Abstract
Shaker channel mutants, in which the first (R362), second (R365), and fourth (R371) basic residues in the S4 segment have been neutralized, are found to pass potassium currents with voltage-insensitive kinetics when expressed in Xenopus oocytes. Single channel recordings clarify that these channels continue to open and close from −160 to +80 mV with a constant opening probability (P o). Although P o is low (∼0.15) in these mutants, mean open time is voltage independent and similar to that of control Shaker channels. Additionally, these mutant channels retain characteristic Shaker channel selectivity, sensitivity to block by 4-aminopyridine, and are partially blocked by external Ca2+ ions at very negative potentials. Furthermore, mean open time is approximately doubled, in both mutant channels and control Shaker channels, when Rb+ is substituted for K+ as the permeant ion species. Such strong similarities between mutant channels and control Shaker channels suggests that the pore region has not been substantially altered by the S4 charge neutralizations. We conclude that single channel kinetics in these mutants may indicate how Shaker channels would behave in the absence of voltage sensor input. Thus, mean open times appear primarily determined by voltage-insensitive transitions close to the open state rather than by voltage sensor movement, even in control, voltage-sensitive Shaker channels. By contrast, the low and voltage-insensitive P o seen in these mutant channels suggests that important determinants of normal channel opening derive from electrostatic coupling between S4 charges and the pore domain.
Keywords: S4 mutations, voltage sensitivity, charge neutralizations, ion-channel gating
introduction
Voltage-gated ion channels are responsible for the electrical excitability of cells in heart, muscle, and throughout the nervous system. In the early 1950's, Hodgkin and Huxley (1952) first demonstrated the ionic mechanism of the transmembrane action potential in the squid giant axon and proposed that such voltage dependence of the K+ and Na+ conductance is due to charged particles within the membrane that move in response to changes in the transmembrane electric field (Hodgkin and Huxley, 1952; Hille, 1992). More recently, the activation process has been modeled as voltage-dependent conformation changes in each of four subunits, followed by cooperative opening transitions with lower voltage sensitivity (Hoshi et al., 1994; Zagotta et al., 1994a,b; Schoppa and Sigworth, 1998a,b,c). The voltage dependence of the activation process results from charge movements within the channel protein, associated with early conformational transitions. These intramolecular charge movements can be directly measured as gating currents (Armstrong, 1981; Hoshi, 1994).
Structural studies in voltage-dependent Na+, K+, and Ca2+ channels have revealed an apparent membrane spanning region, the S4 segment, in which every third amino acid carries positive charge (Noda et al., 1984; Tanabe et al., 1987; Tempel et al., 1987), suggesting that the S4 serves as the primary voltage sensor in these channel types (Greenblatt et al., 1985; Caterall, 1986; Guy and Seetharamulu, 1986; Noda et al., 1986). This hypothesis has been evaluated in potassium channels using a variety of charge neutralizing mutations in segments S2, S3, and S4 (Liman et al., 1991; Lopez et al., 1991; Papazian et al., 1991, 1995; Logothetis et al., 1992, 1993; Tytgat and Hess, 1992; Aggarwal and MacKinnon, 1996; Seoh et al., 1996). Similarly, gating charge measurements from S4 mutations have been widely used to confirm the role of the S4 segment as a voltage sensor in voltage-dependent activation (Bezanilla and Stefani, 1994; Perozo et al., 1994; Seoh et al., 1996). In addition, cooperative transitions leading to potassium channel activation have been identified in Shaker and heteromultimeric mammalian potassium channels (Hurst et al., 1992; Tytgat and Hess, 1992; Bezanilla et al., 1994; Sigworth, 1994; Zagotta et al., 1994b; Smith-Maxwell et al., 1998a). Furthermore, direct evidence for S4 movement during channel activation has come from fluorescence and sulfhydryl-reagent binding studies in which specific residues were replaced with cysteine to probe the environment near the S4 segment in Shaker channels. Such studies indicate that change in membrane potential can alter the internal and external accessibility of charged residues in the S4 segment, clarifying that changes in S4 exposure are involved in the initiation of channel opening (Yang and Horn, 1995; Larsson et al., 1996; Mannuzzu et al., 1996; Yang et al., 1996; Yusaf et al., 1996).
Nevertheless, the transitions closest to the open state appear relatively, or entirely, voltage insensitive even in voltage-gated potassium channels. This understanding has arisen from studies of single channel kinetics (e.g., Zagotta et al., 1989; Hoshi et al., 1994), as well as from macroscopic potassium currents (Zagotta et al., 1994a,b) and gating currents (Bezanilla and Stefani, 1994). Thus, mean open times as well as the three closed times noted from single channel recordings are all essentially voltage insensitive across the physiological range of membrane potentials (Zagotta et al., 1989; Hoshi et al., 1994; Schoppa and Sigworth, 1998a,b,c). The only parameters that have been unequivocally identified as voltage sensitive at the single channel level are the first latency distributions and P o(V) curves.
This paper explores the hypothesis that Shaker mutants in which S4 charges were progressively neutralized might lead to reduced or absent voltage sensitivity in the first latency process. Would the resulting channels continue to open and close via the voltage-insensitive kinetics noted above? Or would these channels be permanently closed in the absence of normal interaction from S4 charges? When expressed in Xenopus oocytes, two of the mutants studied here yielded macroscopic K+ currents that show no evidence of voltage-sensitive gating across the voltage range from −160 to +80 mV. However, single channel recordings show that these mutant channels still open and close, albeit in a voltage-insensitive manner, while retaining normal potassium selectivity and normal mean open times. Using these mutations, it becomes possible to study channel properties in the apparent absence of voltage-sensitive input from the S4 segment.
materials and methods
Mutations and Channel Expression
The sequence of the S4 segment in the Shaker K+ channel is shown in Table I. The seven conserved basic amino acids are indicated in Table I by both sequence numbers (above the sequence) and charge numbers (below the sequence). As noted in Table I, the mutant in which the first and second conserved arginines (R) in the S4 sequence were neutralized to glutamine (Q) will be referred to here as 12Q. Similarly, 124Q means that first, second, and fourth charges were neutralized to glutamines (see Table I), etc. The control NH2 terminus deleted Shaker 29-4 construct will be referred to as ShΔ. Since the amino acid sequence of ShΔ is identical to the Shaker B sequence from S1 through S6, standard Shaker B residue numbering is used throughout. Glutamine-substituted charge-neutralizing mutations within the S4 segment (12Q, 124Q, 1247Q, 127Q, and 147Q) were introduced in an NH2 terminus deleted Shaker 29-4 construct (Iverson and Rudy, 1990; McCormack et al., 1994) by Dr. K. McCormack and were generously made available for this study by Dr. F.J. Sigworth (Yale University, New Haven, CT). An additional mutant, 24Q, was constructed in this laboratory by the synthesized oligomer method, also in NH2-terminus–deleted Shaker 29-4, and assembled in pGEM-9zf(−) vector. Mutation was verified by restriction enzyme digestion and subsequent sequencing.
Table I.
