Abstract
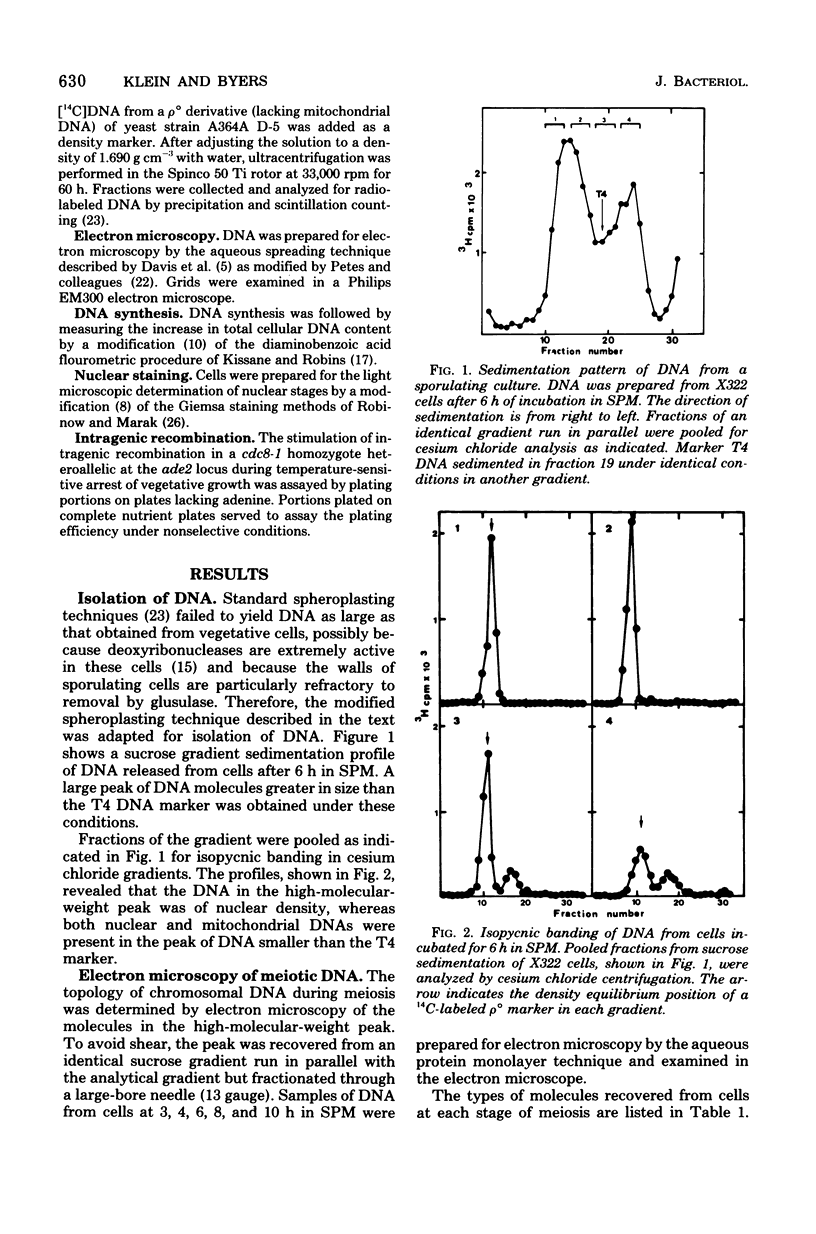
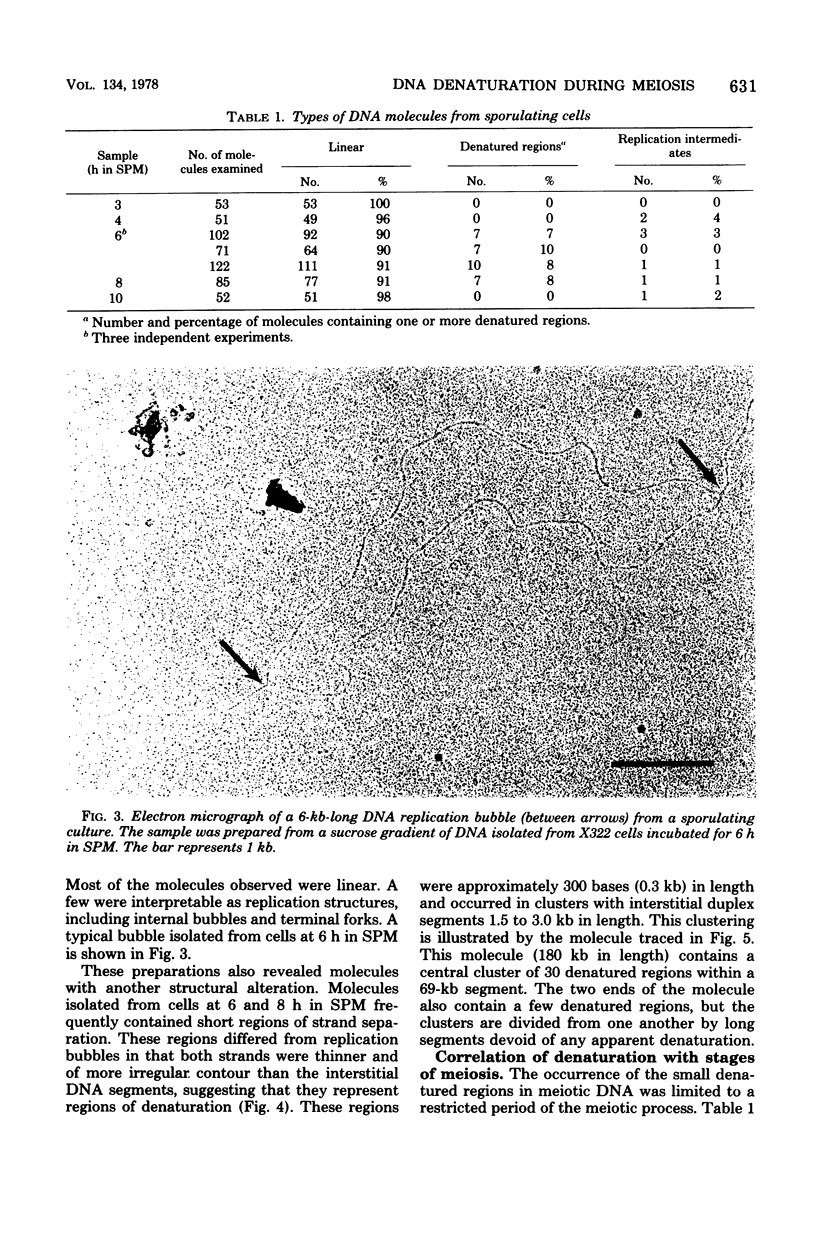
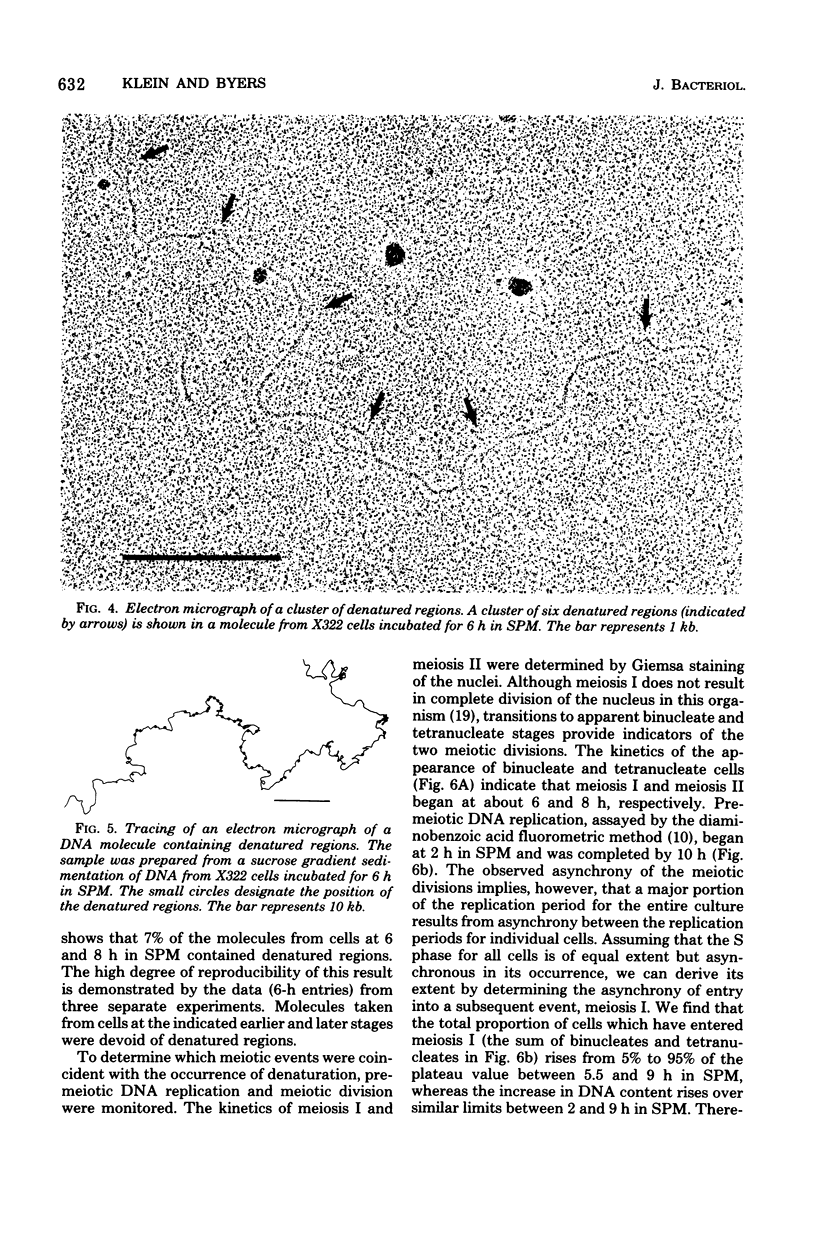
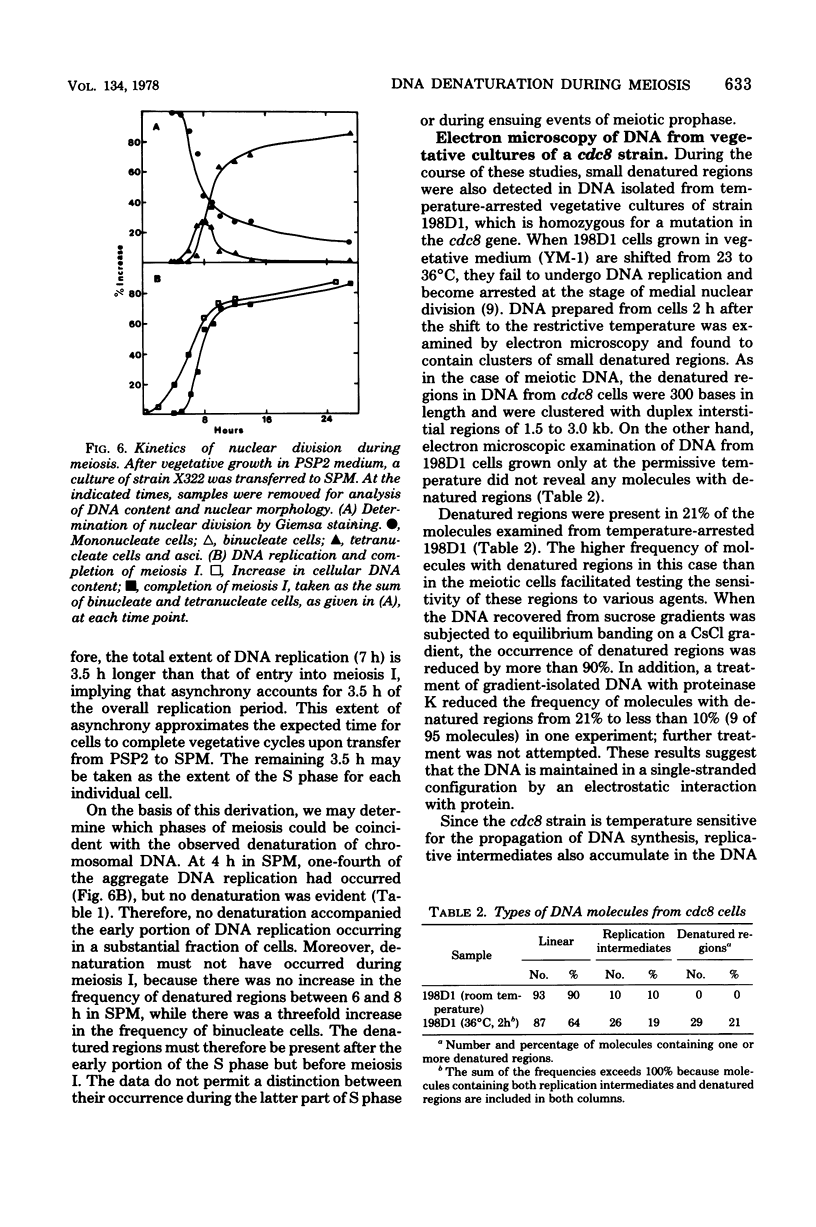
Partial denaturation of Saccharomyces cerevisiae chromosomal DNA was found to occur spontaneously during meiosis. Short regions of strand separation (300 base pairs long) were seen in DNA molecules prepared for electron microscopy by the aqueous spreading technique. These regions were clustered along the DNA. The time course of their appearance indicated that the denatured regions were present during the periods of premeiotic DNA replication and recombination. A similar pattern of denaturation was also detected in the DNA from vegetatively grown cells of a conditional cdc8 mutant, which is defective in DNA replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Bick M. D., Lee C. S., Thomas C. A., Jr Local destabilization of DNA during transcription. J Mol Biol. 1972 Oct 28;71(1):1–9. doi: 10.1016/0022-2836(72)90395-6. [DOI] [PubMed] [Google Scholar]
- Brack C., Bickle T. A., Yuan R. The relation of single-stranded regions in bacteriophage PM2 supercoiled DNA to the early melting sequences. J Mol Biol. 1975 Aug 25;96(4):693–702. doi: 10.1016/0022-2836(75)90146-1. [DOI] [PubMed] [Google Scholar]
