Abstract
The E6 oncoprotein from high-risk HPV types activates human telomerase reverse transcriptase (hTERT) transcription in human keratinocytes. Studies on how E6 regulates hTERT have implicated E-box or X-box elements in the hTERT promoter (Veldman et al., Proc Natl Acad Sci USA 2003;100:8211–14; Oh et al., J Virol 2001;75:5559–66; Gewin et al., Genes Dev 2004;18:2269–82), but the mechanism of activation by E6 is still controversial and not well defined. Here, we demonstrate that induction of both hTERT expression and telomerase activity by HPV-16 E6 in early passage keratinocytes is associated with acetylation of histone H3 at the hTERT promoter, is dependent on the E6 associated protein (E6AP) and is not exclusively reliant on E-box or X-box elements. Further increases in histone acetylation of the hTERT promoter and hTERT transcriptional activity in E6 expressing cells that had been passaged extensively in culture were found to occur only with the endogenous promoter and not with an exogenously introduced hTERT promoter construct. Telomerase activity at both early and late passages, however, was dependent on E6AP expression, implying a continued reliance on E6 function for telomerase activity. Our results demonstrate that E6 induces hTERT promoter acetylation, but that further increases in telomerase activity and histone acetylation in later passage E6 expressing cells are independent of E6 activation of the core hTERT promoter. We also provide evidence that the transcription factor p300 is a potential repressor of telomerase activation and histone acetylation in the context of E6 expression. These studies give insight into how immortalization by HPV results in upregulation of hTERT and furthers our understanding of how telomerase is activated during the process of malignant transformation.
Keywords: E6, hTERT, telomerase, p300, histone acetylation, E6AP
E6 and E7 early viral proteins of the high-risk HPV types are responsible for molecular and cellular changes that potentiate oncogenic transformation. These 2 proteins, which are expressed throughout the viral life cycle, are sufficient for immortalization of many cell types and for oncogenic transformation of some murine cell types. E7 binds and inactivates the retinoblastoma tumor suppressor protein (Rb), promoting progression of the cell cycle.1 E6 further promotes cell cycle progression by binding and directing the degradation of the tumor suppressor protein p53,2 which can also function in normal cells to induce apoptosis under cell stress or DNA damage. The degradation of p53 is accomplished through formation of a complex between p53, E6 and the E6-associated cellular ubiquitin ligase E6 associated protein (E6AP). E6 is a multifunctional protein with many cellular protein targets besides p53. One such protein of potential importance to the cancerous phenotype is the transcription factor p300, which generally has antiproliferative effects.
E6 is also responsible for transcriptional activation of the telomerase reverse transcriptase (TERT) gene,3 which is the catalytic subunit of the enzyme telomerase. Normal somatic cells are TERT negative and are subjected to shortening of telomeric repeats at chromosomal termini upon each cycle of replication. This loss of telomere sequence leads to replicative senescence, thus limiting the lifespan of cells. In addition to allowing immortalization, which is a critical step in the malignant transformation of cells, human telomerase reverse transcriptase (hTERT) expression may also induce proliferation in quiescent cells independently of activity on telomeres.4 In high-risk HPV E6 expressing cells, increased telomerase activity due to transcriptional activation of TERT maintains telomere length, allowing cells to avoid replicative senescence and become immortal. Although hTERT upregulation and telomerase activation are observed in early passage cells that express E6/E7, significant increases in telomerase activity are observed as cells are passaged in culture.5 E6 levels in these cells do not change as they are passaged. This suggests that other cellular changes are necessary for high levels of telomerase in later passage E6/E7 expressing cells; however, the mechanism behind this increase is not known. Studies involving various mutants of E6 indicate that hTERT activation correlates with the ability of E6 to bind E6AP.3,6,7 Recent evidence more strongly implicates the necessity for E6AP for hTERT activation, as knockdown of E6AP levels in human cells or knock-out of E6AP in mouse cells abrogates the ability of E6 to activate hTERT transcription and telomerase activity.8 However, the ability of E6 to activate the hTERT promoter is entirely independent of binding and degradation of p533 and binding of PDZ domain containing proteins.9
In normal cells, telomerase activity is generally regulated by transcriptional control of the hTERT promoter. Although hTERT transcription can be regulated by alternative splicing during fetal development,10 its expression is repressed in fully differentiated somatic tissues. It has been found in differentiated epithelial cells that hTERT is transcriptionally repressed after loss of anchorage in association with recruitment of histone deacetylases (HDACs) to the hTERT promoter.11 Inhibition of HDACs is sufficient to induce histone acetylation and activate the hTERT promoter, and overexpression of HDAC1 is sufficient to repress this hTERT promoter activity in normal human cells.12 The proximal 181 base pairs of the hTERT promoter is a sufficient element for regulation by HDACs.13 Many of the transcription factors that have binding sites at the hTERT promoter are associated with coregulators with histone acetyl transferase (HAT) activity. HAT and HDAC activity are exhibited by a variety of transcriptional coactivators and corepressors, respectively.