Sequence of the S4 Segment in Shaker Potassium Channels
| Shaker No. S4 | L | A | I | L | 362 R | V | I | 365 R | L | V | 368 R | V | F | 371 R | I | F | 374 K | L | S | 377 R | H | S | 380 K | G | L | Q | Net charge | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Charge No. | — | — | — | — | 1 | — | — | 2 | — | — | 3 | — | — | 4 | — | — | 5 | — | — | 6 | — | — | 7 | — | — | — | 7+ | |||||||||||||||||||||||||||
| 12Q | — | — | — | — | Q | — | — | Q | — | — | R | — | — | R | — | — | K | — | — | R | — | — | K | — | — | — | 5+ | |||||||||||||||||||||||||||
| 124Q | — | — | — | — | Q | — | — | Q | — | — | R | — | — | Q | — | — | K | — | — | R | — | — | K | — | — | — | 4+ | |||||||||||||||||||||||||||
| 1247Q | — | — | — | — | Q | — | — | Q | — | — | R | — | — | Q | — | — | K | — | — | R | — | — | Q | — | — | — | 3+ | |||||||||||||||||||||||||||
| 127Q | — | — | — | — | Q | — | — | Q | — | — | R | — | — | R | — | — | K | — | — | R | — | — | Q | — | — | — | 4+ | |||||||||||||||||||||||||||
| 147Q | — | — | — | — | Q | — | — | R | — | — | R | — | — | Q | — | — | K | — | — | R | — | — | Q | — | — | — | 4+ | |||||||||||||||||||||||||||
| 24Q | — | — | — | — | R | — | — | Q | — | — | R | — | — | Q | — | — | K | — | — | R | — | — | K | — | — | — | 5+ |
The numbers below the sequence are charge position numbers for conserved basic amino acids in the S4 segment. Those above the sequence are residue numbers within the Shaker B cDNA sequence. Mutations are noted below the charge numbers.
Plasmids were linearized with NotI and mRNA was transcribed in vitro using mMESSAGE and mMACHINE kits (Ambion, Inc.). mRNA was microinjected into Xenopus oocytes in developmental stage V or VI at a concentration ranging between 0.005 and 0.1 μg/μl (total volume per oocyte, 50 nl). The injected oocytes were incubated in ND96-PGH broth containing (mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.2, plus 2.5 pyruvate, 50 mg/ml gentamicin, and 1% horse serum, at 18°C for 1–7 d before electrophysiological recordings.
Electrophysiology
Channel expression levels in Xenopus oocytes were tested by two-electrode voltage clamp (TEV),1 using a CA-1 High Performance Oocyte Clamp (Dagan Corp.). TEV electrodes were filled with 2 mM KCl and have 0.1–0.3 MΩ resistance. Patch-clamp recordings were obtained using EPC-9 patch clamp amplifier (HEKA Elektronik). Patch pipettes were fabricated from aluminum silicate (for macropatch pipettes) and coated with dental wax to reduce electrode capacitance, or from borosilicate glass (for single channel recordings) and sylgard coated. Macropatch pipettes yield resistances between 0.5 and 2 MΩ in standard solutions. Pipettes for single-channel measurement had resistances between 6 and 20 MΩ in symmetric K+ solutions (115 mM K+). All single-channel currents were recorded from isolated patches in the inside-out configuration. Traces were recorded in steps from holding potential to different test potentials (from −160 to +80 mV) using different pulse durations and sample intervals to address a potentially wide range of channel kinetics. 1-s traces were recorded with a 200-μs sample interval using a 1.25-kHz Bessel filter. 100- and 50-ms traces were recorded at 10-μs sample intervals with a 4-kHz Bessel filter. The digit filter was set ∼1–2 kHz. Data acquisition was controlled using the Pulse+PulseFit software package (HEKA Elektronik). Experiments were carried out at room temperature (between 20° and 22°C).
All data reported here were obtained without P/n subtractions, except where specifically indicated. Such P/n subtractions are commonly used to distinguish ionic current from both capacitance and leak currents by subtraction of “leak pulses” obtained in voltage steps, and from a leak holding potential, at which voltage-gated channels remain closed. This method is not appropriate for channel types that show maintained currents across the full range of experimentally accessible membrane potentials.
Data Analysis
Data analysis was performed using software from PulseFit, PulseTools (HEKA Elektronik), IgorPro (Wave Metrics), Mathematica (Wolfram Research, Inc.), and MacTAC (Skalar Instruments). Single channel openings were identified using the half-amplitude threshold-crossing method (Colquhoun and Sigworth, 1983). Analysis of single-channel fluctuations was carried out following the methods described by Llano et al. (1988) and Perozo et al. (1991).
Single channel open probability was determined from the formula A/(A + B), where A is the total time spent in the open state and B is the total time spent in the closed state. This formula is usable only when a patch shows no evidence of a second channel being present in any of the data traces at all test potentials. However, it is easier to obtain traces with two or three channels present in the patch. We have used a method for estimating channel P o in patch records where the total number of channels, n, can be shown to be no more than three. Statistically, the probability of being either open or closed is equal to 1, thus P closed = 1 − P o.
In multiple channel patch data, it is easy to sum the time spent in closed states, and hence to calculate the probability of no channel being open. Presuming each channel gates independently, the mean probability of any one channel being open and closed is P o and P closed, respectively. In multiple channel patches, the probability of all channels being closed is B, and the probability of at least one channel being open is B o, then we have:
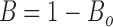 |
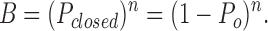 |
Thus:
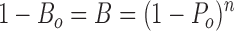 |
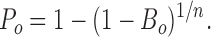 |
This final equation was used to calculate the mean probability of a single channel being open (P o) at different test potentials in symmetric K+ solutions. This equation was checked by analyzing multiple channel data and comparing results with analysis of single channel data from the same channel-type. Since similar results were obtained, some multiple channel traces from the 124Q and 1247Q mutants were analyzed by this method, provided that no more than three channels were detected in any one patch.
Averaged results are reported as mean ± SD (n), where n is the number of patches.
Solutions
For all patch clamp experiments, in addition to monovalent chloride salt, external solutions, referred to as Ringer solutions, always contained 1.8 mM CaCl2 and 10 mM HEPES, pH 7.2. Internal solutions, named EGTA solutions, contained 1.8 mM EGTA and 10 mM HEPES, pH 7.2. The solutions were named according to the content of monovalent cations (mM): normal frog Ringer, 115 NaCl, 2.5 KCl; K-Ringer, 115 KCl; Tris-Ringer, 115 TrisCl; TMA-Ringer, 115 TMA; K-EGTA, 115 KCl; Tris-EGTA, 115 TrisCl. To test channel ion selectivity, K+ was replaced completely by Rb+, NH4+, Cs+, Na+, respectively, in the internal and external solutions.
results
The basic amino acids in the S4 segments of Shaker channels are not functionally equivalent and may make different contributions to the mechanism of channel activation (Liman et al., 1991; Lopez et al., 1991; Papazian et al., 1991; Tytgat and Hess, 1992; Logothetis et al., 1993). Thus, single neutralizations of the even numbered charges (R2 or R4) shift the opening probability [P o(V)] curve to the left along the voltage axis, whereas single neutralizations of R1 or R3 (but not K7) shift the P o(V) curve to the right (Papazian et al., 1991; Logothetis et al., 1992). On the other hand, K5 and R6 play an important role in the proper folding and maturation of the channel protein (Perozo et al., 1994; Papazian et al., 1995). Finally, although Aggarwal and MacKinnon (1996) have shown that neutralization of K7 does not alter charge per channel, an additional 7Q neutralization might affect S4 stability in combination with other charge neutralizations.
Miller and Aldrich (1996) showed that double mutants involving neutralizations of the second and fourth charges induce larger left shifts than would be predicted from additive effects of the two single mutations. Furthermore, their macroscopic current traces from the NH2 terminus–deleted versions of these mutants (see Fig 2 B, Miller and Aldrich, 1996) seemed to show little evidence of activation and deactivation kinetics. These findings were strongly supportive of our own preliminary findings (Bao et al., 1996), and suggested that a more extensive study of S4 charge neutralizations, using single channel methods, might help to elucidate the role of the first four charges in Shaker channel gating.
