Abstract
The policy debate about price, value, and innovation in pharmaceuticals is at a critical stage for the NHS. Claxton and colleagues describe the key principles of value based pricing and consider some of the concerns about such a scheme
Policy background
The NHS spends about £11bn (€15bn, $22bn) annually on pharmaceuticals, of which £8bn is on branded drugs, representing about 13% and 10% respectively of available resources.1 As growth in NHS funding is expected to slow, access to innovative technologies will depend on savings found elsewhere. The UK Department of Health uses the pharmaceutical price regulation scheme to control expenditure on branded drugs. This 50 year old scheme is intended to control the profits companies can earn through periodic and general price cuts. A report by the Office of Fair Trading recommended reform, basing a drug’s price on its health benefits.1 2 The government has confirmed its intention to renegotiate the existing scheme,3 and a recent House of Commons Health Committee report has also welcomed reform.4 5 The policy debate about price, value, and innovation is at a critical stage for the NHS.
The principles of value based pricing
Establishing the value of a drug requires an assessment of whether the additional health expected to be gained from its use exceeds the health forgone as other NHS treatments are displaced by its additional cost—a comparison of the incremental cost effectiveness ratio (ICER, the ratio of the additional health gained to the additional costs) with a threshold for cost effectiveness (see box 1). The National Institute for Health and Clinical Excellence (NICE) uses a threshold of £20 000 to £30 000 per QALY, representing an estimate of the health forgone based on the estimated productivity of other NHS activities.6 This range maybe too high.7 8 Critically, this key determinant of value ought to be removed from the heat of political negotiation and become an empirical question subject to scientific analysis as recently recommended by the health select committee.4 5
Box 1 Value and the cost effectiveness threshold
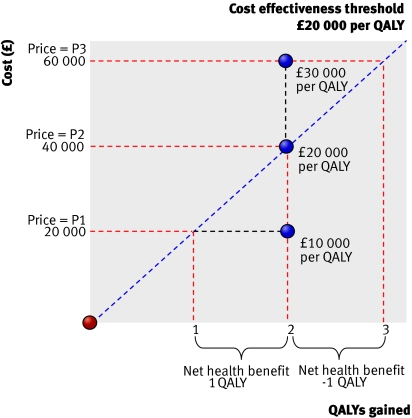
The concept of value and its relation to price, cost effectiveness, and the cost effectiveness threshold is illustrated in figure 1.
Fig 1 Value, price, and cost effectiveness
At price P1 a new drug offers a gain of two units of health outcome, here expressed as quality adjusted life years (QALYs), at an additional cost to the NHS of £20 000—an incremental cost effectiveness ratio (ICER) of £10 000 per QALY gained. The key question is whether the expected gain of two QALYs is greater than the health outcomes forgone elsewhere as other NHS treatments are displaced by the additional cost. In figure 1 this is represented by a cost effectiveness threshold set at £20 000 per QALY—that is, every £20 000 found from existing resources displaces one QALY elsewhere in the NHS. At price P1 the technology is thus expected to improve health by two QALYs and displace only one QALY elsewhere. There is a net health benefit of one QALY to the NHS as a whole and the technology is cost effective (the ICER is less than the cost effectiveness threshold)
If the price of the drug were P2 then the NHS would anticipate the same health gains of two QALYs but at a higher additional cost of £40 000—an ICER of £20 000 per QALY (which is the same as the threshold). These higher additional costs will now be expected to displace two QALYs. The gains in health (two QALYs) are just offset by the health forgone elsewhere (also two QALYs) and overall net health benefits to the NHS are zero
At a higher price, such as P3, the costs increase to £60 000—an ICER of £30 000 per QALY. Now the health forgone (three QALYs) exceeds the health gained from the technology (two QALYs) and overall net health benefits are negative (minus one QALY). The technology is not cost effective at price P3 and does more harm than good in terms of the overall health of the nation.
