Abstract
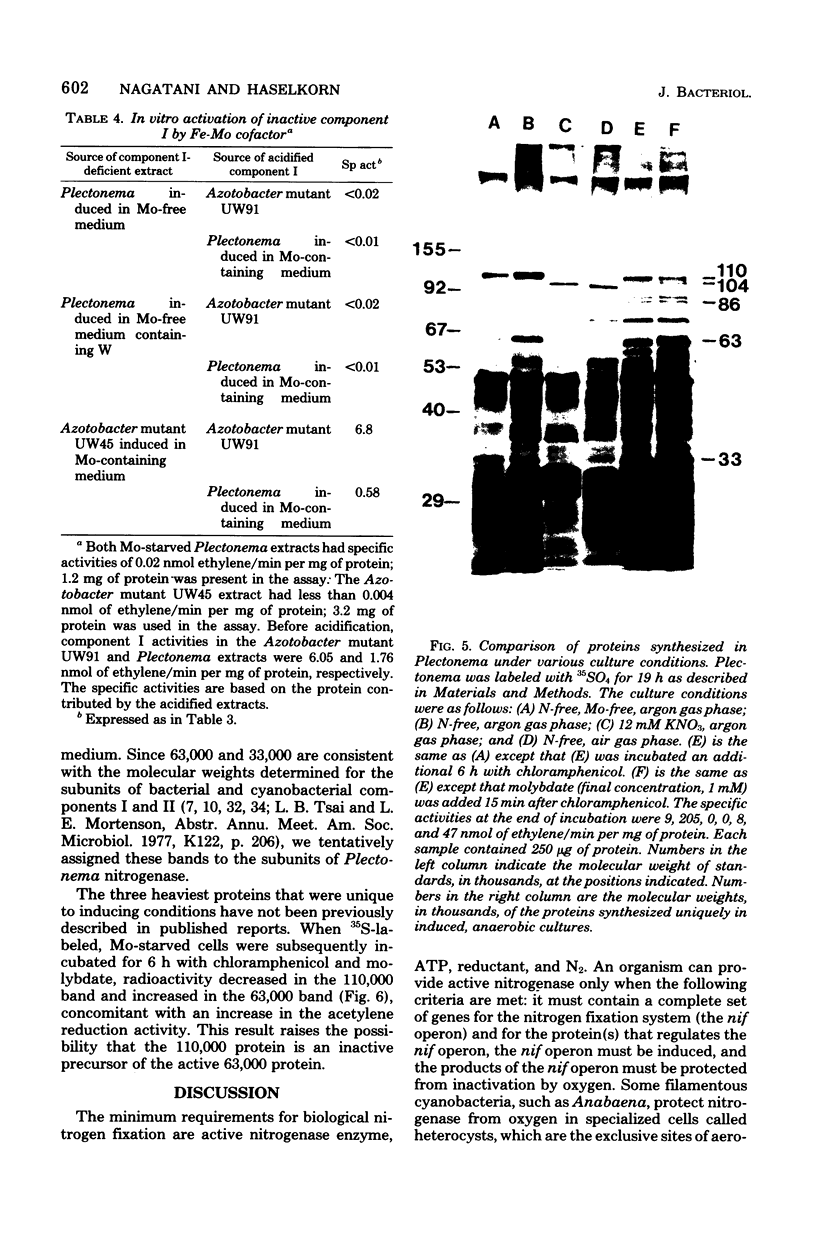
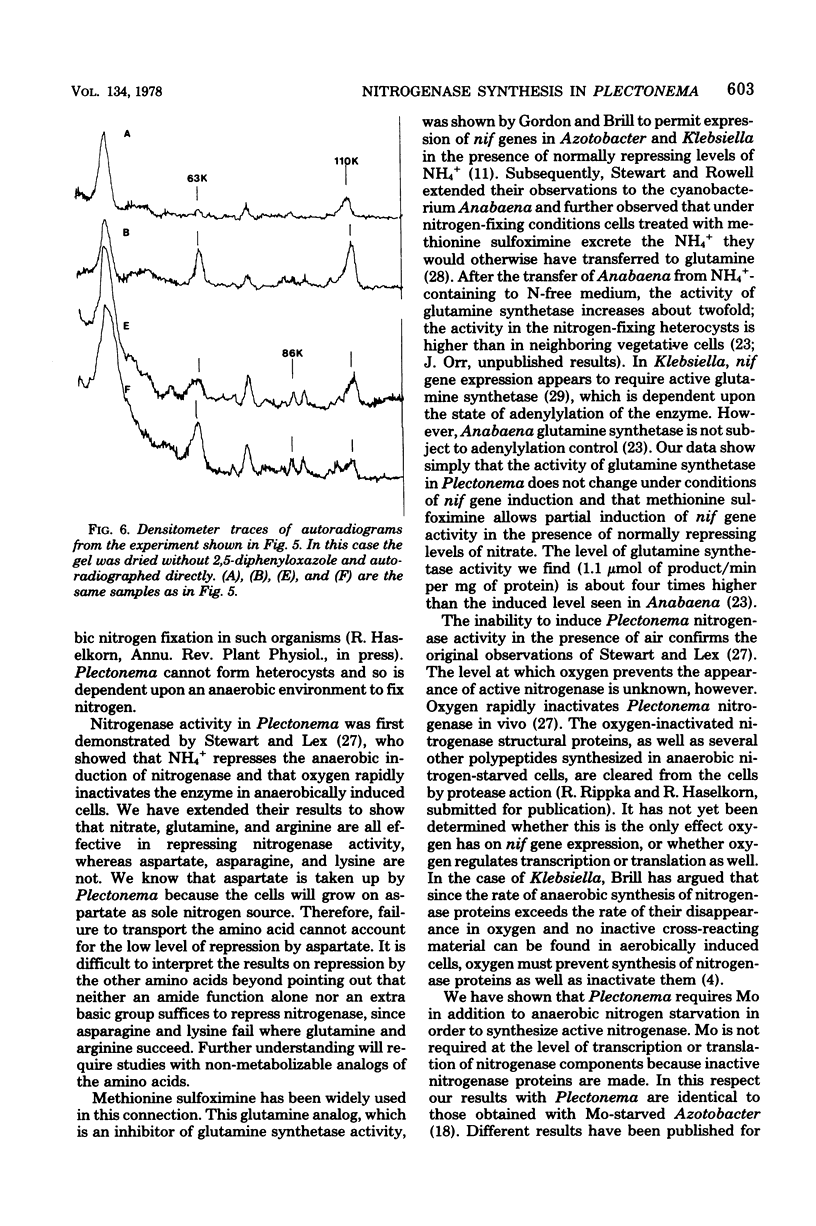
The cyanobacterium Plectonema boryanum (IU 594-UTEX 594) fixes N2 only in the absence of combined N and of O2. We induced nitrogenase by transfer to anaerobic N-free medium and studied the effect of Mo starvation on nitrogenase activity and synthesis. Activity was first detected within 3 h after transfer by the acetylene reduction assay in controls, increasing for at least 25 h. Cells grown on nitrate and Mo and then transferred to N-free, Mo-free medium produced 8% of the control nitrogenase activity. Addition of W to the Mo-free medium reduced the activity to 0.5%. Under both Mo starvation conditions, nitrogenase protein components were synthesized. Component II of the cyanobacterial enzyme was detected by in vitro complementation with Mo-containing component I from Klebsiella pneumoniae or Azotobacter vinelandii but not Clostridium pasteurianum. Component I activity was restored by addition of Mo to cultures in which new enzyme synthesis was blocked by chloramphenicol. Acidified extracts of Plectonema induced in Mo-containing medium contained the Fe-Mo cofactor required to activate extracts of the Azotobacter mutant UW45 in vitro, but they did not activate extracts of Mo-starved Plectonema. Analysis of 35SO4(2-)-labeled proteins by polyacrylamide gel electrophoresis suggested that Mo is required for the conversion of a high-molecular-weight precursor to component I in Plectonema.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Smith G. M., Kostel P. J., McKenna C. E. Tungsten incorporation into Azotobacter vinelandii nitrogenase. FEBS Lett. 1973 Feb 1;29(3):219–221. doi: 10.1016/0014-5793(73)80023-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J. Regulation and genetics of bacterial nitrogen fixation. Annu Rev Microbiol. 1975;29:109–129. doi: 10.1146/annurev.mi.29.100175.000545. [DOI] [PubMed] [Google Scholar]
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J., Mortenson L. E. Role of molybdenum in dinitrogen fixation by Clostridium pasteurianum. J Bacteriol. 1975 Sep;123(3):978–984. doi: 10.1128/jb.123.3.978-984.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Fay P., de Vasconcelos L. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. II. The effect of molybdenum and vanadium. Arch Microbiol. 1974;99(3):221–230. doi: 10.1007/BF00696236. [DOI] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. Differentiation in Nostoc muscorum: nitrogenase is synthesized in heterocysts. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2727–2731. doi: 10.1073/pnas.70.10.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Eady R. R., Kondorosi E., Rekosh D. K. The molybdenum--iron protein of Klebsiella pneumoniae nitrogenase. Evidence for non-identical subunits from peptide 'mapping'. Biochem J. 1976 May 1;155(2):383–389. doi: 10.1042/bj1550383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Postgate J. R. Expression of Klebsiella pneumoniae nitrogen fixation genes in nitrate reductase mutants of Escherichia coli. J Gen Microbiol. 1977 Feb;98(2):551–557. doi: 10.1099/00221287-98-2-551. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Morphology of a virus of blue-green algae and properties of its deoxyribonucleic acid. J Virol. 1967 Apr;1(2):344–361. doi: 10.1128/jvi.1.2.344-361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quin B. F., Brooks R. R. The rapid colorimetric determination of molybdenum with dithiol in biological, geochemical and steel samples. Anal Chim Acta. 1975 Jan;74(1):75–84. doi: 10.1016/S0003-2670(01)82781-1. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. Acetylene reduction by nitrogen-fixing blue-green algae. Arch Mikrobiol. 1968;62(4):336–348. doi: 10.1007/BF00425639. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Rowell P. Effects of L-methionine-DL-sulphoximine on the assimilation of newly fixed NH3, acetylene reduction and heterocyst production in Anabaena cylindrica. Biochem Biophys Res Commun. 1975 Aug 4;65(3):846–856. doi: 10.1016/s0006-291x(75)80463-3. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Thomas J., Meeks J. C., Wolk C. P., Shaffer P. W., Austin S. M. Formation of glutamine from [13n]ammonia, [13n]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J Bacteriol. 1977 Mar;129(3):1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. Y. Some properties of the nitrogenase proteins from Clostridium pasteurianum. Molecular weight, subunit structure, isoelectric point and EPR spectra. Arch Microbiol. 1974;99(1):71–80. doi: 10.1007/BF00696223. [DOI] [PubMed] [Google Scholar]
- Tubb R. S. Glutamine synthetase and ammonium regulation of nitrogenase synthesis in Klebsiella. Nature. 1974 Oct 11;251(5475):481–485. doi: 10.1038/251481a0. [DOI] [PubMed] [Google Scholar]
- Whiting M. J., Dilworth M. J. Legume root nodule nitrogenase. Purification, properties, and studies on its genetic control. Biochim Biophys Acta. 1974 Dec 18;371(2):337–351. [PubMed] [Google Scholar]