Abstract
Intrathalamic oscillations related to sleep and epilepsy depend on interactions between synaptic mechanisms and intrinsic membrane excitability. One intrinsic conductance implicated in the genesis of thalamic oscillations is the H current – a cationic current activated by membrane hyperpolarization. Activation of H current promotes rebound excitation of thalamic relay neurons and can thus enhance recurrent network activity.
We examined the effects of H current modulation on bicuculline-enhanced network oscillations (2-4 Hz) in rat thalamic slices. The adrenergic agonist norepinephrine, a known regulator of H current, caused an alteration of the internal structure of the oscillations – they were enhanced and accelerated as the interval between bursts was shortened. The acceleration was blocked by the β-adrenergic antagonist propranolol. The β agonist isoproterenol mimicked the effect of norepinephrine on oscillation frequency and truncated the responses suggesting that a β-adrenergic upregulation of H current modifies the internal structure (frequency) of thalamic oscillations. Consistent with this, we found that H channel blockade by Cs+ or ZD7288 could decelerate the oscillations and produce more robust (longer lasting) responses. High concentrations of either Cs+ or ZD7288 blocked the oscillations.
These results indicate that a critical amount of H current is necessary for optimal intrathalamic oscillations in the delta frequency range. Up- or downregulation of H current can not only alter the oscillation frequency but also retard or promote the development of thalamic synchronous oscillations. This conclusion has important implications regarding the development of epilepsy in thalamocortical circuits.
Keywords: H current, ZD7288, adrenergic, pacemaking
The thalamus is a well-established neuronal oscillator (Steriade and Deschênes, 1984;Steriade and Llinás, 1988). The results of in vivo and in vitro experiments the last 20 years or so have established the important network and intrinsic cellular excitability components that drive thalamic oscillations. Such oscillatory network responses are important in sleep spindle generation and are also implicated in the development of abnormal hypersynchronous discharges related to some (Williams, 1953;Gloor et al., 1990), but not all (Marcus and Watson, 1966;Steriade and Contreras, 1998) forms of absence epilepsy.
Intrathalamic oscillations depend on reciprocal connections between inhibitory neurons of the thalamic reticular nucleus (nRt) and excitatory thalamocortical relay neurons (McCormick and Bal, 1997). Several studies have used rodent in vitro slice preparations of the somatosensory thalamic region (ventrobasal complex, VB) to study thalamic network activity because of the high degree of circuit connectivity that can be retained within a horizontal thalamic (Huguenard and Prince, 1994a;Huguenard and Prince, 1994b;Ulrich and Huguenard, 1995;Huntsman et al., 1999) or thalamocortical (Warren and Jones, 1994) slice. The ferret dorsal lateral geniculate slice has also been used extensively to study spindle-like and epileptiform thalamic oscillations and the mechanisms responsible for such activities (von Krosigk et al., 1993;Bal et al., 1995a;Bal et al., 1995b). Together these studies have shown that thalamic relay neurons receive powerful inhibitory postsynaptic potentials (IPSPs) that result from activity in cells of nRt (or the analogous perigeniculate nucleus). A characteristic feature of relay neurons is a high level of α1G calcium channel gene expression (Talley et al., 1999) which leads to prominent T-type calcium currents (Coulter et al., 1989) and robust rebound burst responses. Thus relay neurons can respond to IPSPs with a somewhat paradoxical rebound excitation. Through reciprocal connectivity, the rebound bursts in relay cells can then lead to re-excitation of nRt cells and ultimately to a self-propagating recurrent network oscillation.
An interesting property of the isolated thalamic network is that the internal structure of the oscillations (i.e. the frequency of repetitive burst discharges) is largely determined by the duration of the IPSP that occurs in relay neurons as a result of nRt cell activity(Huguenard and Prince, 1994b;Bal et al., 1995a;Bal et al., 1995b). Specifically, most of the interval between bursts during a in vitro spindle sequence is occupied by the duration of an IPSP (Bal et al., 1995a). Further, alteration of the nRt cell activity, which is produced by the GABAA antagonist bicuculline methiodide, results in a dramatic prolongation of the IPSPs in relay neurons and a concurrent slowing of the network activity (von Krosigk et al., 1993;Huguenard and Prince, 1994b;Bal et al., 1995a). Thus the properties of a specific synapse are a central in determining the network response.
In addition to the properties of synapses within the network, the intrinsic excitability of thalamic neurons also strongly contributes to recurrent activities, with both T-type calcium channels (discussed above) and H channels (Huguenard and McCormick, 1992;McCormick and Huguenard, 1992) playing key roles. The so called H current (IH) is a hyperpolarization-activated cationic current that promotes intrinsic rhythmicity in relay neurons (Pape, 1996). Four genes for IH have been identified and one, HCN4, is highly expressed in thalamic relay neurons (Santoro et al., 2000). The properties of this ionic channel are such that during membrane hyperpolarizations (such as those occuring during IPSPs) H channels are opened and this leads to subsequent depolarization of the membrane potential. Activation of IH can thus promotes rebound bursting. Interestingly, it has been shown both experimentally and theoretically that the interaction between IH and the T-type calcium channels in relay cells promotes a self-sustained intrinsic conductance driven oscillation in isolated thalamic cortical relay neurons (McCormick and Pape, 1990b;Soltesz et al., 1991;Huguenard and McCormick, 1992;McCormick and Huguenard, 1992).
Up-regulation of IH during spindle sequences has been proposed to be an important in limiting spindle duration (Bal and McCormick, 1996;Lüthi et al., 1998). However, we found in our theoretical studies of hypersynchronous thalamic activity (Sohal et al., 1998) that IH was important in promoting recurrent network activity and further that up-regulation is unlikely to completely explain the termination of this form of activity. Experimentally we observe a progressive slowing of network responses over their time course – an effect opposite to that expected if IH were up-regulated.
In the current report we examined the effects of several modulators of IH on bicuculline-enhanced in vitro oscillatory activity in rat thalamic slices in an attempt to further examine the role of IH in modulating the slow in vitro synchronous activity (von Krosigk et al., 1993;Huguenard and Prince, 1994b;Huntsman et al., 1999) that resembles the repetitive bursting observed during absence seizures in vivo (Inoue et al., 1990;Slaght et al., 2000). We find that up-regulation of IH can alter the structure of the oscillation by producing a shortening of the period, i.e., an increase in frequency. This can be associated with an overall depression of activity so that the network is less able to sustain a prolonged oscillatory pattern. By contrast, down-regulation of IH by either Cs+ or the bradycardic agent ZD7288 has the opposite effect. Modest reductions in IH lead to a slowing of the oscillation and a concurrent enhancement of the overall network response.
Thus in addition to the previously-described role of IPSP duration in regulating the frequency of network oscillatory (Bal et al., 1995a), these data show that IH has a strong modulatory role in affecting both the frequency and robustness of the network response. Under our recording conditions the relative amount of IH available for activation in relay neurons is in a range such it is susceptible to both and up- and down-regulation. The resting level of IH appears to be suboptimal for the production of the most robust hypersynchronous thalamic oscillations suggesting that endogenous down-regulation of IH would promote the development of synchronous discharge in thalamus and potentially contribute to epileptogenesis.
