb, corresponding quite well to areas we had designated previously as modules “c” and “C” (Fig. 6). We previously reported the association between activity in these modules and the presence of the ketone functional group (Johnson and Leon, 2000a; Johnson et al., 2002; 2004). To determine if the position of the ketone group affected the amount of 2-DG uptake in these modules independently of the overall pattern of activity, we calculated uptake in units of glomerular layer uptake/subependymal zone uptake. The isomers of hexanone and heptanone also did not differ in their stimulation of 2-DG uptake in modules “c” or “C” when uptake was calculated in this way (Fig. 7). Additional analyses of the location of uptake within modules “c” and “C” by way of centroid determinations (Johnson et al., 1998; 1999; 2000b; 2004) also failed to show any difference with ketone position (data not shown).
Fig. 7.
Positional isomers of ketones do not differ in the amount of 2-DG uptake evoked in modules “c” and “C”. Uptake of 2-DG was averaged over modules as described for Figure 5. Error bars denote the standard error of the mean across the different animals.
DISCUSSION
Substitution position can be a molecular feature important to odorant receptors
For ester and alcohol odorants, we found that glomeruli in spatially distinct modules are activated by isomers that have their functional groups in different positions. Thus, substitution position can be represented as a distinct molecular feature that activates distinct sets of odorant receptors projecting to different areas in the bulb. This coding strategy resembles that used to represent distinct functional groups (Johnson and Leon, 2000a; Johnson et al., 2002), enantiomers (Linster et al., 2001), and large differences in hydrocarbon structure (Johnson et al., 1999; 2004; Johnson and Leon, 2000b), and it is distinct from the intra-modular progressions in the location of activity that characterize representations of small differences in molecular length (Johnson et al., 1999; 2004; Rubin and Katz, 1999; Uchida et al., 2000; Johnson and Leon, 2000b).
For ketone odorants, we did not find differences in activity patterns for positional isomers. Given the close relationship that appears to exist between 2-DG uptake patterns and odor perception (Linster et al., 2001; Cleland et al., 2002), the absence of significant differences suggests that rats would not spontaneously distinguish between these ketone isomers.
The magnitude of the effect of functional group position depended greatly on the nature of the functional group. For alcohols, different positions of the hydroxyl group were associated with activity patterns that were nearly non-overlapping. In contrast, differences in ester bond position evoked smaller differences in activity patterns involving glomeruli within individual modules, and differences in carbonyl position in ketones were without detectable effect on activity patterns. These differences indicate that coding strategies used for one set of molecules might not generalize to a different set of chemicals. This variation in coding strategy reinforces the continued need for further studies on the relationship between odorant chemistry and bulbar activity patterns.. Despite the differences in patterns of activity evoked by alcohol isomers, quantitative analysis revealed a global chemotopic organization, where the overall pattern was more similar for the isomers having the most similar substitution positions, a relationship that was also seen for esters in the present study. Similar quantitative similarities were noted for isomeric primary and secondary alcohols during calcium imaging studies of the honeybee antennal lobe (Sachse et al., 1999).
Alcohol odorant ligands are predicted to engage in strong hydrogen bonding as both donors and acceptors, whereas esters and ketones are hydrogen bond acceptors but not donors. The capacity to engage in more hydrogen bonding with odorant receptors may explain why hydroxyl group position had a large effect on alcohol-evoked activity patterns, especially if the receptors detect both the hydroxyl group and the surrounding hydrocarbon structure. The effects of hydroxyl position also could involve electronic properties of the alcohol odorant. Primary alcohols such as 1-heptanol and 1-nonanol should have lower hydroxyl pKa values (i.e., primary alcohols should be more acidic) than the other, secondary alcohols.
