Abstract
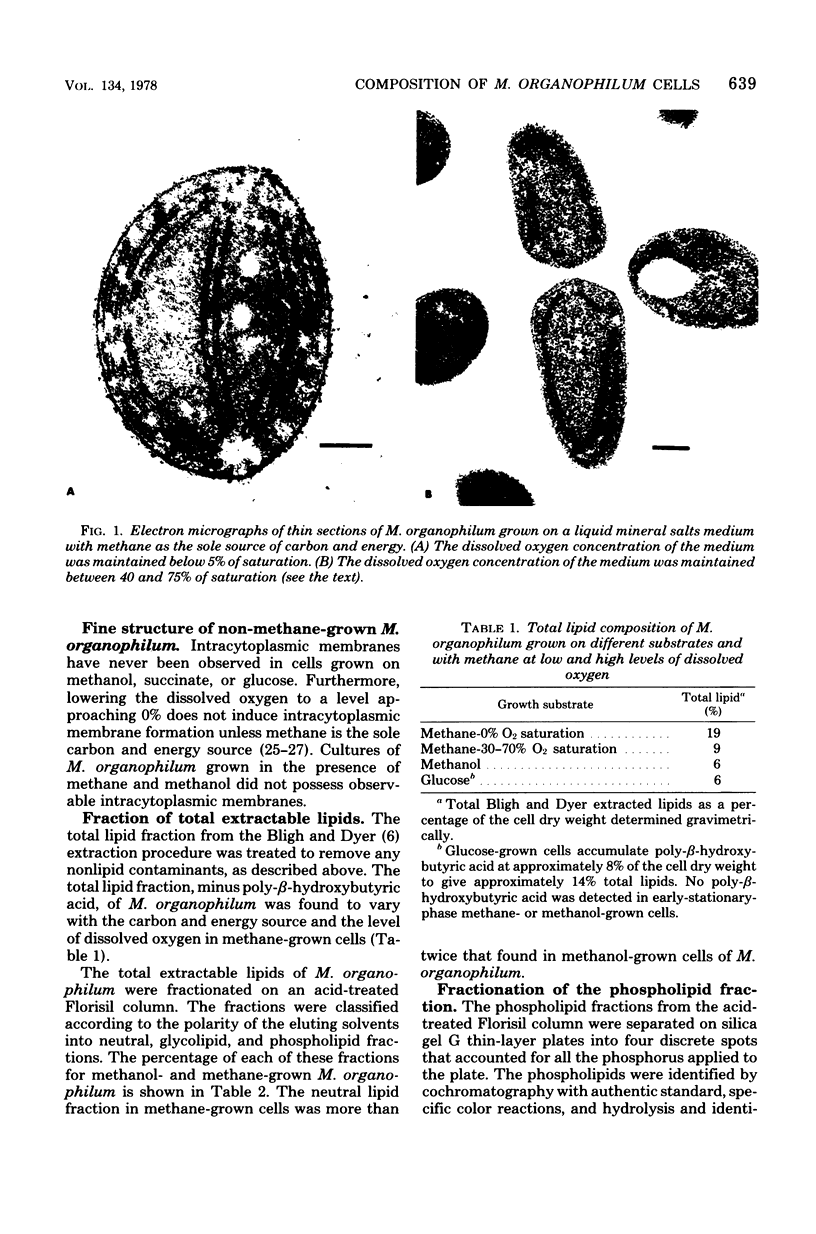
Intracytoplasmic membranes were present in Methylobacterium organophilum when cells were grown with methane, but not methanol or glucose, as the sole carbon and energy source. Cells grown with methane as the carbon and energy source and low levels of dissolved oxygen had the greatest amount of intracytoplasmic membrane. Cells grown with increased levels of dissolved oxygen had less intracytoplasmic membrane. The amount of total lipid correlated with the amount of membrane material observed in thin sections. The individual phospholipids varied in amount, but the same four were present in M. organophilum grown with different substrates and oxygen levels. Phosphatidyl choline was present as a major component of the phospholipids. Sterols were present, and they differed from those in the type I methylotroph Methylococcus capsulatus. The relative amounts of different sterols and squalene changed with the substrate provided for growth. The greatest amounts of sterols were found in methane-grown cells grown at low levels of dissolved oxygen. None of the unusual or usual membrane components assayed was uniquely present in the intracytoplasmic membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Adams B. G., Parks L. W. Evidence for dual physiological formsof ergosterol in Saccharomyces cerevisiae. J Cell Physiol. 1967 Oct;70(2):161–168. doi: 10.1002/jcp.1040700205. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bird C. W., Lynch J. M., Pirt F. J., Reid W. W. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature. 1971 Apr 16;230(5294):473–474. doi: 10.1038/230473a0. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H. Model of interaction of polar lipids, cholesterol, and proteins in biological membranes. Lipids. 1974 Sep;9(9):645–650. doi: 10.1007/BF02532169. [DOI] [PubMed] [Google Scholar]
- CONTI S. F., HIRSCH P. BIOLOGY OF BUDDING BACTERIA. 3. FINE STRUCTURE OF RHODOMICROBIUM AND HYPHOMICROBIUM SPP. J Bacteriol. 1965 Feb;89:503–512. doi: 10.1128/jb.89.2.503-512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- De Boer W. E., Hazeu W. Observations on the fine structure of a methane-oxidizing bacterium. Antonie Van Leeuwenhoek. 1972;38(1):33–47. doi: 10.1007/BF02328075. [DOI] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen P. O., Goldfine H., Williams P. J. Phospholipids of bacteria with extensive intracytoplasmic membranes. Science. 1966 Mar 25;151(3717):1543–1544. doi: 10.1126/science.151.3717.1543. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINETTI G. V., ERBLAND J., STOTZ E. The hydrolysis of lecithins by snake venom phospholipase A. Biochim Biophys Acta. 1959 Jun;33(2):403–414. doi: 10.1016/0006-3002(59)90130-1. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Bond F. T., Thompson E. D., Starr P. R. 8(9),22 -Ergostadiene-3 -ol, an ergosterol precursor accumulated in wild-type and mutants of yeast. J Lipid Res. 1972 May;13(3):311–316. [PubMed] [Google Scholar]
- Pate J. L., Shah V. K., Brill W. J. Internal membrane control in Azotobacter vinelandii. J Bacteriol. 1973 Jun;114(3):1346–1350. doi: 10.1128/jb.114.3.1346-1350.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Hoare D. S., Smith A. J. Ultrastructure of Nitrobacter agilis grown under autotrophic and heterotrophic conditions. J Bacteriol. 1969 Feb;97(2):936–939. doi: 10.1128/jb.97.2.936-939.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Michalover J. L. Methane oxidation by cell-free extracts of Methylococcus capsulatus. FEBS Lett. 1970 Nov 9;11(1):41–44. doi: 10.1016/0014-5793(70)80487-2. [DOI] [PubMed] [Google Scholar]
- SKIPSKI V. P., PETERSON R. F., SANDERS J., BARCLAY M. THIN-LAYER CHROMATOGRAPHY OF PHOSPHOLIPIDS USING SILICA GEL WITHOUT CALCIUM SULFATE BINDER. J Lipid Res. 1963 Apr;4:227–228. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Knowles C. J., Higgins I. J. Properties and partial purification of the methane-oxidising enzyme system from Methylosinus trichosporium. FEBS Lett. 1975 Oct 15;58(1):293–299. doi: 10.1016/0014-5793(75)80282-1. [DOI] [PubMed] [Google Scholar]
- Wadzinski A. M., Ribbons D. W. Oxidation of C1 compounds by particulate fractions from Methylococcus capsulatus: properties of methanol oxidase and methanol dehydrogenase. J Bacteriol. 1975 Jun;122(3):1364–1374. doi: 10.1128/jb.122.3.1364-1374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. L., Patrick M. A., Dugan P. R. Whole-cell and membrane lipids of the methylotrophic bacterium Methylosinus trichosporium. J Bacteriol. 1975 Nov;124(2):602–605. doi: 10.1128/jb.124.2.602-605.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Purification of lipids from nonlipid contaminants on Sephadex bead columns. J Lipid Res. 1966 Jul;7(4):558–561. [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Fine structure of Methanobacterium thermoautotrophicum: effect of growth temperature on morphology and ultrastructure. J Bacteriol. 1973 Jan;113(1):461–467. doi: 10.1128/jb.113.1.461-467.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]