Abstract
Schizophrenia is characterized by subtle but well-replicated total and regional (frontal and temporal) brain tissue volume deficits. Studies of individuals at-risk for developing schizophrenia suggest that the onset of brain volume decrement may closely pre-date overt manifestations of schizophrenia, making brain volume abnormalities potential predictors for early identification. In an ongoing longitudinal morphometric MRI study of young, nonpsychotic first- or second-degree relatives of schizophrenia probands, we compared brain volumes in 46 relatives who are still within age range for developing schizophrenia against comparison groups of 46 schizophrenia patients and 46 healthy volunteers without family history of schizophrenia. Relatives had similar brain volume abnormalities as schizophrenia patients albeit less severe. Relatives had significantly larger whole brain, frontal, temporal and parietal gray matter (GM) volumes than patients. Relatives also had significantly smaller frontal GM volumes than healthy volunteers. Both relatives and patients had significantly larger whole brain WM (specifically parietal WM) volumes compared to healthy volunteers. Abnormally greater WM volumes in relatives and patients are suggestive of genetically-mediated dysmaturation of the age-expected myelination during adolescence through mid adulthood. On prodromal symptoms assessed in relatives one year after MRI brain scans, initial GM deficits as well as larger WM volumes correlated significantly with greater severity of subsequent prodromal symptoms. Together with previous genetic high-risk studies of adolescent or young adult relatives, these findings indicate that premorbid MRI brain abnormalities may be of predictive value for the early identification of schizophrenia.
Keywords: Schizophrenia, Genetics, Magnetic resonance imaging, Prevention, Prodrome, White Matter
Introduction
Schizophrenia is perhaps the most enigmatic and devastating brain disorder. Even though schizophrenia patients experience severe symptoms and impairment, their microscopic and macroscopic brain abnormalities are relatively subtle (Harrison 1999; Shenton et al. 1997). Compared to healthy volunteers, brain volumes in schizophrenia patients are reduced by 1–2% for the whole brain and frontal lobes, between 4–6% reductions in temporal lobe structures (including the hippocampus, amygdala, anterior superior temporal gyrus) (Wright et al. 2000). Although the etiology and pathogenesis of schizophrenia are still not well understood, family studies indicate that the causes of schizophrenia are primarily genetic (Sullivan et al. 2003). Twin studies have estimated heritabilities of around 80%. Prevalence of schizophrenia among relatives is higher than the general population (1%), and diminishes with decreased degree of relatedness to the schizophrenia proband – from 48% in monozygotic twins, to 17% dizygotic twins, 13% off springs, 9% full siblings and down to 2% in first cousins. Besides higher rates of schizophrenia, relatives who do not manifest the disorder are more likely to have similar, but less severe, neuroanatomical, electrophysiological, neurocognitive and behavioral deficits seen in schizophrenia probands (Keshavan et al. 2005).
There are now fairly substantial numbers of in vivo anatomical neuroimaging studies on nonpsychotic relatives of schizophrenia probands (see summary in Table 1). Brain volumes in unaffected relatives are intermediate between schizophrenia probands and healthy controls with no family history of schizophrenia. These volume abnormalities are in similar brain regions as the deficits observed in schizophrenia. Diminished amygdala-hippocampus volumes have been the most frequently reported abnormalities among nonpsychotic relatives.
Table 1.
Family studies of magnetic resonance imaging brain volumes in schizophrenia grouped into studies involving young relatives who are still within age range at-risk for developing schizophrenia versus older relatives
However, as seen in Table 1, most studies have comprised of older adult relatives who are for most part unlikely to develop schizophrenia. To the best of our knowledge, there are only five independent samples in the literature that examined MRI brain volume deficits in nonpsychotic, young relatives who are still in the age range at-risk for developing schizophrenia. Focusing on adolescent or young adult relatives has advantages over studies involving older adult relatives. As volumetric abnormalities in nonpsychotic relatives are partly related to shared genetic vulnerability to schizophrenia, studying brain development of adolescent or young adult relatives who are still in age range at-risk will likely lead to better understanding of the neurodevelopmental etiology of schizophrenia. Since younger samples are likely to contain subjects who will develop schizophrenia in future, and if adequate numbers of the high-risk subjects are followed-up, such genetic high-risk studies can address the issue of whether brain volume abnormalities are present in predisposed individuals before the clinical manifestations of schizophrenia. Brain volume abnormalities together with other biomarkers of increased susceptibility may aid in early identification of schizophrenia and the eventual secondary and primary prevention of the disorder.
To achieve these long term goals, we have begun studying adolescent or young adult first- or second-degree relatives of schizophrenia probands who have been carefully screened for the absence of psychotic disorders. In this report, we compared high-resolution MRI brain volumes of at-risk relatives obtained at intake into the study against two comparison groups of schizophrenia probands and healthy volunteers without family history of schizophrenia. Based on previous studies (Table 1), we hypothesized that these relatives will have gray matter (GM) and white matter (WM) volume deficits that are intermediate between the two comparison groups. In this study, we further assessed the relationships between initial MRI brain volumes and follow-up ratings of prodromal symptoms. We hypothesized that brain volume deficits will be associated with greater severity of prodromal symptoms among relatives.
Experimental/Materials and Methods
Subjects
The 138 subjects in this study comprised of 46 first- (N=30) or second-degree relatives of schizophrenia probands, 46 schizophrenia patients and 46 healthy controls with no family history of schizophrenia. After complete description of the study to subjects, written informed consent was obtained.