Repression of hTERT expression in differentiated cells and activation in E6 expressing cells is dependent on a proximal promoter sequence containing potential and proven binding sites for a variety of transcription factors.14-16 Approximately 250 base pairs of promoter sequence proximal to the transcriptional start site are sufficient for full activation of the hTERT promoter by E6. This core promoter contains 2 Myc binding sites (E-box), 5 Sp1 sites, an E2F site, an NFX binding site (X-box) and putative sites for Ets and NFκB. Both Myc and Sp1 sites have been implicated by some as important for full activation of the hTERT promoter by E6.16,17 More recently, X-box elements have been suggested to have a role in E6 activation of hTERT expression.18 Clearly, hTERT promoter activation by E6 is complex and controversial, and the underlying mechanisms of activation by E6 are not well understood.
In our study, we provide evidence that the hTERT promoter is activated by E6 in association with acetylation of histone H3 at the hTERT promoter. Acetylation of H3 and activation of the hTERT promoter by E6 is dependent on E6AP and enhanced by knockdown of p300. Furthermore, we demonstrate that H3 acetylation of the endogenous hTERT promoter and activation of the promoter are enhanced at late passage in E6E7 immortalized keratinocytes. Our data suggests that the late passage enhancement of hTERT promoter acetylation and activation is independent of E6, and is an exclusive selective property of the endogenous chromatin. The late passage enhancement is associated with a decrease in p300 levels in the cell, which may play a role in upregulation of hTERT transcription. We also provide evidence suggesting that neither E-box nor X-box elements are the sole determinants of hTERT activation by E6.
Material and methods
Cells and culture
Primary human foreskin keratinocytes (HFKs) or those stably transduced with LXSN retroviral vectors expressing wild-type HPV16-E7 or HPV16-E6 and E7 were cultured in E-media with feeder fibroblasts as previously described.19
Plasmid constructs
A pBABE-puro vector expressing shRNA against E6AP was a gift from Denise Galloway, Fred Hutchinson Cancer Research Center, Seattle, WA.18 shRNA constructs for p300 and controls were created using pSIREN-RetroQ vectors (BD Biosciences) according to manufacturer's protocol. The following oligonucleotides were used to create the shRNA sequences: p300-1, top, 5′-GATCC GTACC TCGTG ATGCC ACTTA CTCAA GAGAT AAGTG GCATC ACGAG GTATT TTTTA TCGAT G-3′; bottom, 5′-AATTC ATCGA TAAAA AATAC CTCGT GATGC CACTT ATCTC TTGAG TAAGT GGCAT CACGA GGTAC G-3′; p300-2, top, 5′-GATCC GTTTG TGCTC TTGTG CCCTC CTCAA GAGAG AGGGC ACAAG AGCAC AAATT TTTTA TCGAT G-3′; bottom, 5′-AATTC ATCGA TAAAA AATTT GTGCT CTTGT GCCCT CTCTC TTGAG GAGGG CACAA GAGCA CAAAC G-3′; neg (random insert), top, 5′-GATCC GACAC GAGAT GGTGC ACTGA CGCTT CCAGG TGGTT AGGGA TCAGT TGTTA ACTTC CAAGT AGAGT ACGTC CGGCC GTCAA GACCT GGCCC TACGC TTGTT GACTG TTTTT TATCG ATG-3′; bottom, 5′-AATTC ATCGA TAAAA AACAG TCAAC AAGCG TAGGG CCAGG TCTTG ACGGC CGGAC GTACT CTACT TGGAA GTTAA CAACT GATCC CTAAC CACCT GGAAG CGTCA GTGCA CCATC TCGTG TCG-3′. Underlined sequences represent target sequences.
hTERT promoter constructs in pGL3 luciferase reporter vectors (Promega) were driven by the core 255 base-pair region of the hTERT promoter (TERT-luc). The E-box mutant hTERT construct (TERT-E-luc) was mutated at the downstream E-box from CACGTG to CACCCG. Both wild type and E-box mutant constructs were gifts from Izumi Horikawa, NCI, NIH. The X-box mutant (TERT-X-luc) was created using site-directed polymerase chain reaction (PCR) mutagenesis of the wild type construct with the following primers: forward 5′-CCTGC TGCGC ACGTG CGTCG CCCAT GCCGG GCTCG AGATC TGC-3′, reverse 5′-GCAGA TCTCG AGCCC GGCAT GGGCG ACGCA CGTGC GCAGC AGG-3′, mutating the highly conserved bases of the NFX binding site, GGGAAGCCCTG, to GCGTCGCCCAT.
Retroviral infections
Phoenix cells were transfected with retroviral constructs using GenePorter reagents and protocols (GTS). Keratinocytes were grown to approximately 40% confluence before infection with 1 ml of retroviral supernatant from Phoenix cell cultures in 10 ml of media with 8 μg/ml polybrene. At 24 hr postinfection, cells were washed and fresh media was added. At 48 hr postinfection, cells were split 1:4 with feeders. At 72 hr postinfection, cells were selected for infection with 150 μg/ml neomycin for approximately 7 days, or 1 μg/ml puromycin for 3 days. Fresh media with antibiotics were added each day. Surviving cells were pooled. Mock infected cells were also subjected to selection, none of which survived.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were conducted according to the Acetyl-H3 ChIP Assay Kit (Upstate). Cells were formaldehyde crosslinked for 20 min. Sonication was carried out on ice in ten 10 sec bursts on a probe sonicator. Eighty microliters of protein A-agarose bead suspension were used per IP reaction. Samples were un-crosslinked at 65°C overnight. PCR was carried out using the following primers: exogenous hTERT, forward 5′-TTC ACC TTC CAG CTC CGC CT-3′, reverse 5′-TAC CAA CAG TAC CGG AAT GCC AAG-3′; endogenous hTERT, forward 5′-GGC CGG GCT CCC AGT GGA TTC G-3′, reverse 5′-CAG CGG GGA GCG CGC GGG ATC G-3′; GAPDH, forward 5′-ACC ACT TTG TCA AGC TCA TTT C-3′, reverse 5′-CTC TCT CTT CCT CTT GTG CTC-3′. Cycle numbers that produced linear results were experimentally determined. Actin antibody served as a nonspecific immunoprecipitation control. GAPDH specific primers were used as positive ChIP controls.