Specificities of glomerular modules
The present results, which show differences in the activation of glomeruli in modules “a/A”, “b/B”, and “c/C” for odorants differing in functional group and functional group position, provide further insight into the specificities of glomeruli within these modules. Because the location of individual glomeruli varies across different animals (Strotmann et al., 2000; Schaefer et al., 2001) and because the 2-DG technique does not allow measurements of responses to multiple odorants in the same animal, our data do not allow an analysis of the specificity of individual glomeruli or individual odorant receptors. However, an analysis of the specificity of small regions of the glomerular layer, such as those we have delimited as glomerular modules, is useful in characterizing the rules governing which glomeruli are clustered in the bulb, as these clusters are likely to form local lateral inhibitory circuits participating in odor processing (Yokoi et al., 1995). Although the 2-DG method is capable of detecting the activation of only one or a very few glomeruli (Johnson et al., 1998; 1999), individual straight-chained aliphatic odorants often evoke uptake in areas involving many contiguous glomeruli, which suggests that these glomeruli probably have very similar specificity in order to be activated by the same odorant. Higher concentrations of an odorant tend to activate more glomeruli above any given threshold within these clusters, which also is consistent with the neighboring glomeruli exhibiting similar specificities (Stewart et al., 1979; Guthrie and Gall, 1995; Rubin and Katz, 1999; Johnson and Leon, 2000a). Indeed, within a homologous series of odorants differing either incrementally in carbon number or slightly in molecular length, the most similar molecules appear to stimulate overlapping, but slightly displaced sets of glomeruli within glomerular modules (Johnson et al., 1998; 1999; 2004; Johnson and Leon, 2000b). Similar conclusions regarding the clustering of glomeruli of related specificities have emerged from the very few optical imaging studies that have involved a sufficiently large number of related odorants to truly characterize the specificity of individual glomeruli that are part of the peak response to the odorants (Uchida et al., 2000; Takahashi et al., 2004). Such optical imaging studies also have shown that nearby glomeruli within a response domain can respond with distinct specificity to ketone and ester odorants differing in functional group position (Uchida et al., 2000).
Glomeruli in paired modules “a” and “A” were shown in previous studies to be activated by low concentrations of acid odorants having from one to eight carbons in a great variety of hydrocarbon structures (Johnson et al., 1999; 2002; Johnson and Leon, 2000a;b). A variety of aliphatic methyl and ethyl esters also activate glomeruli within these modules (Johnson et al., 1998; 2002; 2004; Johnson and Leon, 2000a). Although exposures involving aliphatic aldehydes also result in activity in glomeruli in this part of the bulb (Rubin and Katz, 1999; Johnson and Leon, 2000a; Uchida et al., 2000; Wachowiak and Cohen, 2001; Meister and Bonhoeffer, 2001; Johnson et al., 2004), these responses probably are caused by acid contaminants produced by oxidation of the aldehyde material (Johnson et al., 2004).
Our present results confirm that 2-DG uptake in modules “a” and “A” is evoked by methyl and ethyl esters, and they further indicate that the amount of stimulation decreases with increasing length of the alcohol portion of the esters from one to five carbons (methyl caproate to amyl acetate). Additionally, propyl propionate stimulated glomeruli in modules “a” and “A”, while isopropyl propionate did not. From these data, it appears that recognition of the carboxylate feature by receptors associated with modules “a” and “A” may be disrupted by steric hindrance from bulky hydrocarbon structures in the alcohol portion of the molecule.
In earlier papers, we reported activation of glomeruli in modules “a” and “A” by certain small primary alcohols such as 1-propanol and 1-pentanol (Johnson and Leon, 2000a; Johnson et al., 2002). In the current study, we saw no evidence that any heptanol or nonanol isomer stimulated 2-DG uptake in modules “a” or “A”, which is consistent with our recent results showing no activation of glomeruli in these modules by homologous series of primary or secondary alcohols (Johnson et al., 2004). Therefore, it appears that most alcohols are not important stimuli for glomeruli located in these modules.
Glomeruli within paired modules “b” and “B” were shown in previous studies to respond to certain alcohols and aldehydes (Johnson and Leon, 2000a; Johnson et al., 2002, 2004), as well as to isoamyl acetate, isoamyl butyrate, and other large acetates and ethyl esters (Johnson et al., 1998, 2004). In the present study, we found that glomeruli within modules “b” and “B” were stimulated well by seven-carbon esters that had the ester bond on one side or the other of the molecule (i.e., by methyl caproate and amyl acetate), but not as well by isomers that had the ester group in the middle of the molecule. Similar results were obtained for alcohols, where modules “b” and “B” comprised a large part of the response to 1-heptanol and 1-nonanol, but decreased in relative importance when the hydroxyl group was present nearer the middle of the molecules. Therefore, the receptors associated with the areas of the bulb involving modules “b” and “B” may require appreciable polarity in their ligands. One possibility is that these odorant receptors need to bind a hydrophobic stretch of the molecule as well as a molecular feature that can accept a hydrogen bond, and perhaps the placement of the alcohol or ester group in the center of the molecules disrupts the interaction of the receptors with one or the other of these two molecular features.