Figure 2.
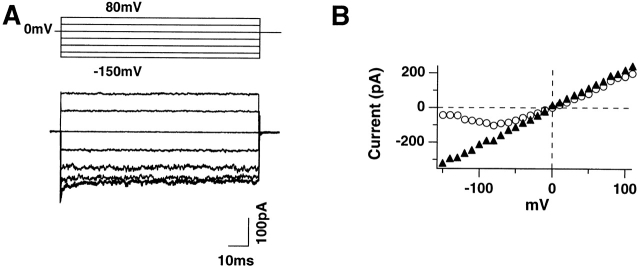
Inside-out patch clamp recordings from 124Q in symmetric (115 mM) K+ solutions. (A) Macroscopic currents at test potentials of −150, −120, −80, −40, 0, +40, and +80 mV, respectively. Holding potential was 0 mV. Capacity currents were subtracted off line. Note the absence of activation or deactivation kinetics in these records. (B) I–V curve for 124Q in standard symmetric (115 mM) K+ solutions containing 1.8 mM Ca2+ (○) is compared with an equivalent curve obtained using 115 mM K+ EGTA solution in the patch pipette (▴), as well as in the bath.
Effects of S4 Mutants 12Q, 127Q, 147Q, and 24Q on Shaker K+ Channel Voltage Sensitivity
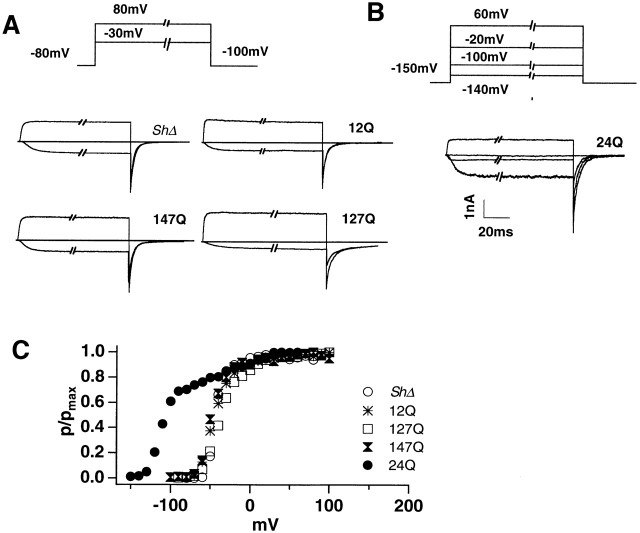
The multiple charge-neutralizing mutations 12Q, 127Q, and 147Q were tested using TEV and patch clamp measurements (Fig. 1 A). These mutants all show voltage-sensitive gating with similar thresholds for channel opening of about −60 mV. Fig. 1 A shows macropatch currents at two different voltages (−30 and +80 mV) from mutants 12Q, 127Q, and 147Q, as well as from the control ShΔ channel. These traces were recorded in symmetric K+ solutions (115 mM) from a holding potential of −80 mV to different test potentials for 100 ms, and then back to a hyperpolarization potential (−100 mV) for another 50 ms. On-line leak and zero subtraction were carried out using a P/4 protocol with the leak holding potential at −120 mV. The peak tail currents (Itail) obtained at different testing potentials were normalized (as Itail/Itail, max) and plotted against test potential to provide macroscopic P o(V) curves for each mutant. None of these mutations shows any marked shift in the voltage dependence of channel opening in comparison with ShΔ (Fig. 1 C). The midpoints of the P o(V) curves for 12Q, 127Q, and 147Q are −35, −45, and −30 mV, respectively, as compared with approximately −45 mV in ShΔ.
Figure 1.
Voltage sensitivity of gating in mutants 12Q, 127Q, 24Q, and 147Q, compared with ShΔ. (A) Macroscopic currents from inside-out patches in steps from −80-mV holding potential to −30 and +80 mV, respectively, returning to −100 mV. Leak holding potential was −120 mV. Note, similar kinetics of activation and deactivation for 12Q, 127Q, 147Q, and ShΔ. (B) Macroscopic currents for the 24Q mutant. Due to the marked left shift in P o(V) curve for this mutant (C), holding potential was −160 mV for this mutant and currents at four test potentials are shown here (see pulse protocol insert). At −140 mV, no significant current is seen. Inward currents occur at −100 and −20 mV, with outward current at +60 mV. Leak holding potential was −160 mV. (C) Normalized voltage dependence of channel open probabilities for 12Q, 127Q, 24Q, and 147Q, as well as for control ShΔ.
The mutant 24Q was also found to show voltage-sensitive gating as demonstrated by the fast tail currents indicating channel closing on return to the holding potential (Fig. 1 B). Threshold opening occurs about −150 mV, and the midpoint of the macroscopic P o(V) curve is close to −100 mV (Fig. 1 C). Thus 24Q (R365Q:R371Q) shows strongly left-shifted voltage dependence, although the shift seen here is considerably smaller than the −180-mV midpoint found by Miller and Aldrich (1996) for their 24 neutralization (R365N: R371I). The traces shown in Fig. 1 B were obtained in steps from a holding potential of −160 mV to the indicated test potentials, with the leak holding potential adjusted to −160 mV, and with negative-going P/4 pulses. No significant current was seen in the step to −140 mV. Activation and deactivation kinetics are readily visible in Fig. 1 B, although apparently at least 80 mV left-shifted by comparison with control ShΔ channels.
Voltage-insensitive Gating in 124Q and 1247Q S4 Mutants
Recordings from 124Q- or 1247Q-injected oocytes, using the two-electrode voltage clamp system with normal frog Ringer as the bathing solution (not shown) did not demonstrate either inward tail currents indicative of time-dependent gating, or voltage-sensitive activation kinetics at any test potential from −100 to +100 mV. Furthermore, large holding currents (by comparison with those seen in uninjected oocytes) were evident in symmetric K+ solutions at −80 mV. Thus, TEV recordings showed no clear evidence of normal voltage-sensitive gating, despite the presence of “leak” currents significantly larger than those seen in uninjected oocytes. However, it seemed possible that rapid voltage-sensitive gating might have been overlooked due to the relatively slow clamp speed (∼0.5 ms) of the TEV system.
Inside-out patch clamp recordings from 124Q and 1247Q channels in symmetric K+ solutions (115 mM K+) were used to assess this possibility (Fig. 2 A). In this figure, which shows records obtained from the 124Q mutant from a holding potential of 0 mV without P/n subtraction, no activation or deactivation kinetics were observed for steps to potentials from +80 to approximately −80 mV. However, the current–voltage (I–V) curve shows progressive reduction of inward current in steps to potentials more negative than −80 mV (Fig. 2 B), as if channels deactivate rapidly at negative potentials in the range −80 to −160 mV.
A possible explanation for this finding would be that significant Ca2+ block was occurring in these channels at very negative potentials. This possibility was explored (see Fig. 2 B) by comparing I–V curves obtained with and without Ca2+ in the external solution. When the normal 1.8 mM external Ca2+ was substituted by 1.8 mM EGTA, the I–V curve is effectively linearized. A Woodhull model for channel block (Woodhull, 1973) was used to fit these data, suggesting a Ca2+ block position ∼40% of the distance from the extracellular end of the channel, with an apparent kD of ∼50 mM Ca2+ (at 0 mV).