Methods
Preparation of thalamic slices
Sprague-Dawley rats of both sexes aged P12-P20 were used. Anesthetized animals were decapitated and brains were blocked, removed, and placed in ice-cold, oxygenated (95% O2-5% CO2) sucrose slicing solution to cool for 2 minutes. The sucrose slicing solution consisted of (in mM): 11 glucose, 234 sucrose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2. Horizontal slices of 400 μm thickness were cut with a vibratome (TPI, St. Louis, MO), hemi-sected, and submerged in pre-heated (33°C), oxygenated physiological saline (in mM): 26 NaHCO3, 2.5 KCl, 10 glucose, 126 NaCl, 1.25 NaH2PO4, 0.63 MgSO4, and 2 CaCl2 for 1 hour. The heat was then turned off and slices were allowed to cool to room temperature.
Electrophysiology
Slices were maintained in an in vitro recording chamber with a continuous perfusion (2 ml/min) of physiological saline at 33°C. Multi-unit extracelluar recordings (Ulrich and Huguenard, 1995) were obtained from thalamic relay neurons in the ventrobasal complex (VB) and from GABAergic neurons of the nucleus reticularis thalami (nRt) via sharpened tungsten electrodes (0.1 MΩ resistance). Recordings were filtered between 100 and 3000 Hz and amplified by 100,000x. Intrathalamic network oscillations driven by synaptic interactions between nRt and VB were evoked by stimulation at the internal capsule (IC). Bicuculline methiodide (1 μM) was used in the saline to enhance the oscillations (von Krosigk et al., 1993;Huguenard and Prince, 1994b;Ulrich and Huguenard, 1995). Various compounds were perfused over the slice by bath application while recording. Norepinephrine, propranolol, S(+)-isoproterenol, ZD7288 [4-(N-ethyl-N-phenylamino)-1, 2-dimethyl-6-(methylamino)pyrimidinium chloride], and Cs+ were tested.
Data Analysis
Recorded extracellular responses were collected with Axotape v.2 (Axon Instruments, Foster City, CA), and action potentials were detected by a software Schmidt trigger algorithm and quantified with a rate-meter output via customized software Metatape v.14, UnitsWin, and Ratemeter (J.R. Huguenard). Rate-meters reflect the activity of multiple units recorded from single electrodes. Contour plots were generated with and Origin 5 (Microcal Software, Inc., Northhampton, MA). Spike output, as measured with the software ratemeter, was analyzed with autocorrelation software, and autocorrelograms were least-squares fitted with a modified Gabor function that provided information regarding the frequency, power and duration of oscillatory and non-oscillatory activity (Ulrich and Huguenard, 1995). The duration of the oscillatory response was best quantified by determining the time constant that characterized the decay of the damped oscillation (oscillation decay τ) in the autocorrelogram.
The rate-meter output produces a post stimulus histogram of unit activity. In this report the rate meters are shown as contour plots (e.g., Figure 2) where the rate-meter from each individual sweep is plotted vertically with time zero at the bottom of the sweep and time within a single sweep progressing upward. These individual rate meters are then plotted adjacent to each other, one for each stimulus, and viewed from above, in the form of a contour plot. Periods of intense spike activity, i.e. high rate-meter output, are associated with black levels and lower levels with progressively lighter shades of gray and then white, which indicates no activity (Jacobsen et al., 2001). Figures 2 and 3 also show examples of multi-unit extracellular recording from VB neurons displayed vertically in order to show the relationship between multi-unit activity and the derived rate-meter contour plot.
Figure 2.
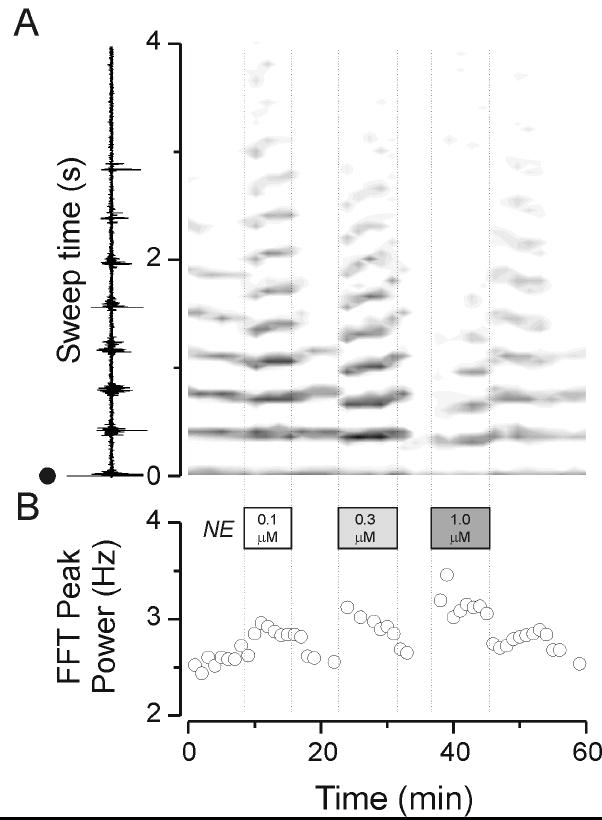
Summary data depicted derived results from the experiment in Figure 1. A. A contour plot of rate-meter output depicting spike activity in the VB nucleus. An individual trace is depicted in the margin of panel A which shows that activity evoked in VB with a shock at time 0 (black dot) and then time during the evoked oscillation (sweep time) progressing towards the top. The gray scale graph on to the right depicts spike activity with denser activities being represented by darker grays and white corresponding to an absence of activity. Note that in control (the portion to the left side of the plot, to the left of the first vertical dashed line) there was repetitive activity that was reliably produced with each stimulus for the first eight minutes or so – six or seven cycles of activity at about 2.6 Hz. Addition of 0.1 μM norepinephrine (NE, indicated by the first vertically dashed line) increased the frequency and duration of network activity in reversible manner. Similarly, 0.3 μM norepinephrine produced an increase in activity that was not as long-lasting. Also note there was a sudden increase in frequency of activity as judged by the gray bars of activity which are bunched closer together. 1 μM norepinephrine largely suppressed activity but still produced a reversible increase in frequency. In the lower panel are shown results of FFT analysis where the progressive increase in frequency with 0.1, 0.3 and 1 μM norepinephrine can be seen.
Figure 3.
