Abstract
Objective To determine the effect of calcium supplementation on myocardial infarction, stroke, and sudden death in healthy postmenopausal women.
Design Randomised, placebo controlled trial.
Setting Academic medical centre in an urban setting in New Zealand.
Participants 1471 postmenopausal women (mean age 74): 732 were randomised to calcium supplementation and 739 to placebo.
Main outcome measures Adverse cardiovascular events over five years: death, sudden death, myocardial infarction, angina, other chest pain, stroke, transient ischaemic attack, and a composite end point of myocardial infarction, stroke, or sudden death.
Results Myocardial infarction was more commonly reported in the calcium group than in the placebo group (45 events in 31 women v 19 events in 14 women, P=0.01). The composite end point of myocardial infarction, stroke, or sudden death was also more common in the calcium group (101 events in 69 women v 54 events in 42 women, P=0.008). After adjudication myocardial infarction remained more common in the calcium group (24 events in 21 women v 10 events in 10 women, relative risk 2.12, 95% confidence interval 1.01 to 4.47). For the composite end point 61 events were verified in 51 women in the calcium group and 36 events in 35 women in the placebo group (relative risk 1.47, 0.97 to 2.23). When unreported events were added from the national database of hospital admissions in New Zealand the relative risk of myocardial infarction was 1.49 (0.86 to 2.57) and that of the composite end point was 1.21 (0.84 to 1.74). The respective rate ratios were 1.67 (95% confidence intervals 0.98 to 2.87) and 1.43 (1.01 to 2.04); event rates: placebo 16.3/1000 person years, calcium 23.3/1000 person years. For stroke (including unreported events) the relative risk was 1.37 (0.83 to 2.28) and the rate ratio was 1.45 (0.88 to 2.49).
Conclusion Calcium supplementation in healthy postmenopausal women is associated with upward trends in cardiovascular event rates. This potentially detrimental effect should be balanced against the likely benefits of calcium on bone.
Trial registration Australian Clinical Trials Registry ACTRN 012605000242628.
Introduction
Evidence suggests that high calcium intakes might protect against vascular disease. Calcium supplementation has been shown to increase the ratio of high density lipoprotein cholesterol to low density lipoprotein cholesterol by almost 20% in healthy postmenopausal women.1 Studies in humans and animals suggest that these effects on cholesterol result from calcium binding to fatty acids and bile acids in the gut, leading to malabsorption of fat,2 3 although direct effects of calcitropic hormones on adipocytes have also been implicated.4 5 Cholesterol changes of this order may be associated with 20%-30% reductions in vascular event rates.6 7 In addition evidence suggests that calcium supplementation causes reductions in blood pressure,8 although these reductions are small and transient.9 Evidence is also inconsistent that high calcium intakes are associated with weight loss.9 These data from interventional studies are supported by observational evidence that calcium intake is inversely associated with vascular disease. The Iowa women’s health study, for example, found a one third reduction in deaths from cardiovascular events in women whose calcium intakes (diet or supplements) were in the highest fourth compared with those in the lowest fourth.10 The Boston nurses health study found that women in the highest fifth for calcium intake had an adjusted relative risk of ischaemic stroke of 0.69 (95% confidence interval 0.50 to 0.95) compared with those in the lowest fifth.11 A study in the United Kingdom reported a strong inverse relation between calcium intake and standardised mortality ratios for ischaemic heart disease.12 Communities with a calcium rich water supply also seem to have lower risks of cardiovascular events.13
Because of the high incidence of vascular disease in postmenopausal women any effects of calcium supplements on vascular health could be as important in terms of their effects on morbidity and mortality as their effects on bone. Although no randomised controlled trials have been designed primarily to assess the effect of calcium supplementation on vascular event rates or deaths, secondary analyses of the women’s health initiative study have recently shown no consistent effects in a population of average age 62.14 Interpretation of the data from this study is hampered by its failure to show consistent effects of calcium on bone density and fractures, poor adherence to study drugs, and a high rate of use of other vasoactive drugs, particularly oestrogen. We recently reported a five year study of calcium supplementation in 1471 healthy older women (mean age 74).15 We tested whether the potential vascular benefits from calcium use were evident in the context of a randomised controlled trial.