Telomerase repeat amplification protocol assay
Telomerase repeat amplification protocol (TRAP) was conducted as previously described19 according to TeloTAGGG Telomerase PCR Elisa Kit (Roche).
Reverse transcription/PCR (RT-PCR)
RNA was collected from AECs stably transduced with LXSN vectors using Tri-Reagent (MRC) per manufacturer's recommendation. RNA concentrations were normalized based on spectrophotometric absorbance. RNA was reverse-transcribed into cDNA using RetroScript reagents and protocols from Ambion. PCR was performed using AmpliTaq Gold and accompanying buffers (Roche) for the following targets using the following primers: hTERT, forward 5′-CGG AAG AGT GTC TGG AGC AA-3′, reverse 5′-GGA TGA AGC GGA GTC TGG A-3′; Actin, forward 5′-GGG GGA AAT CGT GCG TGA CAT T-3′, reverse 5′-GAT GGA GTT GAA GGT AGT TTC GTG-3′; E6AP, forward 5′-CAT AGT ACT GGG TCT GGC-3′, reverse 5′-CAT ACA TCA TTG GGT TAC C-3′; p300, forward 5′ATG AAC GGT TCA ATT GGA GC-3′, reverse 5′-TTC TGA GTA TAT GGT GAA CC-3′; E6, forward 5′-GCT GCA AAC AAC TAT ACA TG-3′, reverse 5′-TGT CTT TGC TTT TCT TCA GG-3′. PCR products were separated on 2% agarose gels at 110 V for 50 min and visualized by staining with ethidium bromide. Reaction mixtures contained 2.5 mM MgCl2, 0.25 mM dNTPs and 2 μM primers. Reactions were carried out at a number of cycles predetermined to give linearly quantitative results.
Luciferase assay
Transfection of luciferase reporter constructs was carried out using GenePorter reagents (GTS) in KSFM without supplements for 5 hr. Transfection media was replaced with fresh KSFM with supplements and cultures were incubated for 24 hr at 37°C. Transfection efficiency was normalized by cotransfection with a constitutive renilla luciferase vector (pRL-null) and dual measurement of renilla and firefly luciferase activity using the dual-luciferase kit (Promega). Both firefly and renilla luciferase activity was measured on a luminometer at 24 hr post infection.
Western blots
Western blot analysis was performed as previously described6 using the following commercially available antibodies: rabbit polyclonal anti-p300 (Santa Cruz, sc-585), goat polyclonal anti-actin (Santa Cruz, sc-1616) and mouse monoclonal anti-p53 (Oncogene Research Products, Ab-6).
Results
Acetylation and activation of the endogenous hTERT promoter are induced by E6 and increased in late passage cells
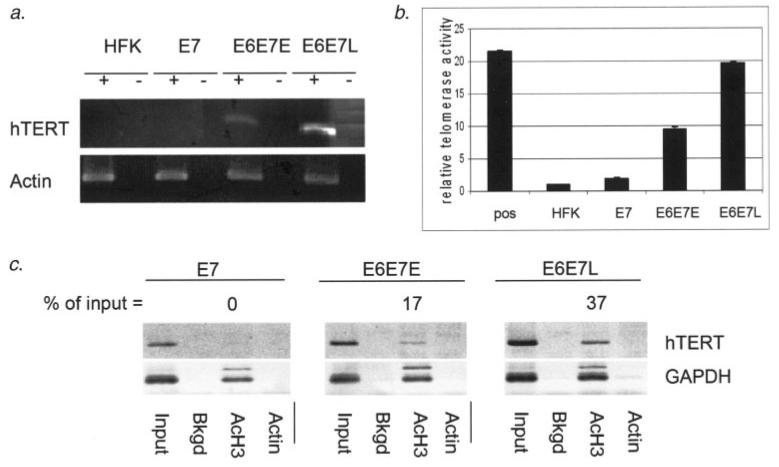
To begin to understand how hTERT expression is regulated in E6 expressing cells, we performed studies on the effects of E6 expression on acetylation and activation of the endogenous hTERT promoter. As previously shown, expression of E6/E7 in HFKs leads to upregulation of hTERT transcript accumulation and telomerase activity (Fig. 1a). At late passage, the level of hTERT transcript is greatly enhanced when compared to that of early passage cells. Concurrent with hTERT transcript levels, telomerase activity is induced in early passage E6/E7 expressing cells approximately 10-fold relative to primary HFKs at the same passage or those expressing E7 alone (Fig. 1b). Like hTERT transcript levels, telomerase activity is greatly enhanced in late passage cells when compared to that in early passage cells. In these cells, telomerase activity is induced to 20-fold over HFKs or those expressing E7 alone, which equates to a 2-fold increase over early passage E6 expressing cells.