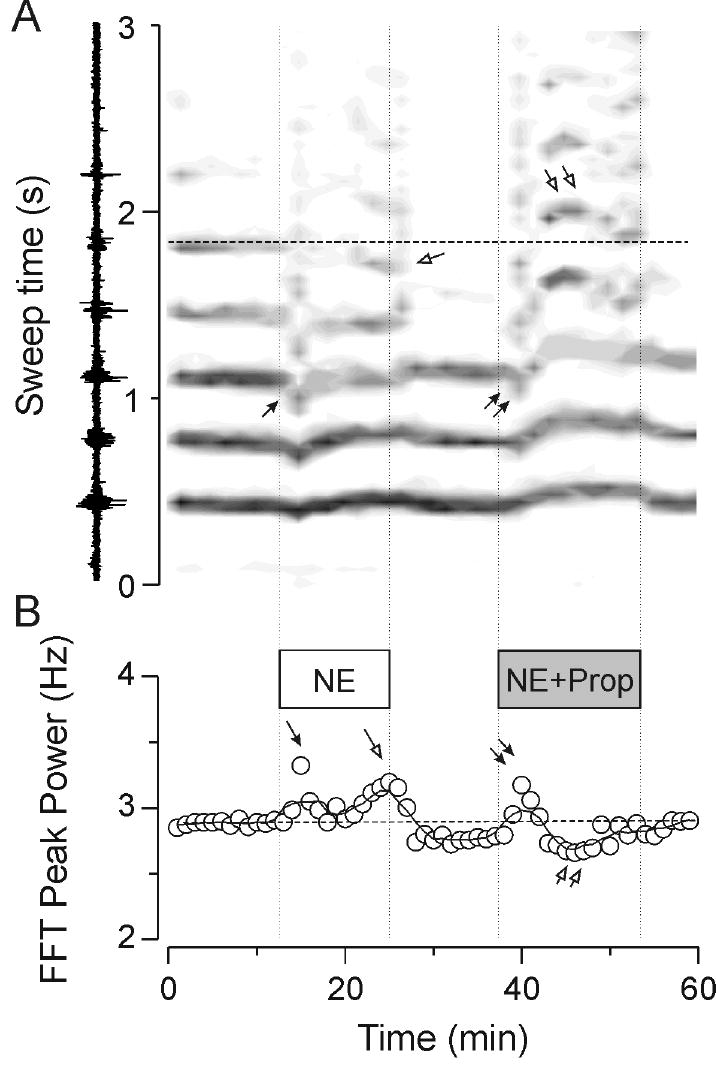
Effects of norepinephrine and norepinephrine plus propranolol on thalamic oscillatory activities. Multi-unit recordings from the VB nucleus. Norepinephrine (NE) at 0.3 μM produced a transient increase in frequency as seen with the downward shift in the gray bars in panel A (single filled arrowhead). With continued application there was also a late frequency increase as judged by the grouping together of later (> 2 sec from stimulus onset) clusters of activity. Note that the fifth cycle of activity (horizontal dashed line in A) occurred at earlier times (single open arrowhead). When norepinephrine was co-applied with 1 μM propranolol (Prop) a similar transient increase in frequency (double filled arrowheads) occurred, but this was subsequently replaced by a later slowing of the response. Note that the fifth cycle of activity (double open arrowheads vs the dashed horizontal line in A) was reversibly delayed. These effects on rate meter output are quantified in the FFT peak power plot in panel B where it can be seen that norepinephrine itself caused early (single filled arrowhead) and late (single open arrowhead) increases in frequency whereas when co-applied with propranolol there is only an early increase in frequency (double filled arrowheads) followed by a marked late slowing activity (double open arrow head).
To account for gradual rundown of network responses, drug effects were quantified as the ratio of the response in the presence of the drug to the mean of the responses in control and wash-out conditions. Because the effects of ZD7288 were irreversible, its effects were quantified by comparison to control only.
Results
Thalamic network activity were obtained in 87 slices. Simultaneous recordings were made in VB and nRt after stimulation of the internal capsule with a bipolar tungsten electrode. Stimuli typically consisted of 40 μs shocks of 40 V intensity. Responses were evoked every 30 seconds and were had a duration 2-8 seconds. Control responses typically consisted of 1-5 repetitive rebound bursts at a frequency of 2-4 Hz (Huguenard and Prince, 1994b;Ulrich and Huguenard, 1995) that could observed in both nRt and VB electrodes (e.g. trace A1 and B1, simultaneously recorded in the two nuclei). These oscillations depend on glutamatergic excitation of nRt cells and GABAB-dependent inhibition of relay neurons (Huguenard and Prince, 1994b;Jacobsen et al., 2001). In control conditions, recordings were typically stable over the course of more than an hour.
Bath application of norepinephrine produced a concentration-dependent increase in neuronal activity. Low concentrations (0.1 μM) produced an enhancement and prolongation of activity (e.g. Figure 1B2) and a slight increase in the internal frequency of the oscillations (Figure 2). This was associated with a modest increase in spontaneous firing in nRt but not VB cells as observed in Figure 1A2 and 1B2. Higher concentrations (0.3 μM) produced larger increases in nRt cell spontaneous firing and further increased the frequency of burst firing (e.g. Figure 1B3 and 2B), and the overall network responses were more sustained compared to those obtained under control conditions, but were not as long lasting as they had been with 0.1 μM norepinephrine. At the highest concentration used (1 μM) norepinephrine produced a large increase in spontaneous firing in nRt and VB and concurrently suppressed repetitive phasic discharges (Figure 1A4 &1B4), and truncated the overall oscillatory response (Figure 1B4 and Figure B2A). These effects were fully reversible in the wash (Figure 1A5,1B5 and Figure 2). Similar concentration-dependent results were obtained in 4 other slices. At a concentration of 0.3 μM norepinephrine decreased the inter-burst period by 6.9 ± 1.7% in nRt (n = 10, p<0.001) and 7.4 ± 2.3% in VB (n = 12, p<0.001) recordings.
Figure 1.
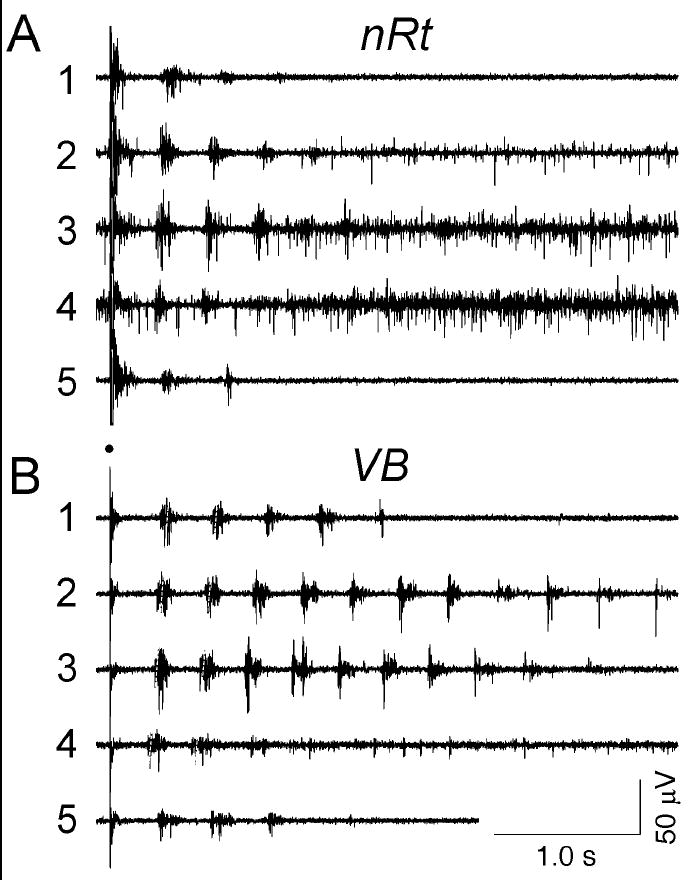
Multi-unit extracellular recording from multiple sites in thalamic slices after enhancement of activity with bicuculline methiodide. Traces in A are from nRt, and traces in B are from the VB nucleus. Each trace number represents a different condition, with trace 1 being control; trace 2, 0.1 μM norepinephrine; trace 3, 0.3 μM norepinephrine; trace 4, 1 μM norepinephrine; and trace 5, wash back to control. Washes were applied between each individual norepinephrine application (not shown but see figure 2). The skewed dashed lines in B indicate the times of the first and second recurrent burst and show that these bursts occurred at progressively earlier times as norepinephrine concentration was increased. Each numbered trace represents simultaneously recorded activity from the two electrodes in one slice.