Thus, the apparent deactivation kinetics of macroscopic currents in the 124Q mutant, as well as equivalent findings for 1247Q (not shown), can be fully explained by calcium block occurring at very negative potentials. This finding suggests that these channels may be essentially voltage-insensitive from −160 to +100 mV. Nevertheless, we chose to complete this study using Ca2+-containing external solutions rather than Ca2+-free external solutions. We note (Fig. 2 B) that Ca2+ block is minimal at potentials greater than −80 mV. Furthermore, as will be seen from single channel recordings (Fig. 3), the Ca2+ block at negative potentials is a fast “flickering block” that has little effect on the apparent kinetics of idealized records obtained using the half-crossing method. However, we report here detailed kinetic analysis of closed time distributions only from potentials greater than −80 mV (see Fig. 5), where fast closings are unlikely to have been contributed by Ca2+ block.
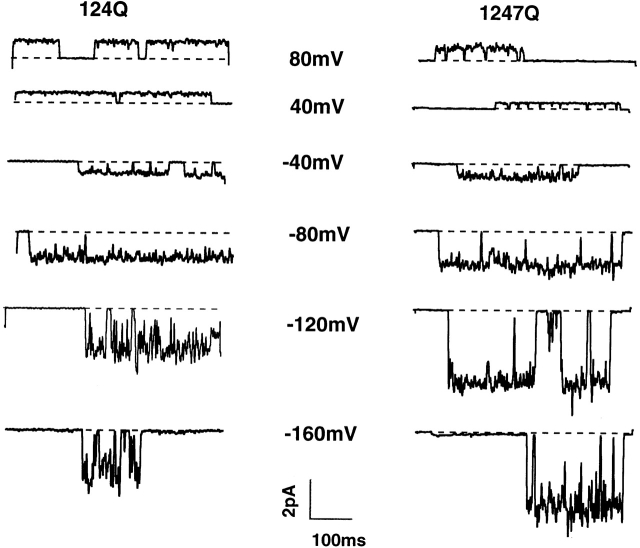
Figure 3.
Typical single channel current traces for 124Q (left) and 1247Q (right) in symmetric 115 mM K+ solutions at various test potentials. Both mutant channels open across the voltage range from −160 to +80 mV. Holding potential was 0 mV for both mutants.
Figure 5.
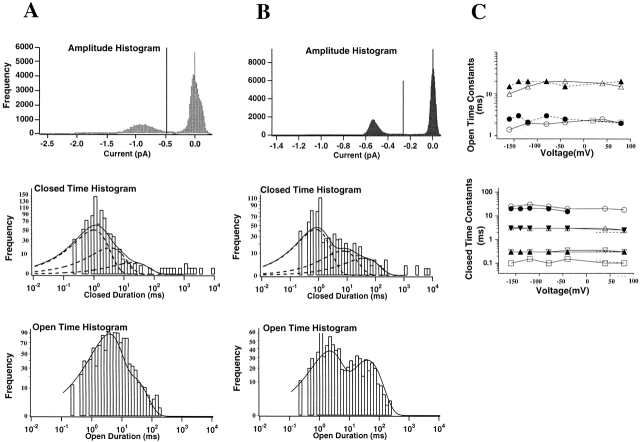
Analysis of single channel currents from the 1247Q mutant at −40-mV test potential in symmetric 115-mM K+ solutions (A), and in external 115-mM Rb+ solution (B). For each solution, single channel amplitude histograms are shown (top) together with logarithmic open time (bottom) and closed time distributions (center). Lines of fit were obtained using a maximum likelihood method. Dashed lines indicate individual exponential components. (C) Open time constants and the four closed time constants are plotted as functions of test potential, data from 124Q (open symbols), and from 1247Q (solid symbols). The mean open time for wild-type ShΔ is indicated as open square at the voltage of 20 mV. The three closed time constants for wild-type ShΔ are indicated as the dashed line. Data in A and B were from two representative patches. Data in C are means.
Single Channel Analysis of the 124Q and 1247Q Mutants
Since macroscopic currents had shown that P o appears constant across voltage, single channel recordings were needed to assess whether these mutant channels were locked in a permanently open state, or whether they might be opening and closing but with voltage-insensitive kinetics. In the latter case, single channel measurements could be used to evaluate the rate-limiting steps for channel gating as well as the potential voltage dependence of transitions between kinetic states. Important conclusions can be drawn from analysis of single-channel behavior even in the absence of a complete kinetic model (Zagotta et al., 1989).
Single 124Q and 1247Q channels open and close in a voltage-insensitive manner.
Single channel currents were recorded from excised patches in an inside-out configuration in symmetric potassium solutions. Preliminary studies of these patches indicated that P o was not affected by changes in either holding or test potentials (from −160 to +80 mV). The apparent absence of any effect of holding potential on P o suggests that these channels do not enter or recover from C-type inactivation at any of the test potentials assessed here. Thus, it should be possible to obtain long continuous recordings by holding at the required “test” potential. Nevertheless, subtle effects might occur from changes in C-type inactivation, and we chose to use repetitive pulse recordings with 5-s intervals between successive traces to maximize the independence between data traces. However, tests with pulses of different durations (50, 100, 200, and 500 ms) all indicated substantial numbers of long openings that outlasted the pulse duration. Hence, 1-s pulses were used to characterize these longest openings, while shorter pulses were used to address brief opening and closing events (see below). Patches were routinely held at reversal potential (0 mV in symmetric K+ solutions) to minimize holding currents and prolong patch lifetime.
Typical single-channel currents in symmetric K+ solutions for 124Q and 1247Q at various test potentials are shown in Fig. 3. These mutant channels clearly open and close, and with apparently similar probabilities, at all test potentials from −160 to +80 mV. Also, the increased “noise” seen only in the open state at the most negative test potentials is consistent with the Ca2+ block noted in macroscopic currents (Fig. 2). Nevertheless, this flickering block had little effect on current records idealized using the half-amplitude crossing method (Colquhoun and Sigworth, 1983) and all single channel data reported here were obtained using Ca2+-containing external solutions.
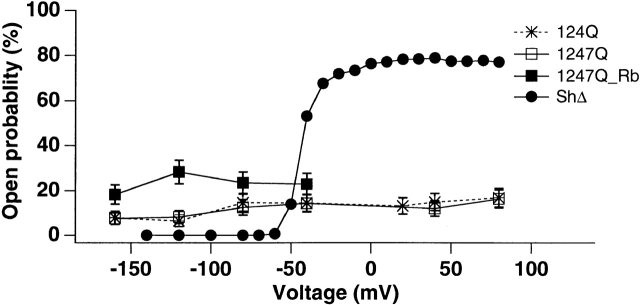
Single channel P o(V) curves (see materials and methods) obtained from these traces are plotted in Fig. 4. Within experimental error, the mean P o for 124Q is the same as that for 1247Q. Both are quite low, P o is ∼0.1–0.15 with little apparent voltage sensitivity. Noting that P o for these channels increases no more than twofold for a 240-mV change of potential, the apparent valence for these channels is <0.1e0. As a control, P o(V) of wild-type Shaker K+ channel was also plotted in Fig. 4 (derived from Fig. 1 A control ShΔ data, scaled to our observed P o,max for ShΔ channels). In wild-type ShΔ, P o increases with test potential from 0 to ∼0.85 across the voltage −60 to +80 mV. These results confirm that 124Q and 1247Q are effectively voltage insensitive, although they open and close spontaneously at all test potentials tested here.
Figure 4.