Figure 1.
Histone H3 acetylation and activation of the endogenous hTERT promoter by E6 are enhanced at late passage. (a) RT-PCR analysis of hTERT transcript levels indicates induction of hTERT transcript accumulation by E6 and an increased accumulation of hTERT transcript in late passage E6/E7 immortalized HFKs. Actin RT-PCR serves as a loading and normalization control. (b) TRAP analysis shows induction of telomerase activity with E6 expression. Like hTERT transcript accumulation, telomerase activity is enhanced approximately 2-fold in late passage E6/E7 immortalized cells when compared to early passage E6/E7 expressing cells. Positive control extract (pos) is provided by the manufacturer of the TRAP kit (Roche). The value for telomerase activity in HFKs in our assay was set to “1” and the values for all other cell types represent fold changes over HFK. Buffer controls were set as a 0 baseline and subtracted from all other values. (c) ChIP assays specific for acetyl H3-associated hTERT promoter were conducted on HFKs stably expressing HPV oncoproteins. Values for acetylation are expressed as percent acetyl-H3 immuoprecipitation signal relative to the preimmunoprecipitation input signal. ChIP assay using primers specific to the endogenous hTERT promoter shows no acetylation in E7 expressing HFKs, while E6/E7 expressing HFKs produce a signal strength of 17% of the input. Histone H3 acetylation is enhanced to 37% in late passage E6/E7 expressing HFKs. This represents a 2.2-fold increase over early passage cells. Background (bkgd) samples were not incubated with an immunoprecipitating antibody and actin antibody was used as an additional negative immunoprecipitation control. The data shown are representative of several experiments.
hTERT can be activated in normal human cells solely by inhibition of HDACs12 and HDACs are recruited to the core promoter during differentiation of epithelial cells.11 With the evident importance of histone acetylation in hTERT promoter regulation, we wanted to ask whether E6 is affecting the histone acetylation status of the core hTERT promoter. To address this question, we employed ChIP assays to determine the acetylation status of the hTERT promoter with and without E6 expression. Chromatin was immunoprecipitated using antibodies recognizing the acetylated form of histone H3. Acetyl-histone H3-associated hTERT promoter was detected using specific PCR primers. The histone H3 acetylation status of the endogenous hTERT promoter correlates directly with hTERT transcript levels and telomerase levels in these cells (Fig. 1c). H3 acetylation is detected only in E6 expressing cells, and the level of acetylation is increased by 2.2-fold in late passage cells versus early passage cells. These data indicate that histone acetylation plays a major role in the regulation of the endogenous hTERT promoter and that the level of acetylation is directly related to the level of hTERT activation in E6 expressing HFKs.
An exogenously introduced hTERT core promoter is acetylated at histone H3 and is activated by E6 independently of passage number
To study activation of hTERT by E6 in a modifiable promoter system and to evaluate the importance of endogenous chromatin structure in increased hTERT promoter activity at late passage when compared to early passage cells, we applied luciferase reporter and ChIP assays to a transiently introduced core hTERT promoter. We hypothesized that the late passage increase in hTERT promoter activity is the result of selection for higher telomerase activity in cell culture independent of the activity of E6 on the promoter. If this selection involves epigenetic changes in chromatin structure at the hTERT promoter, a transiently expressed exogenous promoter should not exhibit the same late passage increases in activity. In HFKs stably expressing E6/E7 at both early passage (16–28 population doublings) and late passage (52–82 population doublings), exogenous hTERT promoter activity is activated to approximately 5-fold over non-E6 expressing controls (Fig. 2a). ChIP of an exogenous hTERT core promoter shows that histone H3 associated with the promoter is acetylated in HFKs stably expressing E6 (Fig. 2b). The level of acetylation of this exogenous promoter is similar whether the cells are at early or late passage. These results, indicating that passage dependent differences in exogenous hTERT promoter activity and histone acetylation, suggest that the late passage increase in histone H3 acetylation and in telomerase activity when compared to early passage cells may be due to selection for cells with higher endogenous telomerase activity and not directly related to E6 expression per se.
Figure 2.
E6 expression results in acetylation and activation of an exogenous hTERT promoter equally at early and late passage. (a) Luciferase assays for hTERT promoter activity were performed on HFKs stably transduced with LXSN:E7 or LXSN:E6/E7 at early (E6E7E) or late passage (E6E7L). Luciferase activity values were expressed as fold changes relative to that exhibited by E7 cells. Error bars represent 1 standard deviation from the mean. hTERT promoter activity was induced approximately 5-fold over E7 expressing cells in both early and late passage E6/E7 expressing cells. (b) Chip assays were performed in HFKs using primers specific to a transiently introduced hTERT core promoter. While only a 3% signal was obtained for E7 expressing cells, early and late passage E6/E7 expressing cells gave signals of 19 and 24% respectively. The data shown are representative of multiple assays.