Relatives were ascertained either through 1) schizophrenia patients who have participated in research studies or have received psychiatric treatment at the University of Iowa Health Care, or 2) advertisements in local newspapers or mental health advocacy groups. Inclusion criteria for relatives were age between 13 to 28 years and having at least one first- or second-degree relative with schizophrenia. Family history of schizophrenia was further verified using Family History-Research Diagnostic Criteria (FH-RDC) (Andreasen et al. 1977), which has well-established reliability and validity. Relatives were interviewed using the SCID (Structured Clinical Interview for DSM-IV), and were excluded if they had a primary psychotic disorder (schizophrenia, schizophreniform disorder, schizoaffective disorder, or delusional disorder), or if they met criteria for substance abuse or dependence currently or within the past year. None of the relatives had schizophrenia-spectrum (schizotypal, schizoid or paranoid) personality disorders. Thirty-five relatives had no lifetime history of any psychiatric disorders. In the remaining 11 relatives, diagnoses included: major depressive disorder (6), depressive disorder, not otherwise specified (1), attention deficit hyperactivity disorder (2), panic disorder (1) and generalized anxiety disorder (1).
Schizophrenia patients and control subjects were selected from neuroimaging studies conducted at the Department of Psychiatry University of Iowa. These two comparison groups were of equivalent gender composition (M:F ratio=20:26) and age range (13 to 28 years) as relatives. Patients were evaluated using a semi-structured interview instrument, Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al. 1992), from which schizophrenia diagnosis meeting DSM-III-R or DSM-IV criteria was based. Thirty of the 46 patients are participants in the Iowa Longitudinal of Recent-Onset Psychoses (Flaum et al. 1992). Mean age of illness onset was 18.3 years (SD=4.8). These patients were still early in their course of illness (Mean duration of illness=2.4 years (SD=3.1), and have had minimal prior antipsychotic treatment (Median duration of treatment=3.0 months (25–75% interquartile range=23)). Healthy controls were evaluated using an abbreviated version of the CASH to exclude subjects with current or past psychiatric illnesses and substance misuse. FH-RDC was also used to assess the absence of family history of schizophrenia in healthy controls. Additional exclusion criteria for all subjects in this study were: neurological disorders, mental retardation, unstable medical conditions or contraindications for magnetic resonance imaging.
All patient subjects and healthy controls in this report were unrelated and were derived from independent families. The 46 relatives were ascertained from 34 independent families: 21 families each contributed 1 relative subject, 4 families each contributed 1 relative subject and 1 schizophrenia proband, 7 families contributed 2 relatives subjects each, 1 family 3 relatives subjects and the last family 4 relatives subjects.
MRI image acquisition and processing
All subjects underwent a high resolution anatomical MR imaging protocol on a 1.5 T GE CVMRI scanner (General Electric Medical Systems, Milwaukee, Wis). This protocol acquired multimodal scans of the whole brain consisting of T1 and T2 weighted sequences. The T1 sequence was obtained as a 3D volume in the coronal plane using a spoiled GRASS sequence with the following parameters: TE = 6 ms, TR = 20 ms, flip angle = 30°, FOV = 160×160×192 mm, matrix = 256×256×124, NEX=2. The T2 images were acquired using a 2D fast spin-echo sequence in the coronal plane with the following parameters: TE = 85 ms, TR = 4800 ms, slice thickness/gap = 1.8/0.0 mm, FOV = 160×160 mm, matrix = 256×256, NEX = 3, number of echoes = 8, number of slices = 124.
After the MR scans were acquired, the images were transferred to our Image Processing Lab for processing using an automated, highly reliable image analysis pipeline. Detailed descriptions of image analysis methods have been provided elsewhere (Andreasen et al. 1996; Harris et al. 1999; Magnotta et al. 2003; Magnotta et al. 2002). In brief, T1 weighted images were aligned horizontally along the interhemispheric fissure in the axial and coronal views and vertically along the anterior commissure (AC) and posterior commissure (PC). Talairach parameters that define the bounding box for the brain as well as the AC and PC points were selected. These parameters define the piecewise linear scaling of the Talairach grid onto the current brain of interest. The T2-weighted images were then co-registered to the AC-PC aligned T1 weighted images. These images were warped into standardized stereotaxic Talairach atlas space (Talairach and Tournoux 1988) to generate automated measurements of frontal, temporal, parietal, and occipital lobes, cerebellum, and subcortical regions (Andreasen et al. 1996). To further classify tissue volumes into gray matter (GM), white matter (WM) and CSF, we employed a discriminant analysis method of tissue segmentation based on automated training class selection that utilized data from the T1 and T2 sequences (Harris et al. 1999). This method identifies the range of values that characterized GM, WM, and CSF in the multispectral MRI data (10–70 for CSF, 70–190 for GM, and 190–250 for WM). Each voxel was given an intensity value based on the weights assigned by the discriminant functions. This reflected the relative combination of GM, WM, and CSF in a given voxel and allows us to correct for partial volume (Harris et al. 1999). Intraclass correlations for this automated tissue segmentation analysis ranged from 0.97 to 0.98.
Assessment of prodromal symptoms at follow-up
After the initial evaluation, a single rater interviewed the relatives at 6-monthly intervals for prodromal symptoms using the Scale of Prodromal Symptoms (SOPS) (Miller et al. 1999). The SOPS provides systematic assessment for the presence and severity of prodromal symptoms. Each of the 19 symptom items is rated on a 7-point ordinal scale (0=Absent, 6=Extreme). The items are further grouped into 4 symptom domains (i.e. Psychotic (5 items), Negative (6 items), Disorganized (4 items) and General (4 items)), which have good construct validity (Hawkins et al. 2004). Domain scores are the sum of individual item ratings within each symptom domain. Total SOPS score is the sum of the 19 symptom ratings. In this report, we present data from the last available SOPS ratings.
Statistical analysis
To reduce type I error, the analyses were conducted in stages. We first performed one joint omnibus multivariate regression test, which assessed the main effects of group membership (healthy controls with no family history of schizophrenia versus relatives of schizophrenia probands versus schizophrenia patients) simultaneously on all 4 primary MRI regions of interest (ROIs) (i.e. whole brain GM, whole brain WM, whole brain CSF and lateral ventricles). A Bonferroni-corrected alpha of .05/4=.0125 was used to control for multiple comparisons. To evaluate which primary ROI and which group contributed to the significant joint omnibus test, we followed up with univariate analyses of covariance (ANCOVA) assessing main effects of group, and with pair-wise group contrasts using the independent sample t statistic on each of the 4 primary ROI. Intracranial volume, age and gender were entered as covariates in the joint omnibus test and univariate ANCOVAs. Pair-wise independent group t-tests compared least-square group means adjusted for these covariates.