A number of noradrenergic effects have been reported in thalamic neurons, including depolarization of relay neurons (McCormick and Prince, 1988) and nRt cells (McCormick and Wang, 1991) and a modulation of IH (Pape and McCormick, 1989). To elucidate the nature of the noradrenergic modulation of thalamic oscillation frequency we applied a pharmacological approach. The β-adrenergic antagonist, propranolol, at a concentration of 1 μM had no effect on frequency or duration of thalamic oscillations (n = 6) but when co-applied with norepinephrine (0.3 μM) it either blocked (in 2 of 8 slices) or sometimes even inverted (in 5 of 8 slices) the shortened period. An example of such an inversion is found in Figure 3. Initially after onset of the drug application, it is seen that norepinephrine (0.3 μM) produces a rapid-onset increase in internal oscillation frequency (single closed arrows in Figure 3A and 3B) followed by a later increase in frequency (single open arrows). These overall effects were reversible, as the frequency of oscillation returned to baseline during washout of norepinephrine. Occasionally there was an undershoot during washout such that the frequency was lower than in control (Figure 3B). Co-application of 0.3 μM norepinephrine with 1 μM propranolol caused a similar, early onset increase in oscillation frequency (double filled arrowheads in Figure 3A and 3B) but the late effects were characterized by a gradual slowing of the network response such that after about 5 minutes the frequency of the oscillation was slower than it had been in control (double open arrow heads). As with application of norepinephrine alone, the latter effects were fully reversible upon drug wash-out (Figure 3).
The reversal of the oscillation-accelerating effects of norepinephrine by propranolol suggested that much of the increase in oscillation frequency observed with norepinephrine was due to activation of β adrenergic receptors. To test this directly we used the specific agonist, isoproterenol, at a concentration of 0.3 μM. Isoproterenol produced a similar increase in frequency to that observed with norepinephrine alone, but unlike norepinephrine it produced a suppression of the overall oscillation. An example of such an experiment is shown in Figure 4 where the rate-meter contour plot shows that isoproterenol produced a reversible shortening of the oscillation decay τ (from 1.3 sec. in control [and wash] down to 0.6 seconds in isoproterenol) and increase in the frequency (from ~ 3.1Hz in control to ~ 3.3 in isoproterenol).
Figure 4.
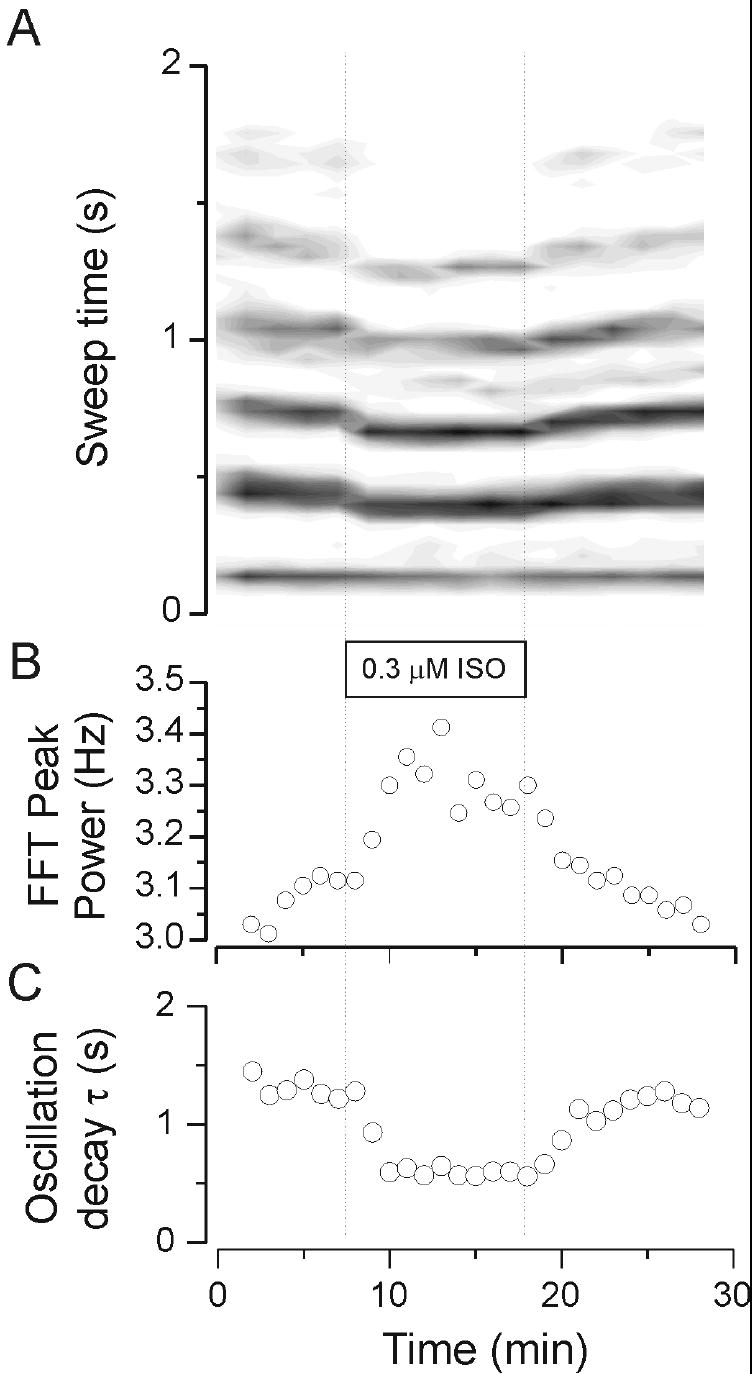
The β adrenergic agonist isoproterenol (ISO) at concentration 0.3 μM caused a marked increase in frequency and a decrease in duration of multi-unit activity in thalamic slices. Activity recorded from a VB multi-unit electrode during thalamic activity. Note in the rate meter of panel A that isoproterenol caused a reversible increase in frequency and a shortening in the duration of the oscillatory response. Panel B shows the effects on frequency where the control levels were approximately 3.1 Hz compared to about 3.3 Hz in the presence of isoproterenol. The oscillation duration, quantified by the decay time constant (C) was reversibly decreased from about 1.3 seconds in control, down to .6 seconds in isoproterenol, and 1.2 seconds in wash.