Methods
This study is a secondary analysis of a randomised controlled trial of calcium supplementation in healthy postmenopausal women, primarily designed to assess the effects of calcium on bone density and fracture incidence over five years. The methods have been described previously.1 15 Briefly, women were included in the study if they had been postmenopausal for more than five years, were aged 55 or more, and had a life expectancy of more than five years. We excluded women who were receiving treatment for osteoporosis or taking calcium supplements; those with any other major ongoing disease including hepatic, renal, or thyroid dysfunction, malignancy, or metabolic bone disease; and those with serum 25-hydroxyvitamin D levels less than 25 nmol/l. Participants gave written, informed consent to take part in the study.
Women were recruited by advertisement, and by post using electoral rolls. In total, 2421 women responded, of whom 641 were ineligible and 309 decided not to participate.
Participants received either 1 g of elemental calcium daily as the citrate (Citracal; Mission Pharmacal, San Antonio, TX) or identical placebo. They were asked to take two tablets (each containing 200 mg elemental calcium) before breakfast and three in the evening. We used a validated food frequency questionnaire to assess dietary calcium intake.16 Compliance was assessed by tablet counts. The women were followed up every six months for five years.
Cardiovascular event assessment
At each visit we recorded adverse events. We compared the frequency of events in the two groups: death, sudden death, myocardial infarction, angina, other chest pain, stroke, and transient ischaemic attack, and a composite cardiovascular end point consisting of sudden death, myocardial infarction, angina, or chest pain. We wrote a more detailed protocol before the analysis of vascular events, and this added an additional composite end point of myocardial infarction, stroke, or sudden death, as this has become the commonly used end point in such analyses. It also enabled adjudication of these events. When a myocardial infarction, stroke, or transient ischaemic attack was recorded we reviewed the participant’s medical records (completed by the family doctor or hospital). For women who died during the study we obtained the cause of death from hospital records or death certificates. To identify events that occurred during the study and were unreported by participants we searched the national database of hospital admissions for cardiovascular events (discharge codes 410-414 and 430-438, international classification of diseases, ninth revision). We also reviewed the hospital records related to these admissions.
We defined myocardial infarction in accordance with the Joint European Society of Cardiology and American College of Cardiology Committee criteria for acute, evolving, or recent myocardial infarction.17 Thus we classified an event as a myocardial infarction if the typical rise and fall of a biochemical marker for myocardial necrosis occurred with at least one of the following: ischaemic symptoms, development of diagnostic Q waves on the electrocardiogram, electrocardiographic changes indicative of ischaemia (ST segment elevation or depression), or coronary artery intervention. We defined stroke as a sudden focal neurological deficit of presumed vascular origin that persisted for more than 24 hours or that led to death, and transient ischaemic attack as a sudden focal neurological deficit of presumed vascular origin that persisted for less than 24 hours. Sudden death was defined in accordance with the international classification of diseases, ninth revision (code 798.2) as death occurring in less than 24 hours from onset of symptoms, not otherwise explained. Data for each event were compiled by a doctor and then adjudicated by a cardiologist (myocardial infarction and sudden death) or neurologist (transient ischaemic attack or stroke). All were blinded to the participants’ treatment group.
Statistical analysis
We used Student’s t test to compare the groups for continuous variables and Fisher’s exact test for the numbers of women experiencing an event. Results are presented as relative risks with 95% confidence intervals. We used Kaplan-Meier survival analysis to compare the proportion of women in each group experiencing an event over time, using Prism v 4.03 (GraphPad Software, San Diego, CA). We calculated the person time rates for events using Open-Epi V1.1 (www.openepi.com) and compared these using an exact midP statistic. Poisson regression was done using the GENMOD procedure of SAS. The number needed to treat or number needed to harm was calculated as the inverse of the absolute difference in event rates between the groups. The significance level was set at P<0.05; all tests were two tailed. Unless specified otherwise statistical analyses were done using the SAS software package version 9.1 and were based on intention to treat.
