Abstract
Objectives
To determine whether there is an association between high levels of telomerase and premalignant cervical disease and to provide a preliminary analysis of telomerase activity as a potential triage strategy.
Materials and Methods
Premenopausal women were invited to participate in the study during routine gynecologic visits as well as visits where colposcopy was performed. Samples were taken from the cervix using a broom device and placed in cold phosphate-buffered saline. A total of 92 samples were evaluated. Cells were counted and lysed, and a semi-quantitative measure of telomerase activity was determined using a commercially available telomerase enzyme-linked immunosorbent assay kit. The presence of human papillomavirus (HPV) types 16 and 18 was assessed by polymerase chain reaction analysis. One-way analysis of variance was used to test for the association of telomerase activity with cytology, HPV type 16 or 18 status, and colposcopy and/or biopsy findings.
Results
When telomerase levels were analyzed according to Pap smear results, there were no differences among four groups of cytology findings (normal, atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion, and high-grade squamous intraepithelial lesion). When colposcopy and/or biopsy results were considered, significantly higher levels of telomerase were detected in cervical intraepithelial neoplasia (CIN) 2,3 samples than in normal Pap smear samples and CIN 1 samples (p = .035). There was no significant difference in telomerase levels between samples that tested positive for HPV type 16 or 18 and those that did not (p = .111).
Conclusions
Telomerase levels were significantly higher in cytologic samples from women with biopsy-proven CIN 2,3 than in samples from women with normal cytology results or CIN 1. These results warrant larger studies to determine whether telomerase activity may be a useful triage tool for abnormal cytologic findings.
Keywords: telomerase, Pap smear, CIN, papillomavirus
Of the estimated 50 million women who undergo routine gynecologic examination each year, 5% to 10% have abnormal Pap smear results [1, 2]. During subsequent follow-up examination by colposcopy and/or biopsy, only 5% to 10% of women with abnormal Pap smears are found to have signs of cervical disease and undergo treatment [1]. Thus, millions of women are left with neither a definitive diagnosis nor a treatment plan [3]. A method to better predict outcomes from Pap smear analyses would be extremely beneficial to both patients and the health care industry [4]. More than 90% of cervical cancers have detectable levels of high-risk human papillomavirus (HPV), suggesting that the presence of HPV could be a potential indicator of malignant disease present in early-stage abnormalities [5, 6]. However, HPV is also widely present in patients without clinically significant disease, thus limiting its use as an indicator of cancer.
Telomeres are at the ends of human chromosomes and consist of the repeated base pair sequence TTAGGG [7, 8]. With each cell division, the telomeres decrease in length until the process results in a signal that triggers senescence [9]. Telomerase is an enzyme that adds a repeated TTAGGG sequence to the telomeres [10]. This prevents senescence by maintaining chromosomal length and function. Telomerase activity is widely detected in human cancers, whereas nonmalignant somatic tissue exhibits little to no telomerase activity [11]. For cervical cancer, a number of studies have demonstrated that telomerase activity can be detected in biopsy specimens from high-grade lesions at a frequency of >90%, but this occurs at much lower levels and frequency in biopsy specimens from lower-grade lesions [12-23]. There is evidence that biopsy specimens that are positive for high-risk HPV types are more likely to contain telomerase activity, although this parameter is difficult to separate from disease grade because nearly all high-grade lesions are positive for HPV.
It has been speculated that measurement of telomerase activity might be a useful method for screening Pap smears [22, 23]. In most studies, it has been found that cytologic samples from patients with high-grade lesions are more likely to contain telomerase activity, although some reports indicate that measurement of telomerase provides no diagnostic value [24-26]. Telomerase activity is usually assayed by using the telomere repeated amplification protocol (TRAP), which involves visualization of radioactive polymerase chain reaction (PCR) products by gel electrophoresis. This type of analysis presents some difficulties for measurement of telomerase activity in a clinical laboratory setting. The objective of the current study was to evaluate the use of a nonradioactive TRAP assay to determine whether measurement of telomerase activity in cytologic specimens would provide a useful means to triage abnormal Pap smears.
MATERIALS AND METHODS
Sample Collection
Samples were obtained from premenopausal women between the ages of 18 and 45 years who visited the University of Iowa Hospitals and Clinics for routine gynecological examination or colposcopy. The Institutional Review Board at the University of Iowa approved the protocol for collection as well as the consent form. Samples were used only from those participants who signed the consent form. Two Pap smears were taken from each volunteer: one for cytologic diagnosis and one for telomerase and DNA analyses. Pap smears were evaluated using standard methods by a cytotechnologist and a cytopathologist and classified according to the Bethesda System [27] with designations of normal, atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), and high-grade squamous intraepithelial lesion (HSIL). For colposcopy, biopsies were performed based on disease impression, with no biopsy specimen taken if there was no sign of disease. Histologic analysis was performed in a blinded fashion by a trained pathologist, with no special handling or multiple reads, and the results were classified into groups of normal, cervical intraepithelial neoplasia (CIN) 1, 2, or 3, or squamous cell carcinoma. For telomerase processing, the samples were collected with a cervical brush, which was then washed in 5 mL of cold 1 × phosphate-buffered solution (PBS). Samples were processed the same day on which they were obtained. Laboratory personnel were blinded to patient identity as well as to the results of clinical diagnosis. Some samples were excluded from certain analyses and comparisons due to a lack of a Pap test diagnosis, insufficient sample for telomerase testing, or inability to amplify β-globin DNA.