Neither E-box nor X-box elements are exclusively responsible for hTERT activation by E6
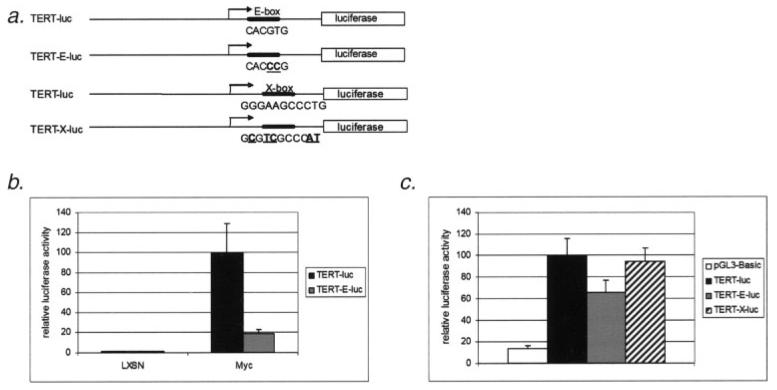
Both E-box and X-box elements of the hTERT promoter have been implicated in the regulation of hTERT transcription by E6.16-18 To investigate these possibilities under our experimental conditions, we employed a reporter assay system to evaluate the importance of E-box and X-box promoter elements in our cells and culture conditions. Mutation of the hTERT promoter at the proximal E-box as described earlier (Fig. 3a) abolishes transactivation by c-Myc (Fig. 3b). Although there is some decrease in E6 activation of the E-box mutated promoter when compared to the wild-type hTERT promoter, E6 retains most of its ability to activate transcription with the E-box mutation (Fig. 3c). Likewise, when the X-box element of the hTERT promoter is mutated at the conserved Nfx binding sequence, E6 activation is also retained (Fig. 3c). We conclude from this data that neither of these elements is exclusively responsible for activation of the hTERT promoter by E6.
Figure 3.
E6 retains its ability to activate the hTERT promoter when an E-box mutated, myc-unresponsive construct (TERT-E-luc) or X-box mutated construct are used. (a) E-box and X-box mutants contain transversion mutations at conserved binding sequences in the promoter. (b) Luciferase assays were performed with transiently transfected wild-type TERT-luc or mutant TERT-E-luc constructs in triplicate. Error bars represent 1 standard deviation from the mean. Activation of the hTERT promoter by a c-Myc expression construct is abolished with the E-box mutation. (c) HFKs were transfected with pGL3-basic, TERT-luc, TERT-E-luc or TERT-X luciferase reporter vectors respectively. Activation of the promoter constructs by E6 when compared to non-E6 expressing vector controls is expressed as percentage of full activation of the wild-type promoter by E6. Approximately 65% activation by E6 is retained when the E-box is mutated. Mutation of the X-box element of the hTERT promoter (TERT X-luc) results in no significant decrease in activation by E6. Where not indicated, E6E7 expressing HFKs are at early passage. Experiments were performed in triplicate. Error bars represent 1 standard deviation from the mean.
Induction of hTERT promoter acetylation and activation is dependent on E6AP
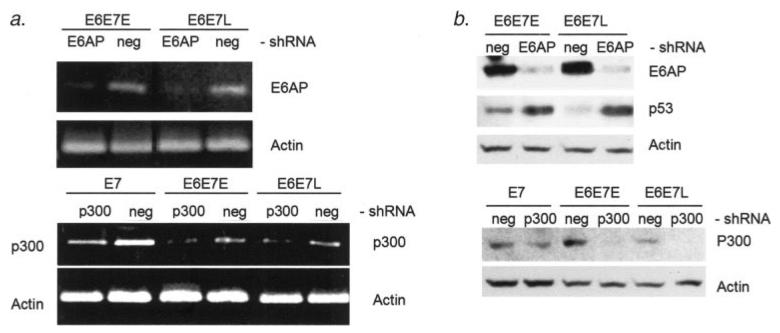
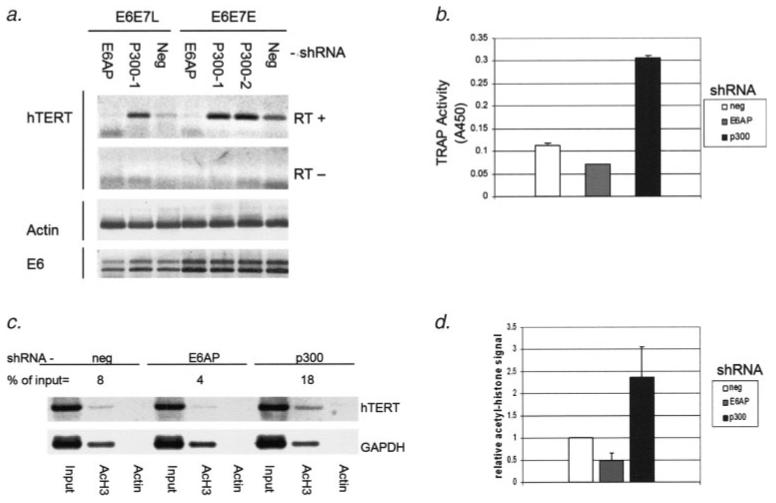
Activation of hTERT transcription and telomerase activity has previously been found to be independent of both p53 degradation3 and PDZ domain binding.9 However, hTERT activation by E6 does correlate with the ability to bind the E6AP ubiquitin ligase.8,20,21 In fact, E6AP has been found to be necessary for hTERT activation by E6.8 Therefore, we hypothesized that E6AP is necessary for the induction of hTERT promoter histone acetylation. To investigate the role of E6AP in the histone acetylation and activation of the hTERT promoter, we have used shRNA knockdown of E6AP. Stable expression of inhibitory shRNA in these cells effectively diminishes levels of both transcripts and protein for E6AP (Figs. 4a and 4b). Abrogation of p53 degradation in E6 positive cells expressing E6AP shRNA also confirms functional E6AP knockdown (Fig. 4b). In cells expressing E6AP shRNA, hTERT transcript levels as well as telomerase activation by E6 are diminished by approximately 2-fold (Figs. 5a and 5b). Likewise, histone H3 acetylation at the hTERT promoter was diminished with E6AP knock-down to a degree similar to that of telomerase activity (Figs. 5c and 5d). These results not only demonstrate a dependence on E6AP for activation of the hTERT promoter, but also confirm the role of the E6 complex in the acetylation of the hTERT promoter.