P o(V) relationships for the 124Q and 1247Q mutants, compared with wild-type ShΔ channels. The wild-type ShΔ data were derived from Fig. 1 A control ShΔ data, scaled to the observed P o,max at +80 mV for ShΔ channels. Single channel data were recorded in inside-out configuration and symmetric (115 mM) K+ solutions or Rb+ (115 mM)//K+ (115 mM) solutions as indicated. All data points are means ± SD, n = 3–6 patches.
A low average P o might arise in 1-s test pulses from an initially higher P o decaying during these long pulses, as a result of C-type inactivation. However, no change in current magnitude was seen in TEV experiments where 5-s pulses were used to look specifically for C-type inactivation. These recordings were made using first normal frog Ringer (2.5 mM [K+]o), and then Tris Ringer (0 mM [K+]o) since reduction of external K+ increases the rate of C-type inactivation in ShΔ (López-Barneo et al., 1993). No change in current magnitude was apparent in these 5-s pulses using either solution (data not shown), and we conclude that the low P o for 124Q and 1247Q does not arise from channels entering an absorbing C-type inactivated state during the test pulses.
On the other hand, unusually long closed intervals must be present in these channels if P o is low, and yet mean open time is similar to control ShΔ values (see below). This finding could arise from either (a) a low, but constant, statistical probability that opening events will occur, as in voltage-sensitive ShΔ channels at negative test potentials, or (b) dynamic equilibrium with a nonabsorbing C-type (or P-type) inactivated state. These alternatives will be considered further in the discussion.
Single channel mean open time is voltage independent in 124Q and 1247Q, but is affected by permeant ion species.
Open time distribution histograms were obtained from 40 idealized 1-s traces (recorded at 1.2 kHz) and concatenated to yield an equivalent 40-s recording. Amplitude and open-time histograms were fitted using a simplex algorithm. Amplitude histograms were fitted to a sum of Gaussian functions using Igor software. Single channel currents were derived from the Gaussian fits to amplitude histograms. Steady state dwell times for 1247Q single channels in symmetric K+ solutions, including amplitude histograms and distribution of open times, are demonstrated in Fig. 5 A. Our data was best fitted as the sum of two exponential distributions, yielding mean open times across the full voltage range from −160 to +80 mV of 2.0 ± 0.4 and 16.3 ± 3.8 ms (n = 6 patches, with data from each patch obtained at eight different test potentials) for 124Q, with these open states having relative probabilities of ∼85 and ∼15%, respectively. Mean open times for 1247Q were 2.5 ± 0.4 and 18.3 ± 2.6 ms (n = 6 patches, each yielding data at six test potentials). These findings suggest the presence of two separable open states. However, amplitude histograms give no indication that the longer open state differs in conductance from the shorter, primary, open state (see Fig. 5 A). Furthermore, 124Q and 1247Q data are not statistically distinguishable.
The longer (∼20 ms) mean open time does not appear in our records from ShΔ channels where mean open time was 2.2 ± 0.1 ms. However, no correction for missed closing events needed to be applied to obtain comparative results from the three channel types used here (124Q, 1247Q, and ShΔ) since identical experimental procedures were used in each case.
The mean open times for both 124Q and 1247Q are presented as functions of voltage in Fig. 5 C. Clearly, the mean open times are voltage independent across the full range of test potentials studied here. Furthermore, the primary (higher probability) mean open times for both mutations are about the same as we find for wild-type ShΔ at +20 mV using this same recording method.
This similarity in mean open times seemed surprising in view of the marked difference in voltage sensitivity between ShΔ channels and the 124Q and 1247Q mutants, suggesting that this finding might well be only coincidental. However, tail current deactivation rates in control Shaker channels are known to be slowed by Rb+ permeation (Zagotta et al., 1994a) and, although it is not possible to address deactivation rates in our voltage-insensitive mutants, it seemed interesting to explore whether the mean open times for ShΔ and these mutant channels would be similarly affected by Rb+. The single channel open probabilities and mean open times were calculated as described above for both mutations and for ShΔ under biionic conditions, with Rb+ (115 mM) in the external solution and K+ (115 mM) as the control cation in the internal solution. Single channel data were recorded at negative potentials where the observed inward currents must reflect Rb+ permeation.
Both P o, measured as total time in the open state divided by total record duration, and mean open time increase (see Figs. 4 and 5 B) when Rb+ is the principal permeant cation. P o increases a little less than twofold to ∼0.3 in Rb+ for both mutants, but remains voltage independent. Similarly, the primary mean open time increased from 2.5 to 4.5 ms for 1247Q, also without voltage dependence (with an equivalent increase in the longer open time from 18.3 to ∼45 ms). However, P o = (τo · n o)/t, where τo is mean open time, t is total recording time, and n o is the number of transitions into the open state. Hence, n o remains constant if P o and τo increase proportionately in the presence of Rb+. Furthermore, for ShΔ channels, a similar approximately twofold increase in mean open time occurs (at −20 mV), when Rb+ replaces K+ as the primary permeant cation, although in ShΔ channels (unlike 124Q and 1247Q channels) n o varies as a function of applied potential (Zagotta et al., 1989). Thus, the increase in P o in Rb+ solutions is fully explained by the increase in mean open time.
Single channel closed times are voltage independent in 124Q and 1247Q.
Closed time distributions were also obtained from the same traces as were used for open time distributions (see Fig. 5, A and B). Best results were obtained from three exponential fits to data from 1-s traces in the 124Q and 1247Q mutant channels (Fig. 5 A). This process was repeated for analysis of 100- and 50-ms pulses recorded using a 4 kHz Bessel filter and sampled at shorter intervals. Mean closed time constants were (ms) 0.11 ± 0.02 (n = 7), 0.32 ± 0.05 (n = 23), 3.1 ± 0.4 (n = 25), and 21.1 ± 4.6 (n = 17 patches) for 124Q. Results for 1247Q channels were again not statistically distinguishable from those reported here for 124Q, and similar results were also obtained from our control ShΔ channels, except that we were unable to resolve the longer ∼20-ms closed time in ShΔ. We conclude that our findings differ from results obtained in wild-type Shaker channels (see also Schoppa and Sigworth, 1998a) by addition of the slower ∼20-ms rate. Although this 20-ms time constant might not have been identified in the 20-ms test pulses used by Schoppa and Sigworth (1998a), it should have been readily detectable in our ShΔ data, given the 100-ms pulses used here and the slow onset of C-type inactivation in high external K+ solutions (López-Barneo et al., 1993). We conclude that this ∼20-ms time constant is not present in control Shaker channels.
Finally, we note that Rb+ has similar effects on both open and closed time distributions, increasing the closed times seen in 1-s traces at −40 mV from ∼0.3, 3.0, and 20 ms in K+ solutions to 1.1, 7.2, and 75 ms in Rb+ for 1247Q channels. Nevertheless, while the increase in P o in Rb+ seems entirely explained by the increase in mean open time (see above), where closed times also increase in Rb+, some additional explanation seems required. Even though the weighting of the longest closed time in our three exponential fits appears to increase in Rb+, the incidence of extremely long (unfitted) closures goes down in Rb+ (compare Fig. 5, A and B), maintaining the internal self consistency of the data set.
Ionic Selectivity in Voltage-insensitive 124Q and 1247Q Mutants
The similar mean open times for ShΔ channels and for the 124Q and 1247Q mutants might suggest that channel structure has not been significantly affected by the apparent loss of voltage sensor input. Additionally, it is already clear from the data reported above that these mutant channels, like ShΔ channels, conduct both K+ and Rb+ ions, giving single channel currents in the pA range. We here report a more complete study of the selectivity of these mutant channels, demonstrating their substantial equivalence to ShΔ controls, both from measurements of reversal potentials (see below) and from the relative conductances observed under simplified ionic conditions (Fig. 6 A). Mutant 124Q and 1247Q have similar properties; therefore, only the ionic selectivity of 124Q is shown here.