The effects of adrenergic agents are summarized in Figure 5 where frequency and duration of the network response is plotted as a percentage of control under different conditions for recordings made in both nRt and VB nuclei. Norepinephrine and isoproterenol produced similar (~ 8%) increases in oscillation frequency whereas the effect of norepinephrine was completely blocked by 1 μM propranolol. Oscillation duration was consistently shortened by isoproterenol, an effect opposite to that seen with norepinephrine (Figure 5B, cf. Figure 3 versus Figure 4). The increase in network response duration observed with norepinephrine was not affected by propranolol (Figure 5b) and is thus likely mediated by α-adrenergic receptors. In guinea pig and cat nRt cells an aminergic depolarization has been demonstrated that results from the closing of membrane K+ channels, and the noradrenergic effects were shown to be mediated by α1-adrenoceptors (McCormick and Wang, 1991). Such an effect would explain the increased spontaneous nRt cell activity that we observed with norepinephrine (Figure 1A2-4), but not isoproterenol. A subtle depolarization of nRt cells could enhance oscillatory network responses while a stronger depolarization would inhibit them as has been shown with cholecystokinin (Cox et al., 1997). The enhancement of thalamic network responses by α-adrenoceptors will not be further considered in this study, as there is no evidence that these adrenoceptors modulation IH.
Figure 5.
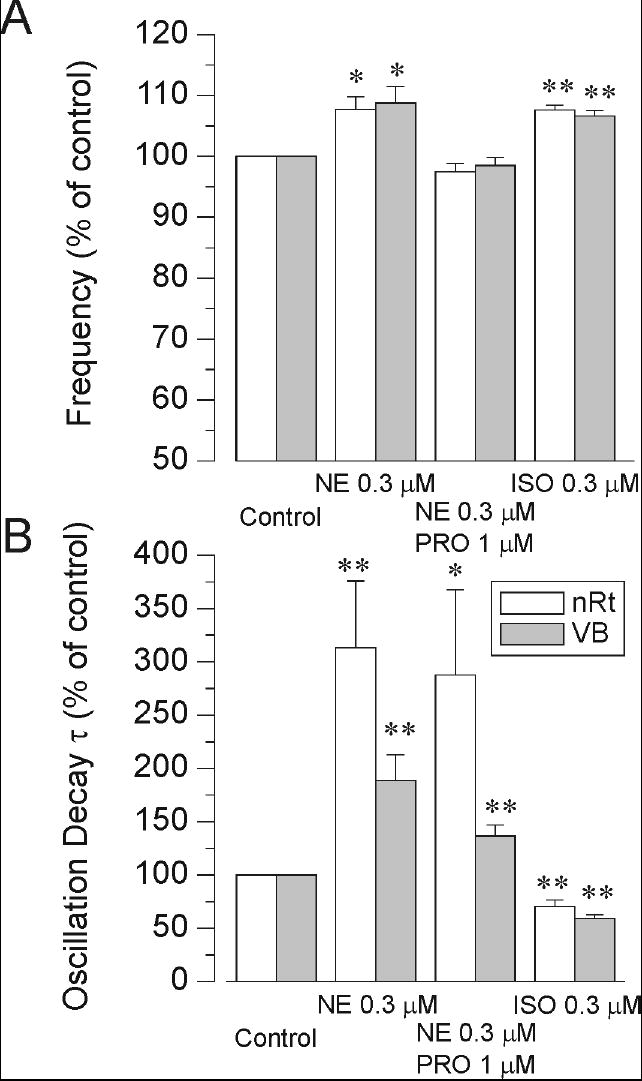
Population data depicting the effects of norepinephrine (NE), norepinephrine plus propranolol (PRO) and isoproterenol (ISO) on the frequency and duration of oscillations. A) Norepinephrine itself caused an increase in frequency, but when co-applied with propranolol these increases were blocked. Isoproterenol itself mimics the effects of norepinephrine on frequency. n = 7 - 14 for each point. B) The oscillation duration, measured by the oscillation decay time constant, was increased by norepinephrine in both the presence and absence of propranolol. Effects tended to be larger with nRt recordings. Isoproterenol had the opposite effect – it shortened the oscillations. *: p < 0.05 compared to control levels; **: p < 0.01; ***: p < 0.001.
The results up to this point indicate that alterations in oscillation frequency can be brought about through activation of β-adrenoceptors. Such activation has previously been shown to produce an up-regulation of IH (Pape and McCormick, 1989;McCormick and Pape, 1990a) mediated through a rightward shift in the activation curve (such that at normal resting membrane potentials near -60 mV more H channels are open) and an acceleration of the activation kinetics for the IH (McCormick and Pape, 1990a).
The finding that isoproterenol caused a shortening of the oscillation duration suggests that resting level of IH can better sustain the bicuculline-dependent oscillations than the IH available after β-adrenergic up-regulation. To further test whether resting H channel availability is optimal for such activities, we altered H channels availability in the opposite direction, by applying antagonists, to test whether such changes might actually enhance the oscillations. Indeed we found that low levels of extracellular Cs+ (e.g., 0.1 mM) caused a slowing of the oscillation frequency (from about 2.8 down to about 2.6 Hz in the example of Figure 6) and this was associated with an increase in the overall duration of the activity. This is especially apparent in the comparison between the activity during the wash-out period following 0.1 mM Cs+ application (3 cycles of oscillation in Figure 6) versus that activity observed in Cs+ (9 cycles of activity). Higher concentrations of Cs+ (0.3 mM) also produced increases in response duration. In the example shown in Figure 6 only 7 cycles of even slower (~2.5 Hz) activity were obtained. This effect was reversible with a slight overshoot in the washout period when frequency transiently rebounding to over 3Hz (Figure 6B). At the highest concentration tested (1 mM) Cs+ disrupted these oscillations and producing a dramatic shortening of the network response and a slowing which could not quantified reliably. These results are summarized in Figure 8, where it can be seen that Cs+ application produced concentration-dependent decreases in oscillation frequency, but increases in duration. This indicates that H channels are indeed an important regulator of oscillation frequency and that suppression of some IH can actually enhance the overall response.
Figure 6.