Uptake of 2-DG in paired modules “c” and “C” was shown in previous studies to be stimulated by high concentrations of small aliphatic ketones as well as by certain cyclic and aromatic ketones, although larger aliphatic ketones and lower concentrations of small aliphatic ketones were ineffective (Johnson and Leon, 2000a; Johnson et al., 2002, 2004). Unlike paired modules “a/A’ and “b/B”, responses within modules “c” and “C” did not show local chemotopic progressions with respect to carbon chain length (Johnson et al., 2004). In the present study, we found that glomeruli in modules “c” and “C” were stimulated by six- and seven-carbon aliphatic ketones independently of carbonyl position. The locations of the responses within the modules also were unaffected by the position of the ketone group. The absence of local chemotopic organization within modules suggests that modules “c” and “C” may be organized differently than other modules (Johnson et al., 2004).
In addition, results of the present study showed that 2- and 3-heptanol, and especially 5-nonanol, stimulated 2-DG uptake in modules “c” and “C”. It is becoming increasingly evident that modules “c” and “C” are unusual not only in their apparent lack of chemotopic organization, but also in their being specific for multiple, but by no means all types of odorant ligand, a topic that we will discuss in greater detail in a subsequent report (Johnson, Farahbod, and Leon, manuscript submitted).
The 2-DG technique
The 2-DG technique has been shown to reveal reliable, distinct patterns of bulbar activity in response to different odorants (Jourdan et al., 1980; Royet et al., 1987; Sharp et al., 1975; 1977; Stewart et al., 1979). The technique has some clear advantages over other techniques and a few disadvantages that would be directly relevant to addressing most issues in olfactory coding. First, the technique allows a quantitative description of activity patterns without the anesthesia that has been suggested to obscure normal responses to odorant stimuli (Motokizawa, 1996), and with the normal respiration patterns that accompany odorant coding in the real world.
The second advantage of the 2-DG technique is its ability to describe the entire pattern of the olfactory response, which we believe is critical to gaining an understanding of the olfactory code. A third aspect of the 2-DG technique is that it is highly replicable because the data are obtained with respect to radioactive standards, which allow quantitative comparisons among any set of brains processed in the same way. At the same time, odorant-evoked 2-DG responses in rats match the odorant-evoked responses seen with Fos, Arc, and Egr1 immunohistochemistry (Guthrie et al. 2000; Sallaz and Jourdan, 1993; Johnson et al., 1995), as well as fos and jun in situ hybridization (Guthrie et al., 1993; Guthrie and Gall, 1995; Schaefer et al., 2001). Moreover, the modular 2-DG response profiles that we have described have been replicated both with optical imaging of intrinsic signals on the lateral aspect of the bulb (Uchida et al., 2000; Takahashi et al., 2004) and with unit recordings of mitral and tufted cells (Nagayama et al., 2004). Therefore, a fourth characteristic of the 2-DG technique is that it has been carefully validated against other techniques, as Bozza et al. (2004) and Wachowiak and Cohen (2003) also have done with their imaging techniques.
A fifth feature of the 2-DG technique is that it has real single-glomerular resolution (Johnson et al., 1998) in that its ability to resolve individual glomeruli does not rely on the selection of a response threshold to gain apparent glomerular resolution.
The peak glomerular responses that we observe can accurately predict the odorant stimulus, knowledge of the odorant stimulus can accurately predict the peak glomerular response, and peak glomerular responses can accurately predict odor perceptions (Leon and Johnson, 2003). This technique also can be used to show glomerular changes following learning or sensory deprivation (Coopersmith and Leon, 1984; Guthrie et al., 1990; Sullivan et al., 1990; Johnson et al., 1996). Therefore, a sixth aspect of the 2-DG technique is that it has predictive value in understanding the olfactory code.
A potential limitation of the 2-DG method is that it takes minutes to be sure that all of the injected 2-DG is completely metabolized to avoid measuring any radioactive label associated with un-metabolized 2-DG (Sokoloff, 1981). Un-metabolized 2-DG would not, of course, reflect neural activity, but most 2-DG is actually metabolized rapidly (Sokoloff, 1981). In addition, rats exposed to an odorant for only seconds within the uptake period have a similar 2-DG uptake pattern as those exposed to the odorant for the entire uptake period (Slotnick et al., 1989). Because of delays inherent in all of the responses that require repeated averaging in an individual, few of the techniques used to date have the temporal resolution required for real-time imaging to follow rapid processing that occurs in this system (Spors and Grinvald, 2002; Uchida and Mainen, 2003). It is also the case that the 2-DG technique does not rely on multiple responses in the same animal, and a seventh advantage of the technique is that it avoids interactions among odorants that may arise by repeated stimulation with various odorants.