Results
Overall 1471 healthy postmenopausal women were randomised: 732 to calcium and 739 to placebo. Three hundred and thirty six women in the calcium group and 296 women in the placebo group stopped the study drug before the five year end point. Baseline characteristics were similar between the groups (table 1). Between baseline and five years significant changes were found in weight (−0.9 kg), systolic blood pressure (10 mm Hg), high density lipoprotein cholesterol levels (0.19 mmol/l), and low density lipoprotein cholesterol levels (−0.76 mmol/l); these did not differ between the groups. The flow chart of study participants has been presented previously.15 Overall, 90% of participants had complete follow-up. Among those still taking the study drugs at five years, tablet compliance was 85% in both groups in each six month period. When participants who discontinued the study drugs were included, compliance over the study was 58% in the placebo group and 55% in the calcium group (P=0.13).
Table 1.
Descriptive and biochemical characteristics of healthy, postmenopausal women assigned to calcium supplementation or to placebo. Values are means (standard deviations) unless stated otherwise
| Characteristics | Calcium group (n=732) | Placebo group (n=739) |
|---|---|---|
| Age (years) | 74.2 (4.2) | 74.3 (4.3) |
| Weight (kg) | 66.8 (11.1) | 67.0 (11.4) |
| Body mass index (kg/m2) | 26.5 (4.3) | 26.4 (4.2) |
| Serum creatinine (mmol/l) | 0.087 (0.015) | 0.086 (0.014) |
| Glomerular filtration rate* (ml/min/1.73 m2) | 61 (10) | 61 (11) |
| Adjusted calcium (mmol/l) | 2.32 (0.071) | 2.30 (0.065) |
| Glucose (mmol/l) | 5.1 (0.7) | 5.1 (0.7) |
| Total cholesterol (mmol/l)† | 6.73 (1.20) | 6.56 (1.04) |
| High density lipoprotein cholesterol (mmol/l)† | 1.65 (0.45) | 1.59 (0.40) |
| Low density lipoprotein cholesterol (mmol/l)† | 4.39 (1.16) | 4.26 (0.98) |
| Ratio of high density lipoprotein to low density lipoprotein† | 0.42 (0.19) | 0.40 (0.17) |
| Triglycerides (mmol/l)† | 1.55 (0.83) | 1.57 (0.73) |
| Dietary calcium (mg/day) | 861 (390) | 853 (381) |
| Physical activity (METS) | 33.6 (4.6) | 33.5 (4.3) |
| No (%) current smokers | 25 (3.4) | 19 (2.6) |
| No (%) former smokers | 295 (40.3) | 275 (37.2) |
| Systolic blood pressure (mm Hg) | 136 (23) | 135 (23) |
| Diastolic blood pressure (mm Hg) | 71 (11) | 70 (10) |
| No (%) with previous hypertension | 220 (30.1) | 207 (28.0) |
| No (%) with previous ischaemic heart disease | 59 (8.1) | 54 (7.3) |
| No (%) with previous dyslipidaemia | 67 (9.2) | 56 (7.6) |
| No (%) with diabetes | 19 (2.6) | 20 (2.7) |
| No (%) with previous stroke or transient ischaemic attack | 12 (1.6) | 7 (1.0) |
METS=metabolic equivalent intensity level.
*Estimated as recommended by the Australasian Creatinine Consensus Working Group.18
†Lipids were measured in a subset of 223 postmenopausal women (111 assigned to calcium supplementation and 112 assigned to placebo). Blood samples were taken fasting.
Table 2 shows the numbers of women with possible cardiovascular events that were self reported or reported by family members. Although no difference was found between groups in the number of women with any cardiovascular event (angina, chest pain, myocardial infarction, or sudden death), a statistically significant increase was found in the number of women who had a myocardial infarction (P=0.01). Similar trends were found in the calcium group compared with the placebo group for stroke, transient ischaemic attack, and sudden death. The composite end point of myocardial infarction, stroke, or sudden death was higher in the calcium group (P=0.008).