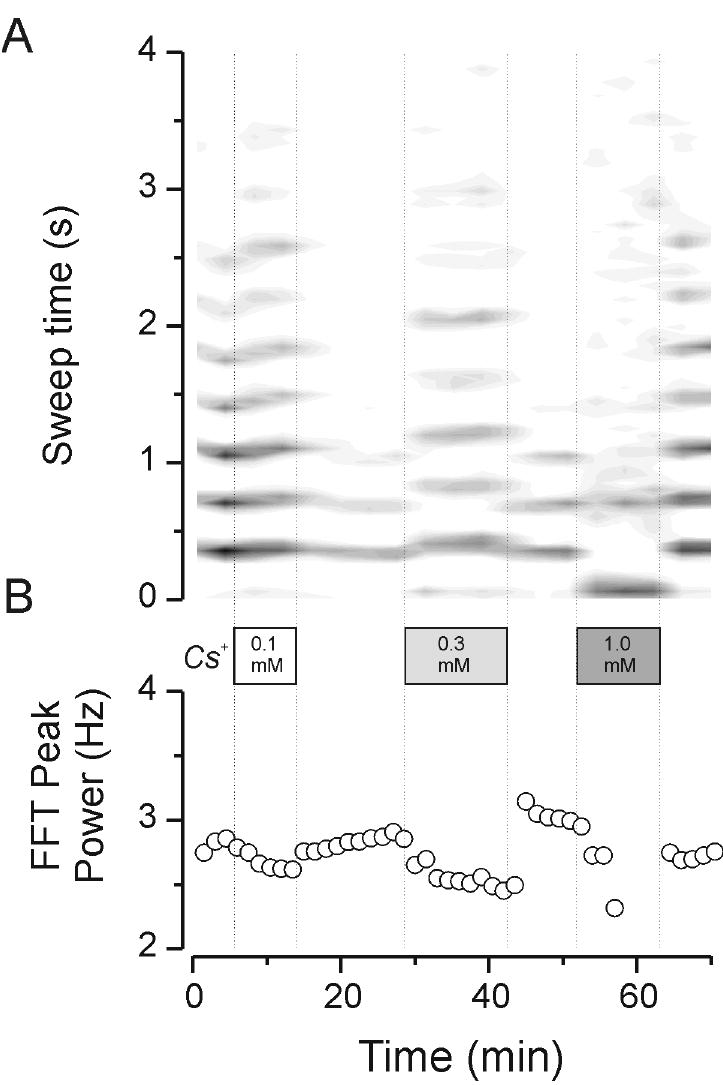
Effects of extracellular Cs+ on thalamic oscillatory activity. Cs+ applied at 0.1, 0.3 and 1 mM. The upper traces depict rate meter outputs in contour plot mode. At a concentration of 0.1 mM Cs+ caused an increase in the duration of oscillatory activity (A) that corresponded with a slowing of activity from about 2.8 down to about 2.6 Hz (B). This was reversible upon wash-out of the Cs+. Note that the duration of the response in the first wash-out was quite abbreviated whereas the addition of 0.3 mM Cs+ caused a prolongation of activity (A) and a further slowing (down to ~ 2.5 Hz, B). Finally, 1 mM reversibly disrupted the oscillatory activity by making it relatively short-lived and poorly organized so that it became difficult to quantify a characteristic oscillation frequency.
Figure 8.
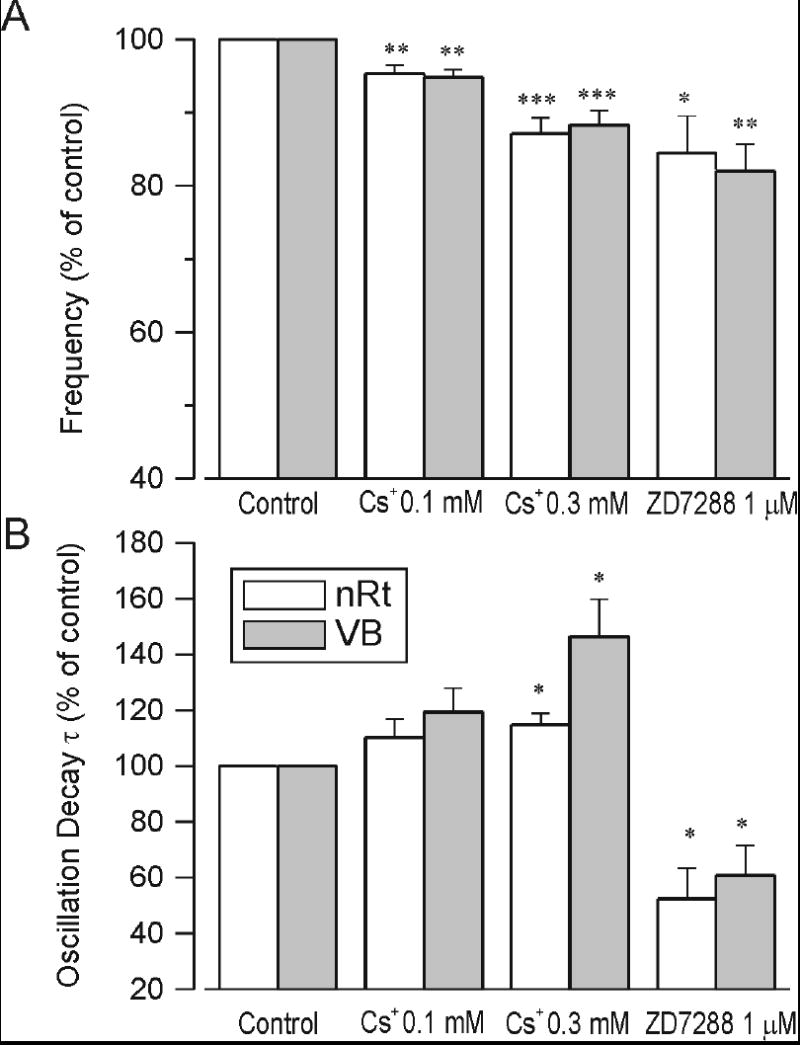
Quantification of H channel blocker effects on oscillation frequency (A) and duration (B). Cs+ at 0.1 and 0.3 mM and ZD at 1 μM all caused significant slowing of the oscillation that was comparable in each recording electrode (nRt and VB). The ZD7288 effects were measured 10 minutes after wash in of the drug when the oscillation frequency could still be reliably measured. This resulted in underestimates of the equilibrium effects of ZD7288 on frequency (A) and duration (B). Cs+ increased the oscillation decay time constant at both concentrations, but effects were only significant with 0.3 mM. n = 6 - 8 for each point. *: p < 0.05 compared to control levels; **: p < 0.01; ***: p < 0.001.
In another test of the influence of IH on intrathalamic oscillations, we applied the specific H channel blocker, ZD7288, at a concentration of 1 uM. This has previously been shown to block IH in thalamocortical relay neurons (Lüthi et al., 1998). We found that ZD7288 had powerful and irreversible effects on intrathalamic oscillations, an example of which is shown in Fig. 7. In this example, stable oscillations lasting between 3 and 4 seconds were observed throughout a prolonged control period of about 30 min. Addition of ZD7288 at 1 uM produced a slowing of the oscillation after a latency of nearly 10 min. Interestingly, the slowing of the oscillation by ZD7288 was transiently associated with an enhanced response. There was an increase in number of cycles from 10 cycles in control up to ~13 cycles once the slowing was initiated (Figure 7A). This was associated with a transient increase in the oscillation decay τ (* in Figure 7C). Although ZD7288 was typically only applied for 15 minutes the effects of ZD7288 were irreversible. Further, during the wash period, the ZD effects continued to progress so that further slowing of the network response was observed. This finally culminated in an abolition of the oscillatory responses, indicating that H channels are necessary for the generation of these network activities. The population data shown in the summary Fig. 8 show that ZD produced reliable and robust decreases in the frequency and duration of the oscillations.