Negotiating a price at which an ICER is just equal to the threshold ensures that the health benefits offered by the drug are just offset by the health displaced elsewhere in the NHS, so all the benefits of the innovation go to the manufacturer in the form of revenue (see box 1)—the net health benefits to the NHS are zero. If cheaper generics become available after the patent expires then the NHS may ultimately benefit. Cost effectiveness, however, will usually vary between subgroups of patients. Consequently, by offering a manufacturer a menu of prices, each associated with complementary guidance on NHS use (higher prices being permitted only when guidance restricts use to more cost effective subgroups), positive net health benefits can be achieved (see box 2). The NHS will share the benefits of innovation with the manufacturers, providing incentives for the NHS to increase uptake.9
Box 2 Price, guidance, and volume
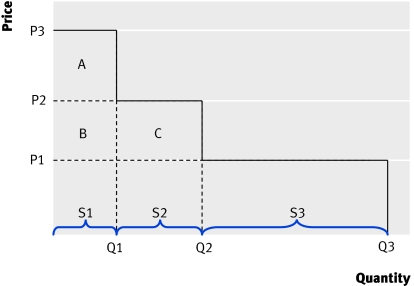
Figure 2 shows the relation between price and guidance, or the “NHS demand curve.”
Fig 2 Price, guidance, and volume
Three subgroups of patients (S1, S2, S3) are represented; the drug is most cost effective for group S1 and least cost effective for S3. A menu of prices and associated guidance is available to the manufacturer:
Choose a high price P3 but with restrictive guidance for use in S1 only, selling Q1 (revenue of P3×Q1)
Choose P2 and get wider approval for S1 and S2, and sell Q2 (revenue of P2×Q2)
Choose a low price (P1) and get unrestricted guidance for this indication (S1, S2, and S3) and sell Q3 (revenue of P1×Q3).
The NHS can gain net health benefits from technological innovation (before the entry of generics) only if the price is set at the level of the least cost effective subgroup. For example, at P2 guidance restricts use to groups S1 and S2. The benefits of the technology for S2 just outweigh the health displaced elsewhere (there is no net health benefit from using the technology in this group). But for group S1 there is positive net benefit equivalent to area A in monetary terms (ICER is lower than the threshold). At the lower price of P1 there is unrestricted guidance, the NHS gets more benefit from technological innovation (area A, B, C) and in this case the manufacturer also gets more revenue by choosing this “lower price-wider coverage” option.
In the short run the principles of value based pricingmean that drugs will be approved for use only at prices that ensure that their expected health benefits exceed the health displaced. In the longer run, prices based on value to the NHS will provide incentives for manufacturers to develop technologies that are more likely to be of value and cost effective. The responsibility of the NHS, through assessment authorities like NICE, is to signal clearly and reliably what is of value. It is then for manufacturers to choose to invest in the development of drugs that they believe will be both cost effective for the NHS and command prices and sales which provide a satisfactory return on investment.
Some concerns
Several concerns can be raised about the consequences of value based pricingfor the pharmaceutical sector.10,11,12,13,14,15
NHS drug spend
Value based pricingmight lead to a lower NHS spend on drugs.12 It would certainly lead to a reallocation of revenue from less to more valuable technologies. This would mean a reduction in price for some drugs, like premium priced branded drugs when an equivalent generic is available. Lower prices for some drugs, however, does not necessarily imply a reduction in the overall NHS drugs spend, which might increase, particularly if new and valuable pharmaceuticals are developed that can command higher prices.
Domestic research and development
Companies that have invested in research in the UK might regard value based pricingas the “last straw” and relocate if they fear a reduction in revenue.10 It is, however, a myth that the current pharmaceutical price regulation scheme incentivises inward investment. In fact the scheme legally cannot distinguish between companies with research located in the UK and those elsewhere.1 Choice of location is directly influenced by a range of incentives including investment in infrastructure, the degree of public investment in research, and local costs. It is difficult to imagine a situation in which the local market would directly influence this choice because products developed in one location can be produced and marketed worldwide. The concern is more readily explicable as a threat: provide easy access to a subsidised domestic market or research will relocate.16 Whether such threats are credible, and whether public policy should respond if they are, is for others to judge. Concern for inward investment is evidently legitimate. The question is whether it is appropriate to use NHS resources for industrial policy rather than the improvement of health, particularly when other more effective incentives and legal policy levers are available.
Incentives for “me too” products
There might also be concerns that drugs that are second or subsequent to market (“me too”) will be disadvantaged.10 Value based pricing,however, means that their manufacturers could charge the same price as the incumbent (if they are equivalent) and have the same NICE guidance, or command a higher price if they can demonstrate additional health benefits. They will necessarily have a shorter period to generate revenue before generics become available but, if industry is hoping to continue to sell at premium prices when equivalent generics become available, then value based pricing, or any other policy to encourage appropriate prescribing, will undermine these expectations.