Table 2.
Potential vascular events self reported by healthy postmenopausal women assigned to calcium supplementation or to placebo or reported by family members. Values are numbers of women (numbers of events) unless stated otherwise
| Vascular event | Calcium group (n=732) | Placebo group (n=739) | P value* | Relative risk (95% CI) |
|---|---|---|---|---|
| Angina | 50 (88) | 71 (99) | 0.058 | 0.71 (0.50 to 1.01) |
| Myocardial infarction | 31 (45) | 14 (19) | 0.0099 | 2.24 (1.20 to 4.17) |
| Other chest pain | 16 (18) | 15 (16) | 0.86 | 1.08 (0.54 to 2.16) |
| Transient ischaemic attack | 33 (42) | 21 (27) | 0.10 | 1.59 (0.93 to 2.72) |
| Stroke | 40 (52) | 28 (34) | 0.14 | 1.44 (0.90 to 2.31) |
| Sudden death | 4 | 1 | 0.22 | 4.04 (0.45 to 36.0) |
| Angina, chest pain, myocardial infarction, or sudden death | 87 (155) | 93 (135) | 0.68 | 0.94 (0.72 to 1.24) |
| Myocardial infarction, stroke, or sudden death | 69 (101) | 42 (54) | 0.0075 | 1.66 (1.15 to 2.40) |
| Death | 34 | 29 | 0.52 | 1.18 (0.73 to 1.92) |
*Differences between groups in numbers of women with reported events, based on Fisher’s exact test.
Table 3 shows the number of women with cardiovascular events that were self reported or reported by family members and were confirmed by the adjudication process. A statistically significant increase was found in myocardial infarction in the calcium group, but there was not a significant increase in stroke or the composite end point (P=0.076).
Table 3.
Verified vascular events self reported by healthy postmenopausal women assigned to calcium supplementation or to placebo or reported by family members. Values are numbers of women (numbers of events) unless stated otherwise
| Vascular event | Calcium group (n=732) | Placebo group (n=739) | P value* | Relative risk (95% CI) |
|---|---|---|---|---|
| Myocardial infarction | 21 (24) | 10 (10) | 0.047 | 2.12 (1.01 to 4.47) |
| Stroke | 31 (34) | 22 (23) | 0.21 | 1.42 (0.83 to 2.43) |
| Sudden death | 3 | 3 | 1.0 | 1.01 (0.20 to 4.99) |
| Myocardial infarction, stroke, or sudden death | 51 (61) | 35 (36) | 0.076 | 1.47 (0.97 to 2.23) |
*Differences between groups in numbers of women with reported events, based on Fisher’s exact test.
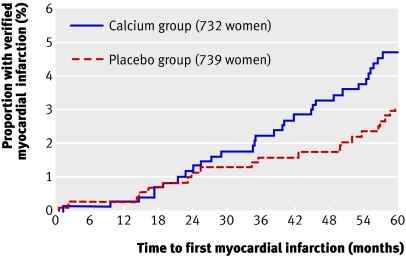
Table 4 shows adjudicated data, including events not reported by participants. A statistically significant increase in the number of women with any of the end points in the calcium group was no longer found. When the data were expressed as event rates, however, the rate ratios for both myocardial infarction and the composite end point were of borderline significance. When these data for myocardial infarction were plotted over time the groups began to diverge at about 24 months, and thereafter continued to separate (figure), although statistical significance was not achieved (P=0.14). Time course analyses of the proportion of women with a verified stroke or a verified composite end point showed a similar temporal pattern (P=0.2-0.3 for both analyses, data not shown).
Table 4.