Figure 4.
shRNA expression effectively knocks down transcript and protein levels for E6AP and p300 targets. (a) RT-PCR analysis shows greatly diminished levels of E6AP transcript in E6/E7 expressing HFKs when E6AP shRNA is stably expressed from retroviral vectors. shRNA of a random, nonspecific sequence (neg) is used as a negative control. Likewise, p300 transcript level is diminished by p300 shRNA expression. (b) Immunoblotting demonstrates greatly reduced levels of E6AP protein under expression of the specific E6AP shRNA. Also, since E6-mediated degradation is dependent on E6AP, knockdown of E6AP in E6/E7 expressing cells rescues p53 accumulation. p300 protein levels are also greatly reduced with p300 shRNA expression. Notably, p300 levels are much lower in late passage cells.
Figure 5.
Histone H3 acetylation and activation of the hTERT promoter is dependent on E6AP and enhanced by loss of p300. (a) hTERT transcript is detectable in HFKs expressing a nonspecific shRNA (neg, lanes 3 and 7) using RT-PCR. Accumulation of hTERT transcript is decreased with E6AP knock-down (lanes 1 and 4), and increased when p300 shRNA is expressed (lanes 2, 5 and 6). p300-1 and p300-2 represent 2 different shRNA constructs targeting 2 distinct sequences within the p300 transcript. RT-PCR was performed to detect the presence of E6 transcript. Double bands represent multiple splicing products of the E6 transcript. The RT- row represents no reverse transcriptase negative controls. (b) Telomerase activity measured by TRAP assay shows decreased activity with E6AP shRNA expression when compared to that with nonspecific shRNA expression (neg). Telomerase activity is increased approximately 3-fold with p300 knock-down. (c) A representative ChIP analysis. hTERT promoter associated acetyl histone H3 signal is expressed as the percentage of the preimmunoprecipitation input signal for each given experimental condition. (d) Histone H3 acetylation as measured by ChIP analysis is decreased by 2-fold with E6AP knock-down and increased by 2.3-fold with p300 knock-down. Triplicate ChIP analysis data is shown in graph form. Error bars represent 1 standard deviation from the mean.
E6-induced hTERT promoter activity and histone acetylation are enhanced by knockdown of p300
Another well defined target of E6 that has not been ruled out as playing a role in activation of the hTERT promoter is p300. We also used shRNA knockdown of p300 to investigate its role in hTERT regulation in E6 expressing cells. Transcript and protein levels of p300 were effectively decreased with stable expression of p300 shRNA (Figs. 4a and 4b). When p300 was downregulated by shRNA in these cells, hTERT transcript accumulation as well as telomerase activity were increased by over 2-fold (Figs. 5a and 5b). Histone acetylation levels were also increased by 2-fold with p300 knock-down (Figs. 5c and 5d). Inhibition of p300 in E7 expressing cells without E6 expression did not result in an increase in hTERT transcript or upregulation of telomerase activity (data not shown). These latter results suggest that the primary effect of E6 on hTERT transcription is not directly through abrogation of p300 function. It is interesting to note that downregulation of p300 was observed in later passage E6E7 keratinocytes that had higher levels of telomerase and acetylation at the hTERT promoter (Fig. 4b). Since we did not observe this phenomenon in all later passage E6E7 cell lines tested, we cannot conclude that p300 downregulation is the sole reason for upregulation of hTERT at later passage (data not shown). Our consistent results from the shRNA p300 knockdown experiments are provocative, however, in that they implicate a potential and previously unknown role for p300 in repressing hTERT transcription in the context of E6 expression.
Discussion
Histone modification plays a central role in the regulation of hTERT expression in relation to both cell cycle progression and differentiation.11-13,22,23 The use of HDAC inhibitors has been shown by many to activate transcription of hTERT and telomerase activity.12,13,24,25 Sp1, E2F and E-box binding repressors have all been shown to recruit HDACs to the hTERT promoter, resulting in transcriptional repression.11,22,23,26 However, a role for histone modification in E6-mediated hTERT activation has not been previously implicated. Our data indicates that E6, in conjunction with E6AP, induces acetylation of histone H3 at the hTERT promoter. The level of hTERT promoter acetylation is directly proportional to the level of hTERT transcript accumulation and telomerase activity in HFKs stably expressing E6. This provides a novel demonstration that histone modification is an integral part of the mechanism of hTERT promoter activation by E6.