Figure 6.
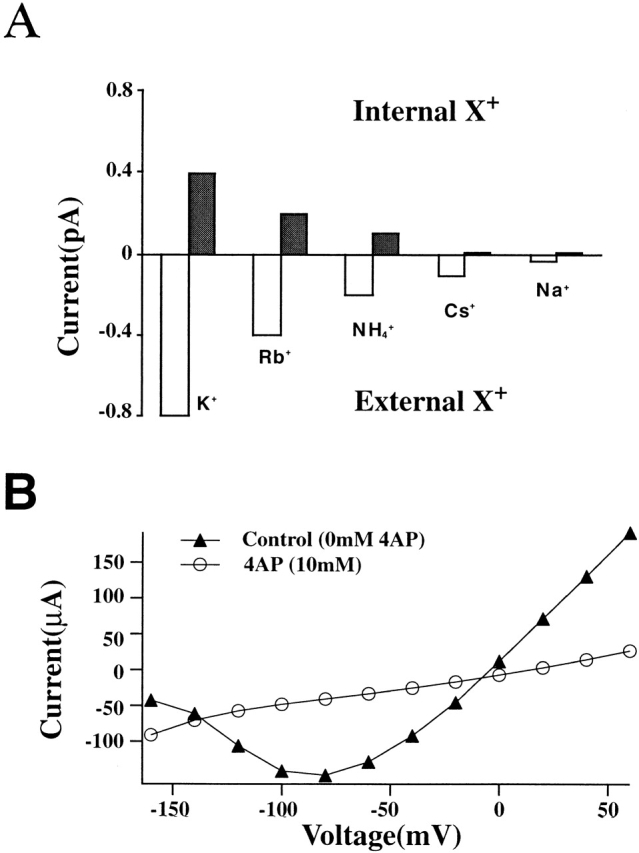
Pore properties of voltage-insensitive 124Q channels. (A) Unidirectional single channel currents measured at +40 mV (Tris Ringer//“X+” EGTA, shaded histograms) and at −40 mV (“X+” Ringer//Tris EGTA, open histograms). The histograms show the following permeability sequence: K+ > Rb+ > NH4+ > Cs+ and Na+. (B) 124Q channels are sensitive to block by 4-AP. I–V data points from one representative oocyte using two-electrode voltage clamp. Macroscopic current seen in 115-mM external K+ solution (▴) appears effectively blocked after addition of 10 mM 4-AP to the bath solution (○).
Ionic selectivity can be determined using the permeability ratio calculated from the reversal potential seen under biionic conditions with the test cation on one side of the membrane and the reference cation (K+) on the other side. For this study, reversal potentials were evaluated by measuring magnitudes of single channel currents across a voltage range from −120 to 80 mV in inside-out configuration, and plotting the observed currents as functions of test potential. The internal solutions contained 115 mM K+ as the reference ion with the external cation being presented in the same concentration. Erev was 0 mV for external K+ solutions, −5 mV for external Rb+, −30 mV for external NH4 +, and less than −100 mV for external Na+. These findings indicate a highly K+-selective channel, in which PX/PK = 0.82 in Rb+; 0.31 in NH4 + and <0.01 in Na+. Clearly, selectivity as defined by the PX/PK ratio is very similar to that found for control Shaker channels, although the ratio for NH4 + seen here is two- to threefold greater than the 0.13 found by Heginbotham et al. (1994). Apparently, multiple charge neutralizations can remove channel voltage sensitivity without necessarily altering the ionic selectivity of the channels, as if the structure of the selectivity filter has been no more than minimally affected by the S4 charge neutralizations.
However, as pointed out by Hille (1992), permeability sequences obtained from reversal potentials are not necessarily identical to those seen from relative single channel conductances. Therefore, single channel data are included where the test ion was presented in the internal solution with the impermeant ion Tris in the external solution, or vice versa (Fig. 6 A). Amplitudes of single channel currents at constant voltages were compared for the following test cations: K+, Na+, Rb+, NH4 +, and Cs+. With Tris Ringer as the external solution and the test ion presented in the internal solution, all single channel currents were outward at +40 mV. Alternatively, when Tris-EGTA was used as the internal reference cation, only inward single channel currents were seen at −40 mV. At both potentials, our results correspond to the permeability sequence seen in the reversal potential data: K+ > Rb+ > NH4 + > Cs+ and Na+.
Sensitivity to channel block by 4-aminopyridine (4-AP; McCormack et al., 1994) was used as an additional test indicating the structural integrity of the ion permeation pathway in the 124Q and 1247Q channels. In whole-cell two-electrode voltage clamp, ionic currents of 124Q decreased dramatically after changing the bath solution from normal K-Ringer to K-Ringer containing 10 mM 4-AP on the same oocyte (Fig. 6 B).
discussion
This study has shown that two multiple charge-neutralizing mutations that involve the first, second, and fourth S4 charges, produce channels that conduct K+ currents without evident voltage sensitivity. Nevertheless, at the single channel level, these mutant channels continue to open and close with a low (0.15) but constant P o across a wide range of test potentials. Their mean open times are not distinguishable from those of control ShΔ channels. Closed time distributions in these channels are also indistinguishable from those of ShΔ channels, except that there is an additional long closed time (∼20 ms) that cannot be identified in the ShΔ controls. Furthermore, these 124Q and 1247Q mutants retain the high K+ selectivity of the parent channel, as if the structure of the selectivity filter has been little affected by these S4 charge neutralizations.
Effects of S4 Charge Neutralization
As initially shown by Papazian et al. (1991), Shaker channels appear tolerant of single neutralizations of all charges other than K5 and R6, which were subsequently found to form electrostatic interactions with negatively charged residues in other segments and appear vital for normal folding of the channel (Papazian et al., 1995). Thus, the multiple charge neutralizing mutations used here avoid charges 5 and 6 and were selected to provide detailed controls for the voltage- insensitive mutants rather than to provide additional information as to the functional roles of each charge in normal Shaker channels. All multiple neutralizations of charges 1, 2, 4, and 7 studied here, other than 124Q and 1247Q, have been shown to give voltage-gated channels. Neither single nor double neutralizations of the first, second, and fourth charges can recreate the effects found when all three of these charges are simultaneously neutralized.
Nevertheless, the mechanisms controlling right and left shifting of the P o(V) curve have not yet been fully elucidated. Thus, two different mutations, both involving neutralization of the second and fourth charges, show substantial quantitative differences in P o(V) midpoint. The R365N:R371I mutant studied by Miller and Aldrich (1996) gave a midpoint of −180 mV, compared with the −100-mV midpoint found here for 24Q (R365Q: R371Q). P o(V) midpoint, which presumably reflects the stability of the S4 in relation to surrounding pore structures, is known to be affected by the size and hydrophobicity of substituted S4 residues (see Sigworth, 1994). Such properties may be more important than the presence or absence of S4 charges.
Are the 124Q and 1247Q Channels C-Type Inactivated?
We have pointed out that these mutants do not enter C-type inactivation during applied test pulses, and this is true both for channels held at 0 and −160 mV (data not shown). However, since the mutant channels open with similar probabilities at all reasonable potentials, it seems clear that they should have reached an equilibrium with C-type inactivation at some time long before their experimental evaluation. Thus, a question arises as to the nature of this equilibrium. Three major possibilities exist.