We have added to the value of the 2-DG method by developing a technique that allows us to map the entire glomerular layer into a standardized data matrix that accurately conforms to the parameters of the lamina. This technique allows us to average the response of groups of rats and perform standard statistics across such groups to compare their evoked responses throughout the bulb. In most cases all details of the patterns are clearly apparent in each bulb of each animal (Johnson and Leon, 1996), which probably explains why we obtain statistically significant results using only 4 or 5 animals in a group. The use of standard statistical methods to analyze the imaged data therefore is the eighth aspect of the 2-DG technique. Reports of imaged data typically do not adequately reflect the reliability of the response across individuals, while our technique is able to show average group responses, along with statistical analyses of those responses. In addition, our technique allows a standardized presentation format such that the glomerular responses to different odorants can be visualized easily and compared across a database of a large number of odorants. It also is not clear from other reports where on the dorsal aspect of the bulb they are recording and whether that area is similar across animals, across studies and across laboratories. We recognize that only techniques that allow repeated recordings in response to different odorants from individual glomeruli can address the questions of response specificity of individual glomeruli, but we suggest that such analyses will only be of value if the recordings are taken from the peak response glomeruli that are actually involved in odor coding, rather than glomeruli activated as part of a background response.
Conclusions
Mapping responses across the entire glomerular layer of rats exposed to odorants differing systematically in chemistry continues to reveal correspondingly systematic changes in spatial activity patterns. These changes in bulbar activity patterns help to reveal the odorant specificity of more elemental components of the olfactory system. In the present study, we have found that the recognition of an odorant by the olfactory system involves an interaction between the nature the odorant’s functional group and its position in the molecule. Future efforts involving differences in other aspects of odorant chemistry, when integrated into and analyzed in the context of our growing archive of spatial activity patterns (http://leonlab.bio.uci.edu) should enhance this understanding even further.
Acknowledgments
We thank Jennifer Kwok, Paige Pancoast, Zhe Xu, Edna E. Hingco, Linh Hoang, and Joanne S. Yihan for technical assistance with exposures, sectioning, mapping, and data analysis.
Supported by United States Public Health Service grant DC03545
LITERATURE CITED
- Araneda RC, Peterlin Z, Zhang X, Chesler A, Firestein S. A pharmacological profile of the aldehyde receptor repertoire in rat olfactory epithelium. J Physiol. 2004;555:743–756. doi: 10.1113/jphysiol.2003.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Coopersmith RM, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Reg Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Functional mapping of odor-activated neurons in the olfactory bulb. Chem Senses. 1995;20:271–282. doi: 10.1093/chemse/20.2.271. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Wilson DA, Leon M. Early unilateral deprivation modifies olfactory bulb function. J Neurosci. 1990;10:3402–3412. doi: 10.1523/JNEUROSCI.10-10-03402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993;90:3329–3233. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie K, Rayhanabad J, Kuhl D, Gall C. Odors regulate Arc expression in neuronal ensembles engaged in odor processing. Neuroreport. 2000;11:1809–1813. doi: 10.1097/00001756-200006260-00003. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Spatial distribution of [14C]2-deoxyglucose uptake in the glomerular layer of the rat olfactory bulb following early odor preference learning. J Comp Neurol. 1996;376:557–566. doi: 10.1002/(SICI)1096-9861(19961223)376:4<557::AID-CNE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H-C, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. 2004 doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980;188:139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco E, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmonson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Motokizawa F. Odor representation and discrimination in mitral/tufted cells of the rat olfactory bulb. Exp Brain Res. 1996;12:24–34. doi: 10.1007/BF00227174. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Royet JP, Sicard G, Souchier C, Jourdan F. Specificity of spatial patterns of glomerular activation in the mouse olfactory bulb: Computer-assisted image analysis of 2-deoxyglucose autoradiograms. Brain Res. 1987;417:1–11. doi: 10.1016/0006-8993(87)90173-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci. 1999;11:3970–3982. doi: 10.1046/j.1460-9568.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Sallaz M, Jourdan F. C-fos expression and 2-deoxyglucose uptake in the olfactory bulb of odour-stimulated awake rats. NeuroReport. 1993;4:55–58. doi: 10.1097/00001756-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001;436:351–362. [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 1977;40:800–813. doi: 10.1152/jn.1977.40.4.800. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Panhuber H, Bell GA, Laing DG. Odor-induced metabolic activity in the olfactory bulb of rats trained to detect propionic acid vapor. Brain Res. 1989;500:161–168. doi: 10.1016/0006-8993(89)90310-7. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1:7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Stewart WB, Kauer JS, Shepherd GM. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979;185:715–734. doi: 10.1002/cne.901850407. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–6938. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004 doi: 10.1152/jn.00236.2004. in press. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nuñez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J Neurophysiol. 2003;89:1623–1639. doi: 10.1152/jn.00747.2002. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]