For the secondary ROIs (frontal, temporal, parietal, and occipital GM volumes and WM volumes), group differences in brain volumes were tested using ANCOVAs. In each general linear model, the respective secondary ROI was the dependent measure. Intracranial volume, age and gender were entered as covariates and grouping as the independent factor in each model. Similarly, pair-wise independent group t-tests compared least-square means of the secondary ROIs. All tests were 2-tailed. Analyses of secondary ROIs as well as all follow-up analyses of primary ROIs were deemed statistically significant at the p≤.05 level.
Given the skewed distribution in total SOPS score (toward low ratings), we used Spearman partial correlations to analyze the relationships between MRI brain volumes and follow-up prodromal symptoms in relatives. Intracranial volume and age were entered as covariates in these correlation analyses.
Results
Relatives, patients and control subjects were comparable with respect to age (Means=19.9 years (SD=4.1), 20.8 years (SD=4.5) and 20.9 years (SD=3.5) respectively) and parental socioeconomic status (modified Hollingshead scale (Hollingshead and Redlich 1958) Means=2.7 (SD=0.6), 2.9 (SD=0.7) and 2.7 (SD=0.4) respectively) (F≤1.57, df=2,137, p≥0.21).
MRI brain volumes in young, non-psychotic relatives
The joint omnibus test assessing all 4 primary ROIs simultaneously found a significant main group effect (F=7.11, df=6,260, p<0.0001). Least square means for these primary ROIs broken down by comparison groups are summarized in Table 2. There were statistically significant main effects of group on whole brain GM, whole brain WM, whole brain CSF and lateral ventricles (F≥4.45, df=2,137, p≤0.01). Compared to patients, relatives had significantly larger whole brain GM volumes and smaller whole brain CSF and lateral ventricles (T≥3.33, df=90, p≤0.001). Whole brain WM volumes did not differ significantly between relatives and patients (T=0.26, df=90, p=0.79). Compared to control subjects, relatives as well as patients had significantly larger whole brain WM volumes (T≥2.42, df=90, p≤0.02). Relatives had smaller whole brain GM volumes than controls; this approached but did not achieve statistical significance (T=1.74, df=90, p=0.08). Whole brain CSF and lateral ventricles did not differ significantly between relatives and controls (T≤0.55, df=90, p≥0.58). Patients had significantly smaller whole brain GM and larger whole brain CSF and lateral ventricles than controls (T≥3.09, df=90, p≤0.002).
Table 2.
Comparison of MRI primary brain regions of interest (least square mean volumes (SD)) between control subjects with no family history of schizophrenia, relatives of schizophrenia probands and schizophrenia patients
| Primary Regions of Interest | Controls (N=46) | Relatives (N=46) | Patients (N=46) | Group a F 2,137 (p) | Pair-wise Group Comparison b T90 (p) |
||
|---|---|---|---|---|---|---|---|
| Relatives v Controls | Relatives v Patients | Controls v Patients | |||||
| Whole Brain Gray Matter | 802.8 (32.5) | 790.0 (33.5) | 762.7 (37.2) | 15.68 (<.0001) | .08 | .0003 | <.0001 |
| Whole Brain White Matter | 499.2 (31.8) | 515.8 (29.0) | 517.7 (35.7) | 4.45 (.01) | .02 | .79 | .008 |
| Whole Brain CSF | 89.9 (32.0) | 85.1 (32.1) | 110.6 (35.0) | 7.58 (.0008) | .58 | .0005 | .002 |
| Lateral ventricles | 11.8 (4.4) | 11.9 (7.4) | 16.8 (7.9) | 7.79 (.0006) | .90 | .001 | .0006 |
Main effects of group membership (covariates of intracranial volume, age and gender)
Independent sample t-tests
Least square means for the secondary ROIs broken down by comparison groups are summarized in Table 3. There were statistically significant main effects of group on frontal, temporal and parietal GM volumes (F≥12.67, df=2,137, p≤0.0001), but not for occipital GM. Post hoc pair-wise group comparisons indicate that relatives had significantly smaller frontal GM volumes than controls, and significantly larger frontal GM volumes than patients (T=2.08 or 3.97 respectively, df=90, p≤0.04). Temporal, parietal and occipital GM volumes among relatives were also intermediate between controls and patients, but did not differ significantly from controls (T≤1.39, df=90, p≥0.17). Compared to patients, relatives subjects had significantly larger temporal and parietal GM volumes as well (T≥3.97, df=90, p≤0.0001).
Table 3.
Comparison of MRI secondary brain regions of interest (least square mean volumes (SD)) between control subjects with no family history of schizophrenia, relatives of schizophrenia probands and schizophrenia patients
| Secondary Regions of Interest | Controls (N=46) | Relatives (N=46) | Patients (N=46) | Group a F2,137 (p) | Pair-wise Group Comparison b T90 (p) |
||
|---|---|---|---|---|---|---|---|
| Relatives v Controls | Relatives v Patients | Controls v Patients | |||||
| Lobar Gray Matter | |||||||
| Frontal | 261.0 (14.9) | 253.8 (16.2) | 240.1 (17.3) | 19.20 (<.0001) | .04 | .0001 | <.0001 |
| Temporal | 164.3 (6.8) | 162.1 (7.0) | 155.7 (8.6) | 15.75 (<.0001) | .17 | .0001 | <.0001 |
| Parietal | 139.0 (8.5) | 138.6 (7.7) | 131.2 (8.3) | 12.67 (<.0001) | .80 | <.0001 | <.0001 |
| Occipital | 74.2 (6.9) | 72.6 (6.3) | 71.4 (6.4) | 2.05 (.13) | .24 | .42 | .05 |
| Lobar White Matter | |||||||
| Frontal | 178.5 (15.4) | 180.6 (15.2) | 182.6 (17.0) | 0.65 (.52) | .52 | .59 | .23 |
| Temporal | 69.9 (7.1) | 71.5 (5.2) | 73.9 (6.3) | 4.59 (.01) | .23 | .08 | .003 |
| Parietal | 109.9 (8.7) | 116.0 (9.8) | 114.6 (9.0) | 5.47 (.005) | .002 | .46 | .02 |
| Occipital | 46.1 (5.0) | 48.0 (4.7) | 48.8 (5.4) | 3.30 (.04) | .08 | .48 | .01 |
Main effects of group membership (covariates of intracranial volume, age and gender)
Independent sample t-tests
There were significant main effects of group on temporal, parietal and occipital WM volumes (Table 3; F≥3.30, df=2,137, p≤0.04), but not for frontal WM. Relatives had significantly larger parietal WM volumes than controls (T=3.15, df=90, p=0.002). Frontal, temporal and occipital WM volumes did not differ significantly between relatives and controls (T≤1.76, df=90, p≤0.08). Patients had significantly larger temporal, parietal and occipital WM volumes (T≥2.42, df=90, p≤0.02) but not for frontal WM (T=1.19, df=90, p=0.23) than controls. Lobar WM volumes also did not differ significantly between relatives and patients (T≤1.78, df=90, p≥0.08).