Figure 7.
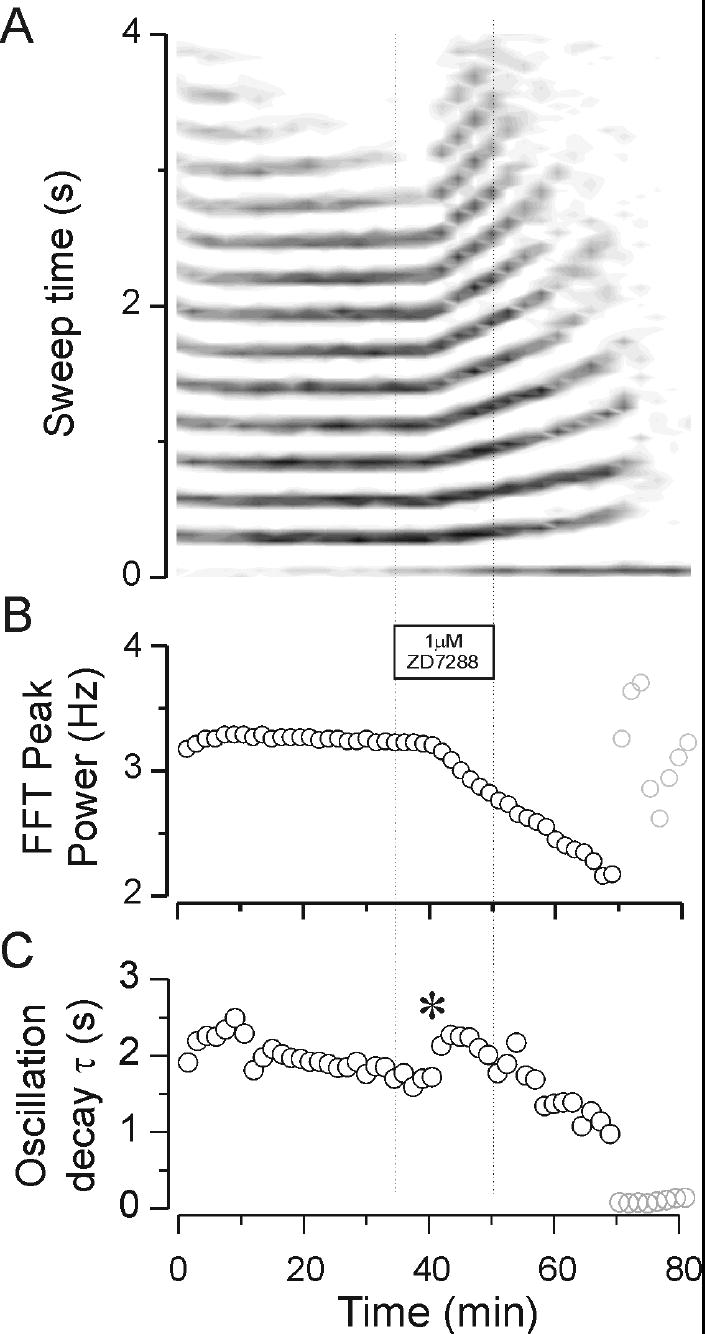
Effects of the H channel blocker ZD7288 on oscillatory thalamic activity. Note the regular pattern of activity that was sustained at a stable frequency of ~3.3 Hz throughout the 30 minute control period. Addition of 1 μM ZD7288 caused a progressive and irreversible slowing of the activity follwing a wash-in delay of about 8 minutes. The slowing was initially associated with an increase in the duration of activity, i.e., a prolongation of the response (A) and increase in the oscillation decay time constant (* in C). Gradually the response became slower and slower. This was associated with a shortening and eventually abolition of the oscillatory response. The final resolvable oscillation was near 2 Hz. The greyed points at the end of the experiment (> 70 minutes) in panels B and C represent the values for which the autocorrelation analysis was unable to produce reliable estimates of duration or frequency.
Discussion
These results provide further support for an important role of specific intrinsic conductances in thalamic cortical relay neurons in regulating a network response that indeed depends on synaptic interactions. Further, these results emphasize that there is a strong interaction between such intrinsic and synaptic mechanisms that regulate such oscillatory phenomenon in the thalamus. Thus it is not just the duration of the inhibitory synaptic responses received by relay cells that determines the frequency of the thalamic oscillation (Huguenard and Prince, 1994b;Bal et al., 1995a), but other factors can contribute. We found that neuromodulators, such as norepinephrine or isoproterenol, as well as direct pharmacological blockers, such as Cs+ or ZD7288, strongly influence these network responses.
Perhaps the most interesting finding of this study is that, under these in vitro conditions, thalamic oscillations have a preferred frequency. Up- or down-regulation of IH, which moves the network frequency further or closer from this preferred frequency, will suppress or enhance the overall network response. This finding is consistent with what is known of the resonant behavior of thalamocortical relay neurons which have a preferred frequency nearer 3Hz (Puil et al., 1994). Activation of IH by the β-adrenergic agent isoproterenol shortened the overall oscillation while modest reductions of IH actually prolonged the oscillation. This indicates that in the baseline state (i.e. in our recording conditions) the thalamic network is in a condition that is sub-optimal for production of 2-4 Hz network oscillatory responses. This leads to a situation where down-regulation of the IH through, for example, purinergic receptors (Pape, 1996) could promote the development of slow thalamic synchrony, whereas other neuromodulators that up-regulate IH, such as norepinephrine and serotonin (Pape and McCormick, 1989;McCormick and Pape, 1990a), increases in intracellular pH (Munsch and Pape, 1999a), or histamine (McCormick and Williamson, 1991) or nitric oxide pathways (Pape and Mager, 1992), will all tend to block the slow synchronous activity.
Pacemaking in individual thalamic neurons has been reported in some of the earliest intracellular in vitro recordings, including (Jahnsen and Llinás, 1984;Leresche et al., 1990;Leresche et al., 1991;Soltesz et al., 1991). The properties of the IH which underlie an important part of this intrinsic oscillation have been fully characterized (Pape and McCormick, 1989;McCormick and Pape, 1990b) and reviewed (Pape, 1996;Lüthi and McCormick, 1998a). The properties of the current are such that it can interact with the low threshold calcium current, so-called T-type calcium current, to produce a yin and yang-type oscillation where calcium-dependent burst responses are followed by an afterhyperpolarization which activates the IH ultimately leading to subsequent depolarization that activates another calcium-dependent burst (McCormick and Huguenard, 1992). Modulation of either calcium current or IH can alter this intrinsic oscillation (Soltesz et al., 1991;McCormick and Huguenard, 1992). The activation properties of the IH can be dramatically modified by various neuromodulators. Normally very few H channels are open at rest, however, with activation of noradrenergic, serotonergic, or histaminergic receptors, the channels begin to open through a cyclic AMP-dependent mechanism (Lüthi and McCormick, 1998b). Opening of these channels allows cations to enter the cell and depolarize the neuron. This can enhance rhythmicity within limits but it is clear that high levels of IH activation can actually abolish in vitro spindle like oscillations (Lee and McCormick, 1996).