In early passage cells, E6 modestly activates hTERT transcription and telomerase activity, while in later passage cells, much higher telomerase activity is seen. In agreement with this, we have found that late passage, E6E7 immortalized HFKs have higher hTERT promoter acetylation, hTERT transcript levels and telomerase activity than their early passage predecessors. We find that only the endogenous hTERT promoter is affected in a passage-dependent manner, while acetylation and activity of an introduced hTERT core promoter remains stable between early passage and late passage cells. This suggests that it is alterations of the endogenous promoter, independent of the activity of E6 on the hTERT core promoter represented by the exogenous construct, that result in increased telomerase activity at later passage. The mechanism accounting for additional epigenetic modifications at the endogenous promoter in late passage cells is unknown. It is apparent that although telomerase activity is enhanced in late passage cultured cells, late passage hTERT activity remains dependent on E6 and E6AP, as evidenced by the abrogation of late passage hTERT activity in E6 expressing cells with shRNA targeting E6AP.
Aberrant transcriptional regulation by histone modification enzymes is common in many cancers.25,27 The majority of the research being done regarding these potential targets for cancer treatment has focused on the use of HDAC inhibitors. Although certain genes related to proliferation, differentiation and the development of cancer are beneficially regulated by the inhibition of histone acetylation, resultant increases in histone acetylation may cause undesirable phenotypes in the context of cancer and other pathological conditions. Inhibition of histone deacetylation is likely to activate hTERT transcription and telomerase activity, which could support cellular immortalization and proliferation.
Our data indicates that p300 may have repressive effects on hTERT transcription and telomerase activity in the context of hTERT activation by E6. Although E6AP is needed for hTERT promoter activation, p300 is not apparently degraded by E6, nor is knock-down of p300 able to activate transcription of hTERT in non-E6 expressing cells. Also, p300 knockdown was not sufficient to activate hTERT transcription in cells expressing E7 alone. Therefore, it is unlikely that inactivation of p300 is the primary means of hTERT promoter activation by E6. There has never been a direct implication of p300 in regard to the regulation of hTERT transcription or telomerase activity in normal cells, although it does have a regulatory effect on factors that may play a role in hTERT regulation. p300 modifies transcription factors associated with growth arrest and differentiation28,29 and positively regulates their function. Studies have shown that p300 and the related CREB-binding protein have repressive effects on c-Myc30,31 and c-Jun transcription factors,31 both of which have been implicated as playing a positive role in hTERT transcription. Antisense knockdown of p300 in human breast epithelial cells led to the increased expression of c-Myc and c-Jun. Another possible link between p300 and hTERT repression is the E2F family of transcription factors. Modification of E2F by the HAT activity of p300 is necessary for E2F function and transition from G1 to S phase of the cell cycle.32 E2F has recently been found to be an important repressor of hTERT transcription in relation to both cell cycle and differentiation-dependent hTERT regulation.11,22 E2F binding sites within the hTERT promoter represent an attractive candidate for a role in E6 activation of hTERT transcription. This is especially true given our findings that histone acetylation correlates with hTERT activation by E6 and recent data suggesting that E2F recruitment of HDACs is a central regulating mechanism of hTERT transcription in normal cells.11,22 Our current findings provide insights into the mechanism of hTERT activation by HPV-E6 and the increase in hTERT activity in immortalized cells that may implicate possible targets for cancer therapy and prevention.
Previous studies on E6 activation of hTERT suggest the involvement of E-box or X-box elements of the hTERT promoter. Although controversial, it is agreed that the proximal promoter containing E-box and X-box elements contains a repressive element or elements, and that this region of the promoter is responsible for HPV-E6 activation of hTERT transcription. The downstream E-box was the first site to be proposed as the element responsible for E6 activation of hTERT transcription.15,18 However, E6 has been found to have no significant effect on Myc levels in the cell.15,16,18 On the other hand, 1 group has reported that the E-box element was not essential for telomerase activation by E6.16 Others have suggested that USF family of transcription factors represses hTERT through E-boxes and that this event is involved in hTERT regulation by E6.33 More recently, NFX-91 has been implicated as a repressor of hTERT and it has been suggested as a putative target for degradation by E6/E6AP.18 Our data provides evidence that the ability of E6 to activate the hTERT core promoter is not exclusively dependent on the function of either E-box or X-box elements. The possibility remains that an unidentified repressor acts on the proximal core promoter of hTERT and is involved in activation and histone acetylation induced by E6.
Acknowledgements
We thank Dr. Denise Galloway, Fred Hutchinson Cancer Research Center, Seattle, WA, for shRNA and viral protein expression vectors, Dr. Izumi Horikawa, NCI, NIH, for hTERT promoter constructs and all members of and contributors to the Klingelhutz lab at the University of Iowa. This work was supported by NIH R01 AG18265 (AJK), and a grant from the Department of Veteran's Affairs Medical Center (JHL).