Because other psychiatric disorders (e.g. major depressive disorder and attention deficit hyperactivity disorder) have also been associated with brain volume deficits, the analyses were repeated after excluding relatives with Axis I disorders. Restricting the relatives sample to only those 35 relatives without any psychiatric disorders did not change the results reported above (details available upon request). Furthermore, to account for potential confounds that may have arisen from correlations of brain volume among family members, we re-ran the analyses with family clustering as an additional covariate. The family clustering covariate was entered as a class variable with 14 levels (1 to 13 coded for the 13 families that contributed at least 2 family members to the study sample; the 14th level was for all remaining unrelated subjects). Again, inclusion of family clustering covariate did not change the results reported above (details available upon request).
Relationships between intake MRI brain volumes and follow-up prodromal symptoms
Thirty-five relatives had SOPS ratings at follow-up assessment. The mean duration between MRI brain scan and last available follow-up SOPS ratings was 1.08 years (SD=0.80). Mean total SOPS score was 2.8 (SD=7.1; Median=0, 25th–75th interquartile range=2). Most subjects either had no prodromal symptoms (N=19) or had prodromal symptoms that were no greater than ‘Mild’ severity (N=13) (Figure 1). If present, prodromal symptoms were predominantly from the Negative or General symptom domains. The only relative subject who endorsed psychotic symptoms has developed probable schizophrenia at follow-up assessment.
Figure 1.
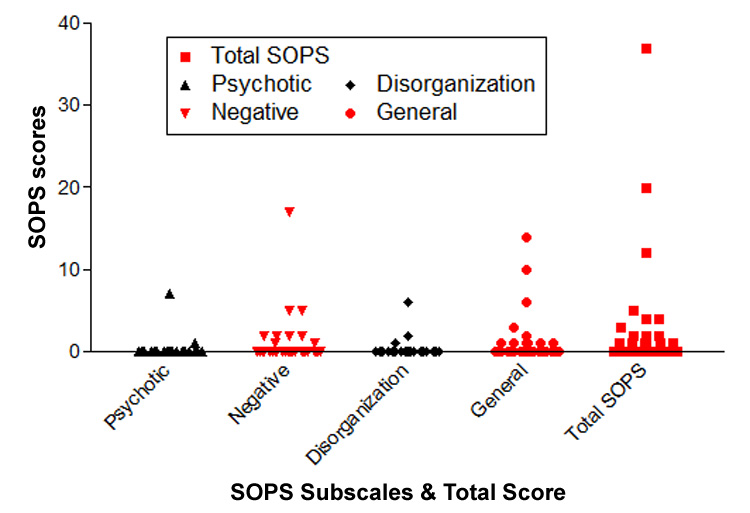
Severity of prodromal symptoms in 35 young, nonpsychotic relatives of schizophrenia probands assessed using the Scale of Prodromal Symptoms (SOPS) on average one year after MRI brain scan.
The associations between MRI brain volumes and the SOPS total scores one year later are summarized in Figure 2 and Figure 3. Greater severity in SOPS total score correlated significantly with smaller total, frontal, temporal and parietal GM volumes (Figure 2; Spearman r ≤ −0.35, df=33, p≤0.04). Greater severity in SOPS total score was also significantly associated with larger total, frontal and temporal WM volumes (Figure 3; Spearman r ≥0.35, df=33, p≤0.05).
Figure 2.
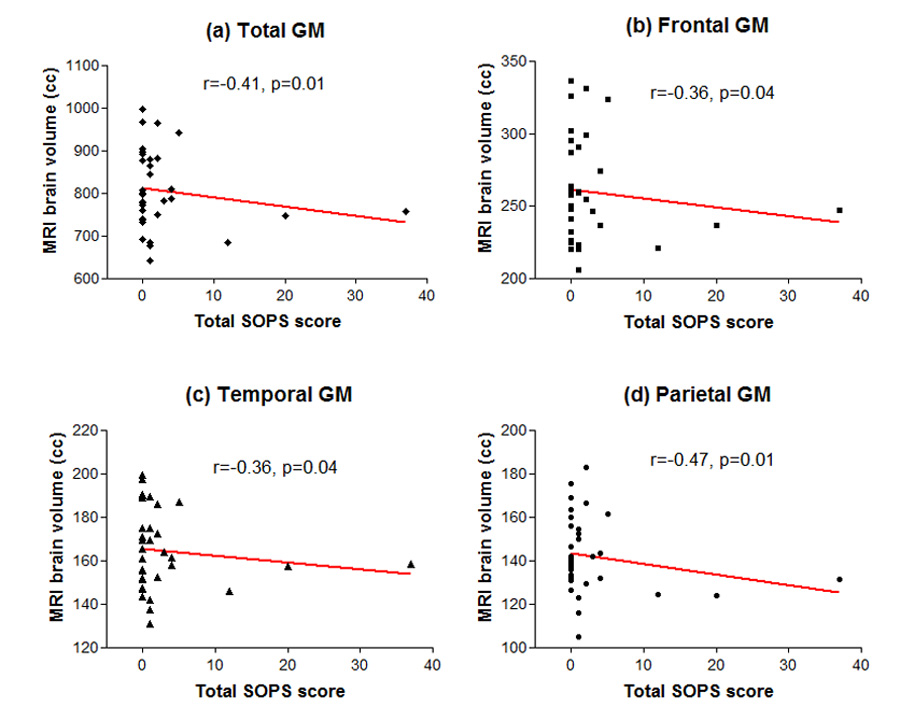
Relationships between initial MRI brain gray matter (GM) volumes and severity of subsequent prodromal symptoms (total score on the Scale of Prodromal Symptoms): (a) Total whole brain GM (b) Frontal Lobe GM (c) Temporal Lobe GM (d) Parietal Lobe GM
Figure 3.