Value of innovation
The potential value of an innovative technology—that is, one that is likely to lead to the development of more valuable future technologies—may not be recognised.17 In other markets private sector companies anticipate the benefits of innovation and the future market returns that flow from better products.
What is different in health care is that neither consumers (patients) nor their agents (doctors) are well placed to identify and synthesise all relevant evidence and undertake the computation required to assess value fully. Even if such individual assessments were possible they are unlikely to lead to decisions consistent with the NHS’s collectively agreed objectives and resource constraints. Instead NICE has responsibility for assessing and signalling value on behalf of the whole NHS. So why should the NHS pay more than the value of the benefits from a new technology in the hope that a more valuable future technology will be developed? To do so is to pay twice for the innovation and to encourage inappropriate investments in innovations that are not worth while.9
Some critical issues
Although many of these concerns seem ill founded, there are dangers in a value based pricingscheme. A poorly specified system could reduce rather than improve health and undermine the evidence base for future NHS practice.
Cost effectiveness threshold and subgroups
The cost effectiveness threshold has a key role in value based pricing(box 1). Therefore, its assessment ought to be transparent and based on independent scientific analysis.4 Moreover, the NHS will share the benefits of innovation only if the assessment body has the power to demand that manufacturers analyse patients by subgroup or else has the resources and access to data to conduct the analysis itself (box 2). Manufacturers may try to offer “all or nothing deals” to get higher prices with an unrestricted NICE recommendation. Avoiding this requires explicit and transparent pricing rules and sufficient independence to say “no” to such offers.
Price and the need for evidence
Positive guidance on a new drug will have an impact on the prospects of acquiring further evidence to support its use because the incentives for manufacturers to conduct evaluative research are removed and the clinical community may regard further experimental research as unethical. Price negotiation and guidance ought therefore to account for both the value of the technology and the value of the evidence that may be forgone for future NHS patients.9 18 19
Additional evidence might be provided while a drug is approved for use in the NHS. However, this type of “risk sharing” will need to establish when additional evidence is needed, the type of evidence required, and whether it can be gathered while the technology is approved for use. For example, the type of observational registry data that are often envisaged will be unable to provide more precise estimates of relative treatment effects because a comparable control group would not be available. If the type of evidence required cannot be provided once a drug is approved, then price ought to be reduced so that its cost effectiveness is less uncertain. The health select committee recommended that price and the value of evidence ought to be linked.4 This would provide incentives for manufactures to invest in the type of evidence needed by the NHS early in the development of their products. Other things being equal, those who do invest, and reduce the uncertainty surrounding the cost effectiveness of their product at launch, would be rewarded with higher prices than those who do not. 9,18
Assessment vehicle
Robust assessments of cost effectiveness are critical, and there are detailed issues of methods, processes, and institutional arrangements that need to be resolved. Much of the assessment needed to implement value based pricingis already being conducted by NICE.20 The existing process, however, would need to be strengthened to form a suitable vehicle for price negotiation.4 5 9 21 The appropriate institutional arrangements seem to follow naturally from the principles of value based pricing. These arrangements suggest that a suitable threshold, common to NICE and value based pricing, should be based on scientific analysis and be the responsibility of an independent authority, and that a single assessment and pricing authority should assess cost effectiveness, establish value based prices, and issue guidance to the NHS or that these functions should be closely linked. A pricing authority with political independence seems essential.
It is not so much price “negotiation” that is required but scientific deliberations between an assessment authority, the manufacturer, and other stakeholders concerning what the available evidence implies for estimates of cost effectiveness, price, and guidance. Any disputes will necessarily turn on explicit scientific questions that can ultimately be resolved through further investigation and a suitable appeal process. Decisions about price and guidance will inevitably be controversial, but responsibility for rejecting or restricting access to a new technology will be more appropriately shared between an NHS that cannot afford wider use of an effective but cost ineffective technology and a manufacturer unwilling to accept a price that would make it cost effective.
Conclusions
Scrapping the current pharmaceutical price regulation scheme provides important opportunities for the NHS. The principles of value based pricing are consistent with recent developments in NHS decision making and healthcare research.4 20 22 An appropriately implemented value based pricingscheme could offer significant benefits to the NHS in the short and longer term. There are, however, some dangers. A poorly specified pricing scheme could damage rather than improve the NHS and could undermine the evidence base for future NHS practice. The current pharmaceutical price regulation scheme is dead. The debate about what principles should guide its renegotiation, the meaning of value, and the relation between guidance, price, value, and evidence is, however, very much alive.