Verified vascular events self reported by healthy postmenopausal women assigned to calcium supplementation or to placebo, reported by family members, and from the national database of hospital admissions in New Zealand.* Values are numbers of women (numbers of events) unless stated otherwise
| Vascular event | Calcium group (n=732) | Placebo group (n=739) | P value† | Relative risk (95% CI) | Calcium event rate/1000 person years (95% CI) | Placebo event rate/1000 person years (95% CI) | Rate ratio (95% CI) | P value‡ |
|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | 31 (36) | 21 (22) | 0.16 | 1.49 (0.86 to 2.57) | 11.1 (7.7 to 15.3) | 6.6 (4.2 to 10.0) | 1.67 (0.98 to 2.87) | 0.058 |
| Stroke | 34 (37) | 25 (26) | 0.23 | 1.37 (0.83 to 2.28) | 11.4 (8.0 to 15.7) | 7.8 (5.1 to 11.5) | 1.45 (0.88 to 2.49) | 0.15 |
| Sudden death | 3 | 6 | 0.51 | 0.51 (0.13 to 2.01) | 0.9 (0.2 to 2.7) | 1.8 (0.7 to 3.9) | 0.51 (0.10 to 2.04) | 0.36 |
| Myocardial infarction, stroke, or sudden death | 60 (76) | 50 (54) | 0.32 | 1.21 (0.84 to 1.74) | 23.3 (18.4 to 29.2) | 16.3 (12.2 to 21.3) | 1.43 (1.01 to 2.04) | 0.043 |
*Includes events not self reported by participants but found through the national database of hospital admissions and review of death certificates.
†Differences in numbers of women with verified events, based on Fisher’s exact test.
‡Differences in event rates between groups, based on Fisher’s exact test.
Kaplan-Meier survival plot showing proportion of healthy postmenopausal women assigned to calcium supplementation or to placebo that had a verified myocardial infarction during the study. Included are events self reported by participants and those from the national database of hospital admissions and review of death certificates (P=0.14 when compared by log rank test)
When the analyses in table 4 were restricted to events occurring in participants with more than 60% compliance since the start of the study no sudden deaths were found. For myocardial infarction, stroke, and myocardial infarction or stroke, event rates were little changed in the placebo group from those for the cohort shown in table 4 (6.6, 11.6, and 18.2), but event rates tended to be higher in the compliant members of the calcium group (15.5, 22.6, and 38.1). This was reflected in the respective rate ratios (2.4, 0.8 to 8.5, P=0.14; 2.0, 0.8 to 5.1, P=0.14; and 2.1, 1.1 to 4.4, P=0.03).
Poisson regression analysis was used to determine whether the effect of treatment on number of events per participant was independent of age and glomerular filtration rate (above or below median for each); history of ischaemic heart disease, stroke, hypertension, dyslipidaemia, and diabetes; and compliance with the study drug. The P values for the effect of treatment allocation were not significantly attenuated by adjustment for any of these potential confounders (table 5), but previous ischaemic heart disease and high compliance were identified as independent risk factors.
Table 5.
Poisson regression models of effects of calcium supplementation or placebo and other variables on composite end point (myocardial infarction, stroke, or sudden death) in healthy postmenopausal women
| Variable | χ2 | P value* |
|---|---|---|
| Model 1: | ||
| Intercept | 370.4 | <0.001 |
| Calcium or placebo | 3.72 | 0.05 |
| Log likelihood −444.4 | ||
| Model 2: | ||
| Intercept | 232.86 | <0.001 |
| Calcium or placebo | 3.77 | 0.05 |
| Glomerular filtration rate | 0.67 | 0.41 |
| Age group (split at median) | 20.07 | <0.001 |
| Log likelihood −432.3 | ||
| Model 3: | ||
| Intercept | 0.23 | 0.63 |
| Calcium or placebo | 2.99 | 0.08 |
| Glomerular filtration rate | 0.31 | 0.58 |
| Age group | 16.91 | <0.001 |
| History of cerebrovascular disease | 14.84 | 0.0001 |
| Dyslipidaemia | 2.69 | 0.10 |
| Diabetes | 2.34 | 0.13 |
| Hypertension | 0.00 | 0.96 |
| History of ischaemic heart disease | 4.31 | 0.04 |
| Compliance with study tablets | 9.32 | 0.002 |
| Log likelihood −419.4 |
*Based on Poisson regression and χ2 test.