(a) Mutant channels may be in equilibrium with a nonabsorbing C-type–inactivated state with a life time of ∼20 ms. Since this C-type (or P-type) state appears “closed” in our channels, the normal Shaker selectivity seen in our data would reflect the properties of noninactivated channels while the inactivated channels would be K+ impermeant. In this case, the low P o,max presumably arises from sequestration of channels in the inactivated state.
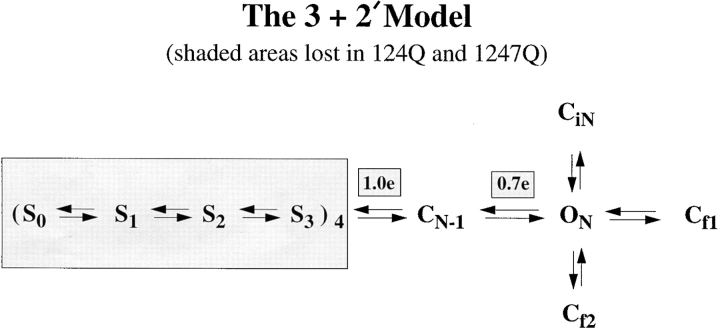
(b) Mutant channels may be incapable of C-type inactivation. Loots and Isacoff (1998) have shown that fluorescent probes inserted at residue 359 report involvement of the region around charge 1 in the P-type conformational change that precedes C-type inactivation. Neutralization of the first, second, and fourth charges could well be sufficient to block this P-type conformational change. Thus, this hypothesis would remove C-type inactivation as a cause of the low P o,max found in our 124Q and 1247Q mutants, suggesting that the reduced P o may be a direct result of the S4 charge neutralizations. Within a model such as the 3+2′ model proposed by Schoppa and Sigworth (1998c), the S4 ↔ CN-1 ↔ ON transitions (Fig. 7) are presumed to be independently voltage sensitive, accounting for voltage-sensitive changes in the frequency of channel opening events at different test potentials. However, it seems likely that the electric field to which the S4 ↔ CN-1 and CN-1 ↔ ON voltage sensors respond would be substantially modified by neutralization of three nearby S4 charges, possibly reducing P o,max in these neutralization mutants.
Figure 7.
The 3+2′ model redrawn from Schoppa and Sigworth (1998c). Estimated reaction valences from their model fits to wild-type ShΔ channel data are shown above each voltage-sensitive reaction step. Shaded areas are presumed to be dysfunctional in voltage-insensitive 124Q and 1247Q mutant channels.
(c) Mutant channels may be permanently C-type inactivated, opening and closing without leaving C-type states. Shaker channels have been found to remain capable of ion conduction while in C-type states (Starkus et al., 1997), although with markedly changed selectivity. This hypothesis would require the selectivity of the inactivated state to have been drastically modified by the charge neutralizations, since wild-type Shaker channels become effectively impermeant to K+ ions when C-type inactivated. Thus, the apparently normal Shaker K+ selectivity seen in these mutants would be highly abnormal for C-type inactivated channels. In this case, the low observed P o,max could be caused by changes in opening probability resulting from conformational changes associated with C-type inactivation.
Unfortunately, the question posed in the heading of this section cannot yet be answered. We can neither force channels to close nor detect kinetic changes that would unequivocally report onset or recovery from C-type inactivation. Thus, alternative approaches will be required to further evaluate these competing hypotheses.
Single Channel Properties of the Voltage-insensitive Mutants 124Q and 1247Q
Despite the uncertainties noted above, the results reported here have considerable implications. The voltage-insensitive behavior seen in 124Q and 1247Q arises when charge 1 is neutralized in addition to charges 2 and 4. We presume that charge 1 must contribute in some substantial way to the normal coupling between S4 movement and voltage-dependent channel gating. Such coupling appears to be a cooperative process (Tytgat and Hess, 1992), which occurs when all four S4 segments have been repositioned by the voltage field (Smith-Maxwell et al., 1998a,b) in control Shaker channels.
Recently, a detailed and comprehensive study of Shaker channel kinetics has been carried out based on macroscopic currents, gating currents, and single channel data in both ShΔ channels and a well-studied S4 mutant (Schoppa and Sigworth, 1998a,b,c). The resulting data was shown to be best fitted by a specific model (see 3+2′ model in Schoppa and Sigworth, 1998c) that delineates the parameters of S4 movement and the kinetic form of the coupling between S4 movement and channel gating, while including the previously identified processes that reflect channel closings to states outside the primary activation pathway (Hoshi et al., 1994). Thus, it seems particularly important to determine how our results fit within this conceptual framework.
The 3+2′ model is shown in Fig. 7, and its relevance to our data should be readily apparent. Our mutants show the same mean open times and three closed times as noted by Schoppa and Sigworth (1998a,c) in control ShΔ channels. Additionally, we see a fourth closed time (∼20 ms) that may represent equilibration with a nonabsorbing inactivated state, as noted above. Nevertheless, the 3+2′ model identifies two independently voltage-sensitive steps between the S4 and ON states, which contribute a total of 1.7 eo to the voltage sensitivity of the Shaker channel. These voltage-sensitive S4 ↔ CN-1 ↔ ON transitions, together with the effects of S4 movement, determine the voltage sensitivities of first latency distributions and single channel P o(V) curves in control ShΔ channels. By contrast, in the 124Q and 1247Q mutants, all traces of independent voltage-sensitivity are lost. Even if all S4 input was removed by these S4 charge neutralizations, the 1.7 e0 reported for the non–S4 voltage sensors of the S4 to ON transitions should have been readily measurable in our P o(V) data. Thus, the low and voltage-insensitive P o in 124Q and 1247Q mutant channels implies additional changes in these S4 to ON transitions caused by direct or indirect effects of the S4 charge neutralizations.
Implications for Ion Channel Gating Mechanisms
The results presented here provide further support for a separation of the channel gating mechanism into three distinct components. Components from the single channel perspective are listed below.
The voltage-insensitive transitions close to the open state.
These transitions seem quantitatively unaffected by the neutralization of S4 charges in the 124Q and 1247Q mutants. However, they are affected to similar extents by transfer from K+ to Rb+ solutions in both control and mutant channels, suggesting that they are substantially determined by interactions between the pore and its permeating ions (see also Swenson and Armstrong, 1981; Matteson and Swenson, 1986; Zagotta et al., 1994a). In view of the tight relationship between these voltage-insensitive kinetics and permeant ions, we presume that these processes involve the “pore gate” proposed by Zheng and Sigworth (1998) to account for effects of the T442 residue on intermediate conductances.
The voltage-sensitive transitions that determine the first- latency and Po(V) distributions.
The voltage-sensitive input, represented by the S4 ↔ ON transitions in the 3+2′ model, appears to be lost after neutralization of the first, second, and fourth S4 charges. These transitions have been associated with a “main” activation gate formed by rearrangements of the S6 helices (Liu et al., 1997) that constrict the inner mouth of the channel in the KcsA structure (Doyle et al., 1998). However, Zheng and Sigworth (1998) point out that both the pore gate and the main gate must be coupled in normal functioning of the Shaker channel. Clearly, such coupling has been broken in the 124Q and 1247Q mutants studied here, as if the S4 ↔ ON transitions (absent in 124Q and 1247Q) provide a parallel drive to both the pore gate and the main gate during normal Shaker channel activation.
The inherently voltage-insensitive transitions involved in the P-type and C-type slow inactivation mechanisms.