Summary points
The NHS spends around £11bn a year on pharmaceuticals
Access to innovative technologies will depend on savings found elsewhere in the NHS budget
A report by the Office of Fair Trading on the pharmaceutical price regulation scheme recommended reform, abandoning profit and general price controls in favour of value based pricing
The government has confirmed an intention to renegotiate the existing pricing scheme over coming months
An appropriately implemented value based pricingscheme could offer significant benefits to the NHS in the short and longer term
Although some concerns do not seem well founded there are dangers associated with a value based pricing scheme
A poorly specified system could lead to the NHS being damaged rather than improved by innovation and could undermine the evidence base for future NHS practice
Contributors and sources: The authors have extensive experience in health economics in general and, specifically, in economic analysis to support decisions about medical technologies. KC, MJB, and MJS are current or past members of the NICE Technology Appraisal Committee, and AJC is a past vice chairman of NICE and the current chair of its research and development committee. KC and AB were consulted by the Office of Fair Trading in the preparation of their report on pharmaceutical pricing. The paper arose out of discussions between the authors and presentations by KC at a symposium on value based pricing held by the Office of Health Economics/University of York and Wiley in 2007. All authors were involved in discussions about the concepts in the paper, together with its format. KC drafted the paper; AB, MJB, AJC, CM, SW, and MJS all contributed to editing. KC is guarantor.
Funding: University of York receives funding from the Medical Research Council for a methodological programme of work on economic evaluation.
Competing interests: AJC chairs NICE’s research and development advisory committee, for which the University of York receives payment.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Office of Fair Trading. The pharmaceutical price regulation scheme. An OFT market study London: Office of Fair Trading, 2007. www.oft.gov.uk/shared_oft/reports/comp_policy/oft885.pdf
- 2.Collier J. The pharmaceutical price regulation scheme. BMJ 2007;334:435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Business Enterprise and Regulatory Reform. Interim government response to Office of Fair Trading (OFT) market study on PPRS London: Department of Business Enterprise and Regulatory Reform, 2007. (URN 07/1247.) www.berr.gov.uk/files/file40756.pdf
- 4.National Institute for Health and Clinical Excellence. First report of the health committee 2007-2008. London: Stationery Office, 2008. (HC27-I.)
- 5.Collier J. Parliamentary review asks NICE to do better still. BMJ 2008;336:56-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culyer AJ, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, et al. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. J Health Serv Res Pol 2007;12:56-8. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Rice N, Smith PC. The link between health care spending and health outcomes: evidence from English programme budgeting data University of York: Centre for Health Economics, 2007. (Research Paper 24.) [DOI] [PubMed]
- 8.National Institute for Health and Clinical Excellence. Clinical and Public Health Directorate progress report London: NICE, 2007. www.nice.org.uk/page.aspx?o=399398
- 9.Claxton K. OFT, VBP: QED? Health Econ 2007;16:545-58. [DOI] [PubMed] [Google Scholar]
- 10.A new prescription. Economist 2007;382:68-9. [Google Scholar]
- 11.Boseley S. Drugs price fixing scheme costs health service millions, says OFT. Guardian 2007. February 20.
- 12.Opinion. OFT’s bitter pill will just have to be swallowed, Business Commentary. Times 2007. February 21.
- 13.Leader. In search of a perfect price for drugs. Financial Times 2007. February 21.
- 14.Brown C. Drug firms accused of ‘ripping off’ the NHS. Independent 2007. February 21.
- 15.Stagg C. Can we afford medical progress. New Statesman 2007. June 11.
- 16.Evans R, Boseley S. Drug firms’ lobby tactics revealed. Guardian 2006. September 28.
- 17.Boseley S. Open up NHS to our drug firms, White House demands. Guardian 2006. November 14.
- 18.Griffin S, Claxton K, Palmer S, Sculpher MJ. Dangerous omissions: the consequences of ignoring decision uncertainty. Med Decison Making 2007;27:E8. [DOI] [PubMed] [Google Scholar]
- 19.Claxton K, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmacoeconomics 2006;24:1055-68. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal London: NICE, 2004 [PubMed]
- 21.Buxton MJ, Akehurst R. How NICE is the UK’s fast-track system? Scrip Magazine 2006;152:24-5. [Google Scholar]
- 22.Cooksey D. A review of UK health research funding London: Stationery Office, 2006