No differences were found between groups in the number of women who had a verified ST segment elevation myocardial infarction or a verified non-ST segment elevation myocardial infarction, or in the number of women in whom the diagnosis of myocardial infarction was made by an elevated troponin level in the context of a recent operation or other important medical illness. Also, no differences were found between groups in the number of women with different clinical categories of stroke (partial anterior circulation infarct, total anterior circulation infarct, lacunar infarct, or posterior circulation infarct).19
The number of women needed to treat for five years to cause one myocardial infarction was 44, to cause one stroke was 56, and to cause one cardiovascular event was 29. By comparison the number needed to treat to prevent one symptomatic fracture was 50.
Discussion
Evidence that calcium might decrease the incidence of vascular events in healthy postmenopausal women is lacking, despite this study also showing a sustained improvement in the ratio of high density lipoprotein cholesterol to low density lipoprotein cholesterol.1 In fact the present data suggest the opposite, showing significant increases in the rates of adjudicated vascular events reported by the women allocated to calcium supplementation. This effect was more noticeable in those with high compliance with the study drug and seems to be progressive during the 30 to 60 months of the study, consistent with an initial latent period during which vascular damage occurs before event rates increase. That calcium supplementation might have adverse effects on vascular disease incidence is of concern because the morbidity and mortality that would follow from even a small adverse effect on vascular event rates is such that the beneficial effects of calcium supplementation on bone loss would be rapidly counterbalanced, as shown by the calculations of numbers needed to treat and harm in this study.
Because of the potential importance of this finding we searched for relevant events that had not been reported by participants. A national database for hospital admissions in New Zealand made this possible, although it is not normal practice to assess adverse events in clinical trials in this way. This process resulted in some attenuation of the calcium effect, although the rate ratios for myocardial infarction and for the composite end point (myocardial infarction, stroke, or sudden death) still had borderline significance (P value about 0.05). Thus the present study does not unequivocally show an adverse cardiovascular effect of calcium but suggests that this matter needs to be considered carefully before calcium supplementation can be broadly advocated.
Strengths and weaknesses of the study
The present study has several limitations, principally its small size for a study with cardiovascular end points. The cohort comprised elderly (10% aged more than 80 at baseline) and white participants, so the findings are not necessarily generalisable to other ages and racial groups. Its strengths are that it is a randomised controlled trial of a substantial dose of a highly bioavailable calcium supplement and has already shown unequivocal effects of this regimen on bone turnover and bone density. This was something the women’s health initiative did not achieve, possibly because of the low bioavailability of the supplement used by that study, the lower compliance with drug taking, or the competing effects of the use of oestrogen and non-trial calcium supplements.
Other studies
Some of the largest studies of calcium supplementation do not mention vascular events,20,21,22 although the randomised evaluation of calcium or vitamin D study did report a trend to higher death rates in those allocated to calcium (18.5%) than to placebo (16.3%).22 The findings in a study by Prince et al of 1460 postmenopausal women (mean age 75) randomised to calcium carbonate or placebo over five years were similar to our own.23 Few details of vascular events are given in that publication, but the hazard ratio for the diagnosis of incident ischemic heart disease was 1.12 (95% confidence interval 0.77 to 1.64). Thus the trend is in the same direction as in the present study although non-significant. These confidence intervals do, however, embrace the relative risk found in the present study.