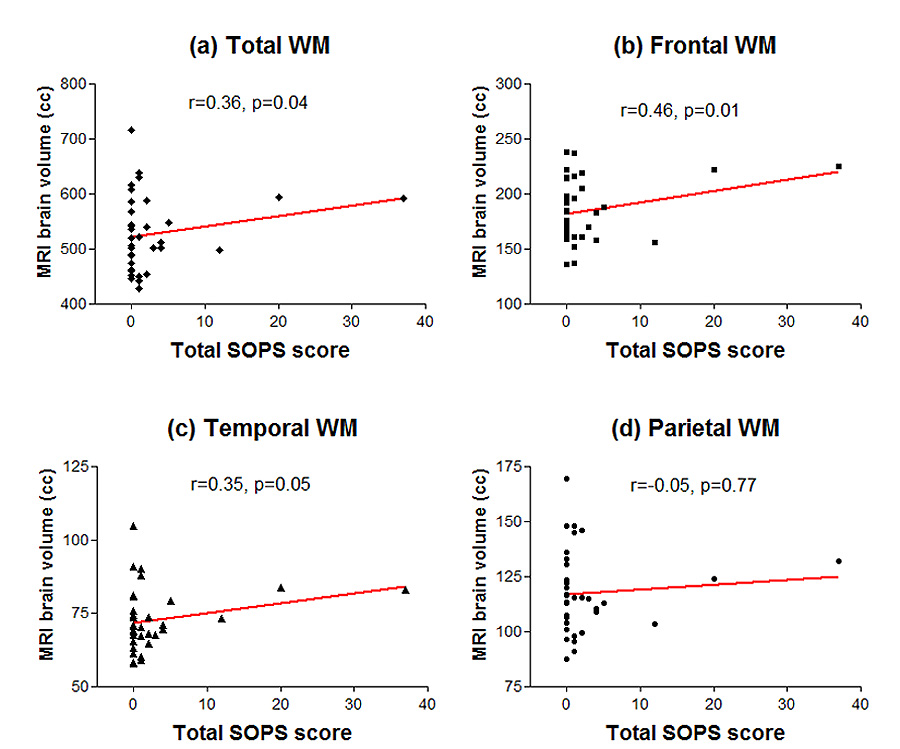
Relationships between initial MRI brain white matter (WM) volumes and severity of subsequent prodromal symptoms (total score on the Scale of Prodromal Symptoms): (a) Total whole brain WM (b) Frontal Lobe WM (c) Temporal Lobe WM (d) Parietal Lobe WM
Discussion
In this study, we examined MRI brain volumes of nonpsychotic, adolescent or young adult relatives of schizophrenia probands who were still within the age range at-risk for developing schizophrenia. Brain volumes in these young, unaffected first- or second-degree relatives were intermediate between those of schizophrenia patients and healthy volunteers without family history of schizophrenia. Compared to patients, at-risk relatives had significantly larger whole brain GM as well as larger frontal, temporal and parietal GM volumes. Compared to healthy volunteers, relatives also had significantly smaller frontal GM volumes, and (at a trend level) smaller whole brain GM. Whole brain WM (specifically parietal WM) volumes were significantly larger in relatives compared to healthy volunteers. WM volumes did not differ significantly between relatives and patients. Relatives had significantly smaller whole brain CSF volumes and lateral ventricles than patients, but these did not differ from healthy volunteers. More interestingly, on prodromal symptoms assessed approximately one year after MRI brain scans, these brain volume abnormalities in adolescent or young adult relatives (i.e. smaller GM as well as larger WM volumes) correlated significantly with greater severity of prodromal symptoms.
In general, our findings are consistent with previous MRI studies from five independent samples (Pittsburgh, Edinburgh, Ulm, Bethesda and Orangeburg/New York) of genetic high-risk individuals still within the age range for developing schizophrenia (DeLisi et al. 2006; Gogtay et al. 2003; Job et al. 2003; Job et al. 2005b; Job et al. 2006; Keshavan et al. 1997; Keshavan et al. 2002b ; Lawrie et al. 1999; Lawrie et al. 2001; Lawrie et al. 2002; Rajarethinam et al. 2004; Schreiber et al. 1999). Even though these young relatives do not have psychotic disorders, they have volume deficits in similar brain regions as schizophrenia patients - albeit less severe. As a group, unaffected relatives have global as well as regional (primarily fronto-temporal) GM brain volume deficits. Unlike our study, most previous studies have examined smaller brain regions, especially structures in the medial temporal lobe. The most consistently reported abnormalities in young relatives of schizophrenia probands have been smaller hippocampus, amygdala and parahippocampus gyrus when compared to healthy volunteers with no family history. Additionally, larger third ventricles (Keshavan et al. 1997; Lawrie et al. 2001), smaller thalamus (Lawrie et al. 1999), smaller anterior cingulate (Job et al. 2003), smaller superior temporal gyrus (Rajarethinam et al. 2004) have also been reported. In this study, relatives subjects did not differ significantly from healthy controls on temporal lobe GM volume even though these were intermediate between the other 2 comparison groups. Since our brain volume measures were only at the lobar level, our study may not have been sensitive enough to detect volume deficits within the smaller medial temporal structures. Despite this limitation, we still found smaller frontal GM volumes in relatives, which is consistent with the Gogtay et al study where siblings of childhood-onset schizophrenia patients also had smaller frontal GM and total GM than healthy controls (Gogtay et al. 2003). Using a novel and potentially more sensitive MR imaging technique of diffusion weighted imaging, DeLisi et al found nonpsychotic young relatives of schizophrenia probands had increased apparent diffusion coefficients suggestive of volume reductions in the left parahippocampal, lingual, superior frontal and middle frontal gyri (DeLisi et al. 2006).