Recent work by Loots and Isacoff (1998) has clarified that slow inactivation involves two distinct conformational changes (P- and C-type), both of which occur around the narrow tunnel region of the permeation path in the Doyle et al. (1998) structure. Like the transitions close to the open state, the kinetics of these transitions appear to be substantially determined by interactions with permeating ions (López-Barneo et al., 1993; Levy and Deutsch, 1996; Starkus et al., 1997). Unfortunately, as noted above, it is not yet clear to what extent these transitions may be affected by S4 charge neutralizations, despite the demonstrated involvement of the outer section of the S4 segment in the C-type conformation change (Loots and Isacoff, 1998).
Nevertheless, as Zheng and Sigworth (1998) point out, both the selectivity changes of slow inactivation and the conductance changes associated with the pore gate appear to take place in a narrow region of the channel “where very small atomic motions would have large effects on conductance and selectivity.”
Acknowledgments
We thank K. McCormack for providing us with ShΔ, and F.J. Sigworth for making available the 12Q, 124Q, 127Q, 147Q, and 1247Q mutations.
This study was supported in part by National Institutes of Health Grant RO1-NS21151, and by Pacific Biomedical Research Center Bridging Funds (to J.G. Starkus). J.G. Starkus and M.D. Rayner were further supported by Grants-in-Aid, while A. Hakeem also received a Predoctoral Fellowship, all from the American Heart Association (Hawaii Affiliate). M.D. Rayner received additional support from the Queen Emma Foundation.
Abbreviations used in this paper
- 4-AP
4-aminopyridine
- I–V
current– voltage
- TEV
two-electrode voltage clamp
Footnotes
Portions of this work were previously published in abstract form (Bao, H., A. Hakeem, K. McCormack, M.D. Rayner and J.G. Starkus. 1996. Biophys. J. 70:A189).
references
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- Armstrong CM. Sodium channels and gating currents. Physiol Rev. 1981;61:644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Bao H, Hakeem A, McCormack K, Rayner M, Starkus J. Voltage-insensitive gating in ShakerB S4 mutants. Biophys J. 1996;70:A189. [Google Scholar]
- Bezanilla F, Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. doi: 10.1146/annurev.bb.23.060194.004131. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of voltage-sensitive sodium channel. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and F.J. Sigworth. 1983. Fitting and statistical analysis of single-channel records. In Single Channel Recording. B. Sakmann and E. Neher, editors. Plenum Publishing Corp., New York. 505 pp.
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Greenblatt RE, Blatt Y, Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricuschannel primary structure. FEBS Lett. 1985;193:125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Guy HR, Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci USA. 1986;83:508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Membranes. 2nd ed. Sinauer Associates, Inc., Sunderland, MA. 59–82.
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Shakerpotassium channel gating I: transitions near the open state. J Gen Physiol. 1994;103:249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Kavanaugh MP, Yakel J, Adelman JP, North RA. Cooperative interactions among subunits of a voltage-dependent potassium channel. J Biol Chem. 1992;267:23742–23745. [PubMed] [Google Scholar]
- Iverson LE, Rudy B. The role of divergent amino and carboxyl domains on the inactivation properties of potassium channels derived from the Shaker gene of Drosophila. . J Neurosci. 1990;10:2903–2916. doi: 10.1523/JNEUROSCI.10-09-02903.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the Shaker K+channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Levy DL, Deutsch C. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys J. 1996;70:798–805. doi: 10.1016/S0006-3495(96)79619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Hess P, Weaver F, Koren G. Voltage-sensing residues in the S4 region of a mammalian K+channel. Nature. 1991;353:752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Llano I, Webb CK, Bezanilla F. Potassium conductance of the squid giant axon. J Gen Physiol. 1988;92:179–196. doi: 10.1085/jgp.92.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Kammen BF, Lindpaintner K, Bisbas D, Nadal-Ginard B. Gating charge differences between two voltage-gated K+channels are due to the specific charge content of their respective S4 regions. Neuron. 1993;10:1121–1129. doi: 10.1016/0896-6273(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Movahedi S, Satler C, Lindpaintner K, Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of voltage-gated K+channel result in corresponding decreases in gating charge. Neuron. 1992;8:531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- Loots E, Isacoff EY. Protein rearrangements underlying slow inactivation of the Shaker K+channel. J Gen Physiol. 1998;112:377–389. doi: 10.1085/jgp.112.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GA, Jan YN, Jan LY. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K+channel. Neuron. 1991;2:327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shakerpotassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Matteson DR, Swenson RP., Jr External monovalent cations that impede the closing of K+channels. J Gen Physiol. 1986;87:795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AG, Aldrich RW. Conversion of a delayed rectifier K+ channel to a voltage-gated inward rectifier K+channel by three amino acid substitutions. Neuron. 1996;16:853–858. doi: 10.1016/s0896-6273(00)80105-1. [DOI] [PubMed] [Google Scholar]
- McCormack K, Joiner WJ, Heinemann SH. A characterization of the activation structural rearrangements in voltage-dependent Shaker K+channels. Neuron. 1994;12:301–315. doi: 10.1016/0896-6273(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Kayano T, Suzuki H, Takeshima H, Kurasaaki M, Takahashi H, Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M, Tanabe T, Takai T, Kayano T, Ikeda T. Primary structure of the Electrophorus electricussodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Shao XM, Seoh SA, Mark AF, Huang Y, Wainstock H. Electrostatic interactions of S4 voltage sensor in Shaker K+channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shakerpotassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Perozo E, Santacruz-Toloza L, Stefani E, Bezanilla E, Papazian DM. S4 mutations alter gating currents of ShakerK channels. Biophys J. 1994;66:345–354. doi: 10.1016/s0006-3495(94)80783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Vandenberg CA, Jong DS, Bezanilla F. Single channel studies of the phosphorylation of K+channels in the squid giant axon. J Gen Physiol. 1991;98:1–34. doi: 10.1085/jgp.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels I. Characterization of voltage-dependent transitions. J Gen Physiol. 1998a;111:271–294. doi: 10.1085/jgp.111.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels II. Kinetics of the V2 mutant channel. J Gen Physiol. 1998b;111:295–311. doi: 10.1085/jgp.111.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Sigworth FJ. Activation of Shakerpotassium channels III. An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998c;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J Gen Physiol. 1998a;111:399–420. doi: 10.1085/jgp.111.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Maxwell CJ, Ledwell JL, Aldrich RW. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J Gen Physiol. 1998b;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shakerchannels. J Gen Physiol. 1997;110:539–550. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson RP, Armstrong CM. K+ channels close more slowly in the presence of external K+ and Rb+ . Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flovkerzi V, Takahashi H, Kangawa K, Kojima M, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. . Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Tytgat J, Hess P. Evidence for cooperative interaction in potassium channel gating. Nature. 1992;359:420–423. doi: 10.1038/359420a0. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, George AL, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yang N, Horn R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Yusaf SP, Wray D, Sivaprasadarao A. Measurement of the movement of the S4 segment during the activation of a voltage-gated potassium channel. Pflügers Arch. 1996;433:91–97. doi: 10.1007/s004240050253. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Dittman J, Aldrich RW. Shakerpotassium channel gating II: transitions in the activation pathway. J Gen Physiol. 1994a;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Dittman J, Aldrich RW. Shakerpotassium channel gating III: evaluation of kinetic models for activation. J Gen Physiol. 1994b;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with ShakermRNA. Proc Natl Acad Sci USA. 1989;86:7243–7247. doi: 10.1073/pnas.86.18.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Sigworth FJ. Intermediate conductances during deactivation of heteromultimeric Shakerpotassium channels. J Gen Physiol. 1998;112:457–474. doi: 10.1085/jgp.112.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]