- Broker T. R. An electron microscopic analysis of pathways for bacteriophage T4 DNA recombination. J Mol Biol. 1973 Nov 25;81(1):1–16. doi: 10.1016/0022-2836(73)90243-x. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971 Jul 14;59(1):183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Periodic density fluctuation during the yeast cell cycle and the selection of synchronous cultures. J Bacteriol. 1970 Dec;104(3):1280–1285. doi: 10.1128/jb.104.3.1280-1285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Stern H. A DNA-binding protein in meiotic cells of Lilium. Dev Biol. 1971 Sep;26(1):87–99. doi: 10.1016/0012-1606(71)90110-2. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Structure of branch points in replicating DNA: presence of single-stranded connections in lambda DNA branch points. J Mol Biol. 1971 Mar 14;56(2):319–325. doi: 10.1016/0022-2836(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Kleinschmidt A. K. Locating interrupted hydrogen bonding in the secondary structure of PM2 circular DNA by comparative denaturation mapping. J Virol. 1974 Jun;13(6):1176–1185. doi: 10.1128/jvi.13.6.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. K., Pinon R., Esposito R. E., Esposito M. S. Single-strand scissions of chromosomal DNA during commitment to recombination at meiosis. Proc Natl Acad Sci U S A. 1975 May;72(5):1887–1891. doi: 10.1073/pnas.72.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kavenoff R., Zimm B. H. Chromosome-sized DNA molecules from Drosophila. Chromosoma. 1973;41(1):1–27. doi: 10.1007/BF00284071. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic Mapping in Saccharomyces IV. Mapping of Temperature-Sensitive Genes and Use of Disomic Strains in Localizing Genes. Genetics. 1973 May;74(1):33–54. doi: 10.1093/genetics/74.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Gray R. H., Ris H. Meiotic spindle plaques in Saccharomyces cerevisiae. J Cell Biol. 1972 Jun;53(3):837–841. doi: 10.1083/jcb.53.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Byers B., Fangman W. L. Size and structure of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3072–3076. doi: 10.1073/pnas.70.11.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Sedimentation properties of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1188–1191. doi: 10.1073/pnas.69.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Newlon C. S. Structure of DNA in DNA replication mutants of yeast. Nature. 1974 Oct 18;251(5476):637–639. doi: 10.1038/251637a0. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: electron microscopic observation of recombination intermediates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow C. F., Marak J. A fiber apparatus in the nucleus of the yeast cell. J Cell Biol. 1966 Apr;29(1):129–151. doi: 10.1083/jcb.29.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G., Friedmann A. Structure of DNA molecules in yeast meiosis. Nature. 1975 Sep 4;257(5521):64–66. doi: 10.1038/257064a0. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. S., Inman R. B. Visualization of a novel junction in bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3024–3028. doi: 10.1073/pnas.72.8.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Regions of single-stranded DNA in the growing points of replicating bacteriophage T7 chromosomes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2682–2686. doi: 10.1073/pnas.69.9.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Electron microscopic analysis of DNA replication in main band and satellite DNAs of Drosophila virilis. J Mol Biol. 1976 Dec;108(2):305–331. doi: 10.1016/s0022-2836(76)80123-4. [DOI] [PubMed] [Google Scholar]