While GM volume deficits in our young nonpsychotic relatives are consistent with previous studies, the finding of enlarged WM volumes was not predicted initially. To the best of our knowledge, this is the first study reporting enlarged WM volumes in nonpsychotic adolescent or young adult relatives of schizophrenia probands. Previous studies examining WM abnormalities in nonpsychotic relatives involved relatives who were already in their 30s to 40s, and have found either smaller WM volumes (McIntosh et al. 2006) or no difference (Cannon et al. 1998; McIntosh et al. 2005; Schneider-Axmann et al. 2006; Staal et al. 2000). Our observation of significantly larger parietal WM volumes among relatives is unlikely to be a chance finding since schizophrenia patients in this study also had significantly greater whole brain, temporal, parietal and occipital WM volumes than healthy volunteers. More importantly, both GM deficits as well as WM enlargements in relatives were associated with greater everity of subsequent prodromal symptoms. Taken together, enlarged WM volumes in relatives subjects are likely to be of relevance to the pathogenesis of schizophrenia.
Although a recent meta-analysis estimated a 1% reduction in whole brain WM volume among schizophrenia patients (Wright et al. 2000), patient-control MRI volumetric studies have reported conflicting findings: no significant group differences in WM volumes (e.g. (Gur et al. 1999; Gur et al. 2000; Zipursky et al. 1992), regional WM reductions (e.g. (Buchsbaum et al. 1998; Marsh et al. 1997) as well as WM enlargements (e.g. (Highley et al. 2003; Shenton et al. 1992; Wible et al. 1995) in schizophrenia patients. That both unaffected relatives and schizophrenia patients in our study had larger WM volumes in the posterior (temporal, parietal and occipital lobes) but not the anterior (frontal lobe) portions of the brain than healthy volunteers may be related to aberrant WM maturation during adolescence and early adulthood. Unlike GM brain volume, WM volume continues to increase during adolescence in a back-to-front wave (Giedd et al. 1999; Paus et al. 1999; Pfefferbaum et al. 1994; Sowell et al. 1999; Sowell et al. 2002). During early to mid-adulthood, WM volumes plateau during the fifth decade of life with the frontal lobe being the last to mature (Mathalon et al. 2001; Sowell et al. 2003). Schizophrenia patients do not show this normal age-related expansion in WM volume (Bartzokis et al. 2003). In fact, schizophrenia patients may actually start out having abnormally enlarged WM brain volumes during adolescence and early adulthood (Bartzokis et al. 2003). Because patients do not show the normal age-related WM volume expansion during early to mid-adulthood, patient-control differences in WM volumes become increasingly more evident with age such that WM volume reductions are most marked by the fifth decade of life (Bartzokis et al. 2003). Thus, our finding of enlarged WM volumes in nonpsychotic relatives and in schizophrenia patients, all of whom were either adolescents or still in their early twenties, may be related to dysmaturation in myelination.
The underlying basis for these brain volume deficits observed in young nonpsychotic relatives of schizophrenia probands are not well understood. A plausible and often cited explanation is that these less severe morphometric abnormalities are primarily due to the genetic factors of schizophrenia, and with additional contributions from yet unknown environmental effects that relatives and schizophrenia probands share (Seidman and Wencel 2003). That schizophrenia susceptibility genes have major influences on these intermediary brain volume abnormalities in nonpsychotic relatives is supported by indirect evidence from several lines of investigations: schizophrenia is a genetic disorder (Kendler and Robinette 1983; Sullivan et al. 2003), subtle but well-replicated global and regional brain volume deficits are a core feature of schizophrenia (Honea et al. 2005; Pantelis et al. 2005; Steen et al. 2006; Vita et al. 2006; Wright et al. 2000), and brain volume is a heritable trait (Narr et al. 2002; Pfefferbaum et al. 2004; Thompson et al. 2001). In line with the prevailing concept of schizophrenia as a neurodevelopmental disorder (Murray and Lewis 1987; Weinberger 1987), it has been further postulated that aberrant synaptic pruning, programmed cell death and/or synaptic plasticity affecting schizophrenia patients (Feinberg 1982) may also have similar but less severe effects on reducing fronto-temporal brain volumes in nonpsychotic relatives (Keshavan et al. 1997). With recent advances in the complex genetics of schizophrenia (Harrison and Weinberger 2005) and the current interest in genotype-phenotype association studies (Ho et al. 2005b), this hypothetical relationship between schizophrenia susceptibility genes and brain structural and functional abnormalities in nonpsychotic relatives are now beginning to be tested more directly (Hall et al. 2006; McIntosh et al. 2007). Of particular interest would be genes whose products mediate neurobiological processes involved in late maturational events of synaptic remodeling and myelination.
While shared genetic factors and aberrant neurodevelopment may be the underlying basis for intermediary brain volume abnormalities in unaffected young relatives of schizophrenia probands, there is now increasing evidence that such abnormalities ascertained using morphometric MR imaging may also be pertinent biomarkers useful for the early identification of schizophrenia. In our study, GM volume deficits and larger WM volumes in young relatives subjects assessed at intake correlated significantly with greater severity of prodromal symptoms a year later. Although limited by small sample size, short follow-up duration and the absence of prodromal symptom ratings at baseline assessment, these associations between brain volume abnormalities and prodromal symptoms are intriguing. And as we enroll more subjects into our study, and as we continue our longitudinal assessments of these relatives, initial GM volume deficits and greater WM volumes may prove to be indicators of future development of schizophrenia.