A much larger randomised controlled trial of the effect of calcium carbonate and vitamin D supplementation has recently been published by the women’s health initiative investigators.14 This study of 36 000 women, followed over seven years, showed no overall effect of the supplements on cardiovascular event rates, with a hazard ratio for myocardial infarction or coronary heart disease death of 1.04 (95% confidence interval 0.92 to 1.18). When the composite end point of myocardial infarction, coronary heart disease death, coronary artery bypass graft, or percutaneous coronary intervention was used, however, the hazard ratio approached significance (1.08, 0.99 to 1.19). The participants in that study differed in several respects from those in the present study: they were younger (mean age 62), heavier (mean body mass index 29), had higher calcium intakes (mean 1150 mg/day), were taking vitamin D with the calcium, and 50% were taking hormone replacement therapy. Hazard ratios for vascular events in the supplemented group tended to be higher in older women and the interaction with body mass index was significant such that women with lower values had a higher cardiovascular risk associated with the use of calcium and vitamin D. Thus the hazard ratio for myocardial infarction for women with a body mass index less than 25 was 1.16 and for death from coronary heart disease for women with a body mass index of 25-30 was 1.18, findings similar to those in the present study (relative risk of myocardial infarction, stroke or sudden death, 1.21) in which the mean body mass index was 26.5. Also, in the women’s health initiative the trend was towards an adverse effect of calcium supplementation in those with baseline calcium intakes of less than 800 mg/day (hazard ratio 1.12). If calcium supplementation does contribute to vascular adverse events, then the use of a less bioavailable supplement in that study may have reduced the size of any adverse effect, as may the poor compliance. The use of oestrogen by half the participants and the administration of vitamin D with calcium might have also obscured any vascular effects of the calcium supplementation. Thus the results of the present trial are not incompatible with those of the randomised evaluation of calcium or vitamin D study, the study by Prince et al, or the women’s health initiative. Taken together these four studies raise major concerns about the cardiovascular safety of calcium supplementation, particularly with respect to myocardial infarction in older postmenopausal women.
Possible mechanisms
The finding of an adverse trend in vascular events with calcium supplementation is not necessarily surprising, since calcium supplements acutely elevate serum calcium levels24 possibly accelerating vascular calcification, which is predictive of vascular event rates.25 High calcium intakes have also been associated with brain lesions on magnetic resonance imaging scans26 and with vascular calcification27,28,29,30 and mortality31 32 in patients who receive dialysis. Data from patients requiring dialysis may be relevant to the present study population because of the age of our participants and the associated reduced renal function. The trend to more frequent cardiac events in the calcium group does not seem to be secondary to changes in lipids or other risk factors, since calcium supplementation was associated with beneficial trends in levels of high density lipoprotein cholesterol and low density lipoprotein cholesterol in the present study,1 and these were sustained until four years’ follow-up, although the differences between groups attenuated in the final year (data not shown). Similar beneficial trends in cholesterol fractions were also shown in the women’s health initiative.14
Unanswered questions and future research
The data on vascular events from a secondary, preplanned analysis of the Auckland calcium study are not conclusive but suggest that high calcium intakes might have an adverse effect on vascular health. The similarities between these findings and those from the dialysis literature suggest that this might be a particular concern in those with poor renal function, particularly elderly people. The subgroup analyses available within the women’s health initiative would be consistent with this hypothesis. The present data do not permit definitive conclusions to be reached in this regard but do flag cardiac health as an area of concern in relation to calcium use and mandate that this is assessed carefully in future studies of calcium supplementation. In the meantime this potentially detrimental effect should be balanced against the likely benefits of calcium on bone,33 particularly in elderly women.
What is already known on this topic
Calcium supplementation produces changes in serum lipids that might impact favourably on the incidence of cardiovascular disease
Although observational data support this idea, evidence from trials is lacking
What this study adds
Healthy older women randomised to calcium supplementation showed increased rates of myocardial infarction
This effect could outweigh any benefits on bone from calcium supplements
Contributors: PAB and RD adjudicated the cases. MJB, BM, AH, and RA collected the data. MJB, GDG, and IRR analysed the data. IRR conceived the study. He is guarantor for the paper. All authors designed the study and drafted and revised the manuscript.
Funding: This study was supported by the Health Research Council of New Zealand. Mission Pharmacal supplied the calcium citrate tablets and placebo.
Competing interests: IRR has received research support from and acted as a consultant for Fonterra and Mission Pharmacal.