Grant sponsor: NIH; Grant number: R01 AG18265; Grant sponsor: Department of Veteran's Affairs Medical Center.
References
- 1.Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 2.Elbel M, Carl S, Spaderna S, Iftner T. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillomaviruses with p53 and E6AP in correlation to their transforming potential. Virology. 1997;239:132–49. doi: 10.1006/viro.1997.8860. [DOI] [PubMed] [Google Scholar]
- 3.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 4.Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–52. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baege AC, Berger A, Schlegel R, Veldman T. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am J Pathol. 2002;160:1251–7. doi: 10.1016/S0002-9440(10)62552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–8. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 7.Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280:10807–16. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J Virol. 2003;77:6957–64. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–72. [PubMed] [Google Scholar]
- 11.Crowe DL, Nguyen DC, Ohannessian A. Mechanism of telomerase repression during terminal differentiation of normal epithelial cells and squamous carcinoma lines. Int J Oncol. 2005;27:847–54. [PubMed] [Google Scholar]
- 12.Hou M, Wang X, Popov N, Zhang A, Zhao X, Zhou R, Zetterberg A, Bjorkholm M, Henriksson M, Gruber A, Xu D. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp Cell Res. 2002;274:25–34. doi: 10.1006/excr.2001.5462. [DOI] [PubMed] [Google Scholar]
- 13.Takakura M, Kyo S, Sowa Y, Wang Z, Yatabe N, Maida Y, Tanaka M, Inoue M. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001;29:3006–11. doi: 10.1093/nar/29.14.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura-Arioka Y, Ohtani K, Hara T, Iwanaga R, Nakamura M. Identification of two distinct elements mediating activation of telomerase (hTERT) gene expression in association with cell growth in human T cells. Int Immunol. 2005;17:207–15. doi: 10.1093/intimm/dxh201. [DOI] [PubMed] [Google Scholar]
- 15.Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–72. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75:5559–66. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci USA. 2003;100:8211–16. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–82. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprague DL, Phillips SL, Mitchell CJ, Berger KL, Lace M, Turek LP, Klingelhutz AJ. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology. 2002;301:247–54. doi: 10.1006/viro.2002.1542. [DOI] [PubMed] [Google Scholar]
- 20.Foster SA, Demers GW, Etscheid BG, Galloway DA. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J Virol. 1994;68:5698–705. doi: 10.1128/jvi.68.9.5698-5705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann H, Degenkolbe R, Bernard HU, O'Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–19. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won J, Chang S, Oh S, Kim TK. Small-molecule-based identification of dynamic assembly of E2F-pocket protein-histone deacetylase complex for telomerase regulation in human cells. Proc Natl Acad Sci USA. 2004;101:11328–33. doi: 10.1073/pnas.0401801101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Won J, Yim J, Kim TK. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J Biol Chem. 2002;277:38230–8. doi: 10.1074/jbc.M206064200. [DOI] [PubMed] [Google Scholar]
- 24.Tsurutani J, Soda H, Oka M, Suenaga M, Doi S, Nakamura Y, Nakatomi K, Shiozawa K, Amada YY, Kamihira S, Kohno S. Antiproliferative effects of the histone deacetylase inhibitor FR901228 on small-cell lung cancer lines and drug-resistant sublines. Int J Cancer. 2003;104:238–42. doi: 10.1002/ijc.10921. [DOI] [PubMed] [Google Scholar]
- 25.Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, Kawabata S, Kasai T, Yamada Y, Kamihira S, Tei C, Kohno S. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer. 2002;97:621–5. doi: 10.1002/ijc.10082. [DOI] [PubMed] [Google Scholar]
- 26.Cong YS, Bacchetti S. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J Biol Chem. 2000;275:35665–8. doi: 10.1074/jbc.C000637200. [DOI] [PubMed] [Google Scholar]
- 27.Hess-Stumpp H. Histone deacetylase inhibitors and cancer: from cell biology to the clinic. Eur J Cell Biol. 2005;84:109–21. doi: 10.1016/j.ejcb.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–41. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–8. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 30.Rajabi HN, Baluchamy S, Kolli S, Nag A, Srinivas R, Raychaudhuri P, Thimmapaya B. Effects of depletion of CREB-binding protein on c-Myc regulation and cell cycle G1-S transition. J Biol Chem. 2005;280:361–74. doi: 10.1074/jbc.M408633200. [DOI] [PubMed] [Google Scholar]
- 31.Kolli S, Buchmann AM, Williams J, Weitzman S, Thimmapaya B. Antisense-mediated depletion of p300 in human cells leads to premature G1 exit and up-regulation of c-MYC. Proc Natl Acad Sci USA. 2001;98:4646–51. doi: 10.1073/pnas.081141998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ait-Si-Ali S, Polesskaya A, Filleur S, Ferreira R, Duquet A, Robin P, Vervish A, Trouche D, Cabon F, Harel-Bellan A. CBP/p300 histone acetyl-transferase activity is important for the G1/S transition. Oncogene. 2000;19:2430–7. doi: 10.1038/sj.onc.1203562. [DOI] [PubMed] [Google Scholar]
- 33.McMurray HR, McCance DJ. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J Virol. 2003;77:9852–61. doi: 10.1128/JVI.77.18.9852-9861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]