Studying such a genetic high-risk population of nonpsychotic, young relatives, who are still within age range for developing schizophrenia, is a potentially fruitful research strategy from the standpoint of early identification of schizophrenia (Johnstone et al. 2005; Keshavan et al. 2005). By contrasting the neurodevelopmental trajectories between young relatives who go on to develop schizophrenia against relatives who remain well, we can better appreciate the early manifestations of schizophrenia which, in turn, could lead to early identification and intervention. However, such a longitudinal study of young relatives is relatively inefficient since a large sample is needed to obtain a sufficient numbers of schizophrenia subjects. Nevertheless, convergent findings from genetic high-risk studies and from clinical high-risk samples identified through having at-risk mental states (Falloon 1992; McGlashan and Johannessen 1996; McGorry et al. 1996) suggest that such a longitudinal study of young relatives is feasible, and can aid in the development of multi-stage screening programs for early identification of schizophrenia. In the Edinburgh high-risk study, longitudinal reductions in temporal and cerebellar gray matter densities prior to overt clinical symptoms of schizophrenia appear to have good predictive power in identifying young relatives who went on to develop schizophrenia (Job et al. 2005a; Job et al. 2006). These premorbid medial temporal lobe changes in genetic high-risk subjects are consistent with findings from the clinical high-risk or prodromal studies (Borgwardt et al. 2007; Pantelis et al. 2003), where ultra high-risk subjects who subsequently developed psychotic disorders had smaller baseline hippocampus, parahippocampus gyrus, and superior temporal gyrus volumes as well as longitudinal reductions in parahippocampus and fusiform gyri (Pantelis et al. 2003).
Thus, structural brain volume abnormalities, especially longitudinal medial temporal lobe volume reductions, have predictive validity in at-risk individuals. Its predictive value will likely further improve when combined with other known clinical risk factors (e.g. prodromal symptoms, poor scholastic test scores) and biological/genetic risk factors (e.g. endophenotypic traits and genetic allelic variations) into more sophisticated, multifactorial and multi-stage screening programs for the early detection of schizophrenia (Ho et al. 2005a).
Acknowledgement
This research was supported in part by NIMH Grants MH68380 and MH31593, NARSAD Young Investigator Award and NIH /NCRR GCRC Program M01-RR-59. The author thanks Lindsey Fuhrmeister, Helen Keefe, Michael Kiguta, Julie Koeppel, and Ronald Pierson for their kind assistance with imaging data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (cash). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49(8):615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, 2nd, Flashman LA, O'Leary DS, Ehrhardt JC, Yuh WT. Automatic atlas-based volume estimation of human brain regions from mr images. J Comput Assist Tomogr. 1996;20(1):98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Baare WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58(1):33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: A magnetic resonance imaging study. Biol Psychiatry. 2003;53(5):412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pfluger M, Rechsteiner E, D'Souza M, Stieglitz R-D, Radu E-W, McGuire PK. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61(10):1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon JH, Atlas SW. Mri white matter diffusion anisotropy and pet metabolic rate in schizophrenia. Neuroreport. 1998;9(3):425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55(12):1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59(1):35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- Chua SE, Sharma T, Takei N, Murray RM, Woodruff PW. A magnetic resonance imaging study of corpus callosum size in familial schizophrenic subjects, their relatives, and normal controls. Schizophr Res. 2000;41(3):397–403. doi: 10.1016/s0920-9964(99)00081-x. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, Branch CA, Ardekani BA. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry Res. 2006;148(1):61–66. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Boronow JJ, Ringel JJ, Parente F. Social functioning and neurocognitive deficits in outpatients with schizophrenia: A 2-year follow-up. Schizophr Res. 1999;37(1):13–20. doi: 10.1016/s0920-9964(98)00134-0. [DOI] [PubMed] [Google Scholar]
- Falloon IR. Early intervention for first episodes of schizophrenia: A preliminary exploration. Psychiatry. 1992;55(1):4–15. doi: 10.1080/00332747.1992.11024572. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Flaum MA, Andreasen NC, Arndt S. The iowa prospective longitudinal study of recent-onset psychoses. Schiz Bull. 1992;18(3):481–490. doi: 10.1093/schbul/18.3.481. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal mri study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17(2):415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Greenstein D, Lenane M, Gochman PA, Zijdenbos A, Rapoport JL. Structural brain mri abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am J Psychiatry. 2003;160(3):569–571. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gu RE. Sex differences inbrain gray and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57(8):761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, Lawrie SM. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9(12):1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in mri: A three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23(1):144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Harris JG, Young DA, Rojas DC, Cajade-Law A, Scherzinger A, Nawroz S, Adler LE, Cullum CM, Simon J, Freedman R. Increased hippocampal volume in schizophrenics' parents with ancestral history of schizophrenia. Schizophr Res. 2002;55(1–2):11–17. doi: 10.1016/s0920-9964(01)00272-9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(pt4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, McGlashan TH, Quinlan D, Miller TJ, Perkins DO, Zipursky RB, Addington J, Woods SW. Factorial structure of the scale of prodromal symptoms. Schiz Res. 2004;68(2–3):339. doi: 10.1016/S0920-9964(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Highley JR, DeLisi LE, Roberts N, Webb JA, Relja M, Razi K, Crow TJ. Sex-dependent effects of schizophrenia: An mri study of gyral folding, and cortical and white matter volume. Psychiatry Res. 2003;124(1):11–23. doi: 10.1016/s0925-4927(03)00076-3. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Fuller R, Arndt S, Cadoret RJ. Secondary prevention of schizophrenia: Utility of standardized scholastic tests in early identification. Ann Clin Psychiatry. 2005a;17(4):11–18. doi: 10.1080/10401230590905272. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-o-methyl transferase val158met gene polymorphism in schizophrenia: Working memory, frontal lobe mri morphology and frontal cerebral blood flow. Mol Psychiatry. 2005b;10(3):229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: A community study. New York: John Wiley & Sons, Inc; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64(1):1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005a;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Structural imaging as an early diagnostic test in people at high risk of schizophrenia. Schiz Res. 2005b;81 Suppl:S14–S15. [Google Scholar]
- Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: Findings from the edinburgh high-risk study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Robinette CD. Schizophrenia in the national academy of sciences-national research council twin registry: A 16-year update. Am J Psychiatry. 1983;140(12):1551–1563. doi: 10.1176/ajp.140.12.1551. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, Sweeney JA. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: Preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1285–1295. doi: 10.1016/s0278-5846(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002a;58(2–3):173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol Psychiatry. 2002b;26(6):1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: A selective review and update. Schizophrenia Research. 2005;79(1):45–47. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353(9146):30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJ, Owens DG, Johnstone EC. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49(10):811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Miller P, Best JJK, Owens DGC, Johnstone EC. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181(2):138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- Magnotta V, Johnson HJ, Eccher D, Pfalzgraf C, Keefe H. Brains2 users guide University of Iowa. Iowa City. 2003 [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WTC, Heckel D. Structural mr image processing using the brains2 toolbox. Comput Med Imaging Graph. 2002;26(4):251. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122(3):153–167. doi: 10.1016/s0925-4927(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, Csernansky JG, DeMent, S, Faustman WO, Sullivan EV, Pfefferbaum A. Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Arch Gen Psychiatry. 1997;54(12):1104–1112. doi: 10.1001/archpsyc.1997.01830240060009. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(2):148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- McDonald C, Grech A, Toulopoulou T, Schulze K, Chapple B, Sham P, Walshe M, Sharma T, Sigmundsson T, Chitnis X, Murray RM. Brain volumes in familial and non-familial schizophrenic probands and their unaffected relatives. Am J Med Genet. 2002;114(6):616–625. doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163(3):478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Johannessen JO. Early detection and intervention with schizophrenia: Rationale. Schizophr Bull. 1996;22(2):201–222. doi: 10.1093/schbul/22.2.201. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ. Eppic: An evolving system of early detection and optimal management. Schizophr Bull. 1996;22(2):305–326. doi: 10.1093/schbul/22.2.305. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56(8):544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. Whitematter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58(3):254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141(1):76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GKS, Moorhead TWJ, Owens DGC, Miller P, Porteous D, Lawrie SM, Johnstone EC. Relationship of catechol-o-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol Psychiatry. 2007;61(10):1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran R, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J Clin Res Ed. 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerksjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis. 2002;11(1):83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR. Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res. 1996;22(1):27–40. doi: 10.1016/0920-9964(96)00046-1. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Florencio PS, Gagnon D, Wolff AV, Benkelfat C, Mikula L, Lal S, Evans AC. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res. 2001;107(2):75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis : A cross-sectional and longitudinal mri comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31(3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiol Aging. 2004;25(2):175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161(6):1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Schneider-Axmann T, Kamer T, Moroni M, Maric N, Tepest R, Dani I, Honer WG, Scherk H, Rietschel M, Schulze TG, Muller DJ, Cordes J, Schonell H, Steinmetz H, Gaebel W, Vogeley K, Kuhn KU, Wagner M, Maier W, Traber F, Block W, Schild HH, Falkai P. Relation between cerebrospinal fluid, gray matter and white matter changes in families with schizophrenia. J Psychiatr Res. 2006;40(7):646–655. doi: 10.1016/j.jpsychires.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Baur-Seack K, Kornhuber HH, Wallner B, Friedrich JM, De Winter IM, Born J. Brain morphology in adolescents at genetic risk for schizophrenia assessed by qualitative and quantitative magnetic resonance imaging. Schizophr Res. 1999;40(1):81–84. doi: 10.1016/s0920-9964(99)00026-2. [DOI] [PubMed] [Google Scholar]
- Schulze K, McDonald C, Frangou S, Sham P, Grech A, Toulopoulou T, Walshe M, Sharma T, Sigmundsson T, Taylor M, Murray RM. Hippocampal volume in familial and nonfamilial schizophrenic probands and their unaffected relatives. Biol Psychiatry. 2003;53(7):562–570. doi: 10.1016/s0006-3223(02)01910-8. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, Hoge EA, Kennedy D, Makris N, Caviness VS, Tsuang MT. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: A pilot magnetic resonance imaging study. Am J Med Genet. 1997;74(5):507–514. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT. Thalamic and amygdale-hippocampal volume reductions in first-degree relatives of patients with schizophrenia : An mri-based morphometric analysis. Biol Psychiatry. 1999;46(7):941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: A magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59(9):839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Wencel HE. Genetically mediated brain abnormalities in schizophrenia. Curr Psychiatry Rep. 2003;5(5):135–144. doi: 10.1007/s11920-003-0030-4. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives--the maudsley family study. Schizophr Res. 1999;40(2):111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Wible CG, McCarley RW. A review of magnetic resonance imaging studies of brain anomalies in schizophrenia. In: Krishnan KRR, Doraiswamy PM, editors. Brain imaging in clinical psychiatry. New York: Marcel Dekker; 1997. pp. 297–380. [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural mri study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack H, van der Schot AC, Kahn RS. Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry. 1998;155(12):1784–1786. doi: 10.1176/ajp.155.12.1784. [DOI] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157(3):416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- Steel RM, Whalley HC, Miller P, Best JJ, Johnstone EC, Lawrie SM. Structural mri of the brain in presumed carriers of genes for schizophrenia, their affected and unaffected siblings. J Neurol Neurosurg Psychiatry. 2002;72(4):455–458. doi: 10.1136/jnnp.72.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322(12):789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54(11):1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy J, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159(9):1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: A meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82(1):75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Tepest R, Pfeiffer U, Schneider-Axmann T, Maier W, Honer WG, Falkai P. Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: A morphometric mri study. Am J Psychiatry. 2001;158(3):494–496. doi: 10.1176/appi.ajp.158.3.494. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW. Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch Gen Psychiatry. 1995;52(4):279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49(3):195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]