Ethical approval: This study was approved by the Auckland ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reid IR, Mason B, Horne A, Ames R, Clearwater J, Bava U, et al. Effects of calcium supplementation on serum lipid concentrations in normal older women: a randomized controlled trial. Am J Med 2002;112:343-7. [DOI] [PubMed] [Google Scholar]
- 2.Govers MJAP, Vandermeer R. Effects of dietary calcium and phosphate on the intestinal interactions between calcium, phosphate, fatty acids, and bile acids. Gut 1993;34:365-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr 1993;123:1047-53. [DOI] [PubMed] [Google Scholar]
- 4.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J 2000;14:1132-8. [PubMed] [Google Scholar]
- 5.Kelly KA, Gimble JM. 1,25-dihydroxy vitamin D-3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology 1998;139:2622-8. [DOI] [PubMed] [Google Scholar]
- 6.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9. [PubMed] [Google Scholar]
- 7.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301-7. [DOI] [PubMed] [Google Scholar]
- 8.Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure—an updated metaanalysis of randomized controlled trials. Am J Hypertens 1999;12:84-92. [DOI] [PubMed] [Google Scholar]
- 9.Reid IR, Horne A, Mason B, Ames R, Bava U, Gamble GD. Effects of calcium supplementation on body weight and blood pressure in normal older women: a randomized controlled trial. J Clin Endocrinol Metab 2005;90:3824-9. [DOI] [PubMed] [Google Scholar]
- 10.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol 1999;149:151-61. [DOI] [PubMed] [Google Scholar]
- 11.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999;30:1772-9. [DOI] [PubMed] [Google Scholar]
- 12.Knox EG. Ischaemic-heart-disease mortality and dietary intake of calcium. Lancet 1973;ii:1465-7. [DOI] [PubMed]
- 13.Dawson EB, Frey MJ, Moore TD, McGanity WJ. Relationship of metal metabolism to vascular disease mortality rates in Texas. Am J Clin Nutr 1978;31:1188-97. [DOI] [PubMed] [Google Scholar]
- 14.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846-54. [DOI] [PubMed] [Google Scholar]
- 15.Reid IR, Mason B, Horne A, Ames R, Reid HE, Bava U, et al. Randomized controlled trial of calcium in healthy older women. Am J Med 2006;119:777-85. [DOI] [PubMed] [Google Scholar]
- 16.Angus RM, Sambrook PN, Pocock NA, Eisman JA. A simple method for assessing calcium intake in Caucasian women. J Am Diet Assoc 1989;89:209-14. [PubMed] [Google Scholar]
- 17.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-69. [DOI] [PubMed] [Google Scholar]
- 18.Mathew TH, Australasian Creatinine Consensus Working Group. Chronic kidney disease and automatic reporting of estimated glomerular filatration rate: a position statement. Med J Aust 2005;183:138-41. [DOI] [PubMed] [Google Scholar]
- 19.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. [DOI] [PubMed] [Google Scholar]
- 20.Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 1994;308:1081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D-3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 2002;13:257-64. [DOI] [PubMed] [Google Scholar]
- 22.Grant AM, Anderson FH, Avenell A, Campbell MK, Cooper C, Donaldson C, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365:1621-8. [DOI] [PubMed] [Google Scholar]
- 23.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure—results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 2006;166:869-75. [DOI] [PubMed] [Google Scholar]
- 24.Reid IR, Schooler BA, Hannon S, Ibbertson HK. The acute biochemical effects of four proprietary calcium supplements. Aust N Z J Med 1986;16:193-7. [DOI] [PubMed] [Google Scholar]
- 25.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004;164:1285-92. [DOI] [PubMed] [Google Scholar]
- 26.Payne ME, Anderson JJB, Steffens DC. Calcium and vitamin D intakes are positively associated with brain lesions in depressed and non-depressed elders. FASEB J 2007;21:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62:245-52. [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 2005;68:1815-24. [DOI] [PubMed] [Google Scholar]
- 29.Asmus HG, Braun J, Krause R, Brunkhorst R, Holzer H, Schulz W, et al. Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dialysis Transplant 2005;20:1653-61. [DOI] [PubMed] [Google Scholar]
- 30.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478-83. [DOI] [PubMed] [Google Scholar]
- 31.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 2007;71:438-41. [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 2004;15:770-9. [DOI] [PubMed] [Google Scholar]
- 33.Tang BMP, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657-66. [DOI] [PubMed] [Google Scholar]