There is now increasing evidence that long-term regulation of IH may regulate the duration of individual sleep-related spindle sequences. Calcium entry that occurs during an individual spindle as a result of repetitive calcium-dependent burst discharges can lead to long-term changes in activation of H-type channels (Lüthi and McCormick, 1998b) so that progressive activation of the IH leads to a strong depolarization that prevents recurrent activity in relay cells and thus terminates the spindle. Blockade of the IH by cesium or ZD7288 can lead to prolonged spindle sequences (Bal and McCormick, 1996;Lüthi et al., 1998). Interestingly, IH is also modulated by changes in intracellular pH (Munsch and Pape, 1999a) which occur during neuronal activity (Meyer et al., 2000) and can be brought about by blockade of the carbonic anhydrase (Munsch and Pape, 1999b). These findings of activity-dependent regulation of IH, either by pH or Ca2+ indicate ways that the thalamic network can be regulated during brain activity. It remains to be determined what are the physiological or pathophysiological conditions under which such use-dependent regulatory mechanisms come into play.
Overall, these results provide new information about the specific types of interaction that can occur between H channels and the slow (2-4 Hz) thalamic oscillations that resemble the hypersynchronous activity during thalamocortical seizures. It should be emphasized that it is not at this point clear what mechanisms lead to the putative slowing and synchronization of thalamic oscillations as is thought to occur at the onset of absence seizures (Gloor et al., 1990). These might include a breakdown of intra-nRt connectivity (Huntsman et al., 1999) or a specific pattern of cortical input (Destexhe et al., 1998;Blumenfeld and McCormick, 2000;Bal et al., 2000). Once the thalamus becomes recruited into a network, characterized by a slow synaptic interaction dominated by GABAB receptors (von Krosigk et al., 1993;Huguenard and Prince, 1994b), then the interactions with the IH may become particularly critical. It thus seems likely that IH modulation may not necessarily be an initiating mechanism that would lead to the development of spontaneous seizures in vivo. Such modulation of the IH may on the other hand predispose the thalamic portion of the thalamocortical circuit towards the generation of slow synchronous discharges under conditions when the underlying synaptic circuitry is capable of driving slow network activities.
Acknowledgments
This work was supported by NINDS NS 06477 and NS 34774 and a Stanford University major URO grant.
Reference List
- Bal T, Debay D, Destexhe A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. J Neurosci. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol (Lond) 1995a;483:665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol (Lond) 1995b;483:641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol (Lond) 1989;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Peptidergic modulation of intrathalamic circuit activity in vitro: actions of cholecystokinin. J Neurosci. 1997;17:70–82. doi: 10.1523/JNEUROSCI.17-01-00070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- Gloor P, Avoli M, Kostopoulos G. Thalamo-cortical relationships in generalized epilepsy with bilaterally synchronous spike-and-wave discharge. In: Avoli M, Gloor P, Kostopoulos G, Naquet R, editors. Generalized epilepsy: neurobiological approaches. Boston: Birkhäuser; 1990. pp. 190–212. [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Clonazepam suppresses GABAB-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994a;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T current modulation causes robust anti-oscillatory effects. J Neurosci. 1994b;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Inoue M, Peeters BW, Van Luijtelaar EL, Vossen JM, Coenen AM. Spontaneous occurrence of spike-wave discharges in five inbred strains of rats. Physiol Behav. 1990;48:199–201. doi: 10.1016/0031-9384(90)90285-c. [DOI] [PubMed] [Google Scholar]
- Jacobsen RB, Ulrich D, Huguenard JR. GABAB and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001 doi: 10.1152/jn.2001.86.3.1365. in press. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron. 1996;17:309–321. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Leresche N, Jassik-Gerschenfeld D, Haby M, Soltesz I, Crunelli V. Pacemaker-like and other types of spontaneous membrane potential oscillations of thalamocortical cells. Neurosci Lett. 1990;113:72–77. doi: 10.1016/0304-3940(90)90497-w. [DOI] [PubMed] [Google Scholar]
- Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol (Lond) 1991;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, Bal T, McCormick DA. Periodicity of thalamic spindle waves is abolished by ZD7288, a blocker of Ih. J Neurophysiol. 1998;79:3284–3289. doi: 10.1152/jn.1998.79.6.3284. [DOI] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. H-current: Properties of a neuronal and network pacemaker. Neuron. 1998a;21:9–12. doi: 10.1016/s0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- Lüthi A, McCormick DA. Periodicity of thalamic synchronized oscillations: The role of Ca2+-mediated upregulation of Ih. Neuron. 1998b;20:553–563. doi: 10.1016/s0896-6273(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Marcus EM, Watson CW. Studies of the bilateral cortical callosal preparation. Trans Amer Neuro Assn. 1966;91:291–293. [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol (Lond) 1990a;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol (Lond) 1990b;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol (Lond) 1991;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TM, Munsch T, Pape HC. Activity-related changes in intracellular pH in rat thalamic relay neurons. Neuroreport. 2000;11:33–37. doi: 10.1097/00001756-200001170-00007. [DOI] [PubMed] [Google Scholar]
- Munsch T, Pape HC. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol (Lond) 1999a;519:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch T, Pape HC. Upregulation of the hyperpolarization-activated cation current in rat thalamic relay neurones by acetazolamide. J Physiol (Lond) 1999b;519:505–514. doi: 10.1111/j.1469-7793.1999.0505m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pape HC, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Puil E, Meiri H, Yarom Y. Resonant behavior and frequency preferences of thalamic neurons. J Neurophysiol. 1994;71:575–582. doi: 10.1152/jn.1994.71.2.575. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaght S, Charpier S, Deniau JM, Leresche N, Crunelli V. In vivo intracellular recordings in neurones of the nucleus reticularis thalami during spike and wave discharges in the gaers genetic model of absence epilepsy. Soc Neurosci Abstr. 2000;26:735. [Google Scholar]
- Sohal VS, Cox CL, Huguenard JR. Localization of CCK receptors in thalamic reticular neurons: A modeling study. J Neurophysiol. 1998;79:2820–2824. doi: 10.1152/jn.1998.79.5.2820. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol (Lond) 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschênes M. The thalamus as a neuronal oscillator. Brain Res Rev. 1984;8:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. Purinergic inhibition of GABA and glutamate release in the thalamus: implications for thalamic network activity. Neuron. 1995;15:909–918. doi: 10.1016/0896-6273(95)90181-7. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Warren RA, Jones EG. Glutamate activation of cat thalamic reticular nucleus: Effects on response properties of ventroposterior neurons. Exp Brain Res. 1994;100:215–226. doi: 10.1007/BF00227192. [DOI] [PubMed] [Google Scholar]
- Williams DA. A study of thalamic and cortical rhythms in petit mal. Brain. 1953;76:50–69. doi: 10.1093/brain/76.1.50. [DOI] [PubMed] [Google Scholar]