Sample Processing
Samples were spun at 1,200 rpm and 4°C for 5 minutes. The PBS was carefully removed before adding 2 mL of trypsin to dissociate cell clusters. The samples were incubated at 37°C for 5 minutes, at which time 2 mL of PBS/2% fetal bovine serum was added to inhibit the trypsin. At this point, the squamous cells in each sample were counted by using a hemacytometer (Hausser Scientific, Horsham, PA), and aliquots of 50,000 to 200,000 cells were pipetted into sterile 1.5-mL Eppendorf tubes. Samples were washed one more time in PBS, pelleted, and stored at −80°C after the supernatant was removed.
TRAP
TRAP was followed as instructed in the TeloTAGGG Telomerase PCR enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Biochemicals, Mannheim, Germany). Pelleted samples were lysed using 1 μL of lysis buffer (supplied by the kit) per 1,000 cells. One microliter (i.e., 1,000 cell equivalents) was used for each TRAP assay. For standardization, a positive control (i.e., an hTERT transduced human keratinocyte cell line) was run with each assay. Using dilution analyses, telomerase could be detected in as few as five positive control cells (data not shown). To account for background, values obtained from a negative control (lysis buffer) were subtracted from those obtained from experimental samples. Each sample and control was run a minimum of three times for each assay, and average values were used for comparison.
DNA Isolation
DNA was isolated either from stored pellets or directly from the TRAP lysate using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Both pellets and lysates were resuspended in PBS to a final volume of 200 μL to begin the blood and body fluid spin protocol. Samples were eluted in 50 μL of warm nanopure water or a proportional amount if fewer cells were present. The concentration was determined using a 1:50 dilution of the sample in a SmartSpec 3000 (Bio-Rad).
HPV Typing
DNA was detected by PCR amplifications of the β-globin gene using the primers GH20 and PC04 [28]. Each 50-μL reaction included 5 μL of PCR buffer plus MgCl2, 4 μL of dNTPs, and 2.5 U of SuperTaq polymerase (Ambion, Austin, Texas); 10 ng of sample DNA; and 50 pmol each primer. Each reaction was subjected to the following conditions in a Thermal Cycler (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany): denaturation at 94°C for 3 minutes; 40 cycles of 93°C for 30 seconds, 55°C for 30 seconds, and 72°C for 45 seconds; and extension at 72°C for 10 minutes. Amplification products were run on an agarose gel, developed in ethidium bromide, and photographed under ultraviolet light. Amplification of the β-globin fragment indicated that there were no inhibitors to the PCR reaction in the sample and that there was adequate DNA for HPV detection.
The L1 region of HPV types 16 and 18 was subsequently amplified by PCR assay using HPV type 16 L1– and HPV type 18 L1–specific primers, as previously described [29]. The reactions were performed as described above for the β-globin reaction. Negative and positive controls were run with each PCR reaction. The negative control for all PCR reactions was 1 μL of nanopure water in place of DNA. The positive control for the β-globin and HPV type 16 L1 reactions was 1 ng of HPV type 16 containing cervical cells [30]. The positive control for the HPV type 18 L1 reactions was 1 ng of HeLa DNA.
Statistical Analyses
To assess variability of the telomerase assay, the intra-class correlation was calculated. Because telomerase values were not normally distributed, a natural log transformation was applied to the data to normalize the data distribution for other analyses. One-way analysis of variance was then used to compare telomerase levels among patients grouped by Pap smear results (normal, LSIL, HSIL, and ASCUS) and by colposcopy results (normal, CIN 1, CIN 2,3, and higher). This was followed by the Tukey test for multiple pairwise comparisons among groups. In addition, a receiver-operating characteristic curve was constructed, and the area under the curve was computed to evaluate the utility of telomerase activity as a predictor of colposcopy positivity (CIN 2,3 or higher). To test the association of HPV with Pap smear or colposcopy findings, the Fisher exact test was used.
RESULTS
Telomerase Levels and Pap Smear Findings
The results of both Pap smear and telomerase analysis were available for 92 women who visited the University of Iowa Clinics for routine gynecologic examination or colposcopy. Breakdown of the different Pap smear results are shown in Table 1. In this group, Pap smears were classified as follows: 39, normal; 15, ASCUS; 27, LSIL; and 11, HSIL. After standardization and subtraction of background using positive and negative controls, telomerase values, as measured by TRAP ELISA, ranged from 0 to 0.237 for all samples. The intraclass correlation, which measures the degree of agreement between different assays of the same sample, was calculated to be 0.60. This represents fair to good agreement between the different repeats. Because telomerase values were not normally distributed, a natural log transformation was applied to the data to normalize the data distribution. From one-way analysis of variance comparing telomerase levels among different groups, there was no statistically significant difference in telomerase levels among the four groups (p = .325), although the observed data suggested higher telomerase activity in the HSIL group than in all other groups (p = .08).
Table 1.
Telomerase Analysis and Pap Smear Results
| Pap Smear result | No. | Median | 25th–75th Percentile | Minimum–maximuma |
|---|---|---|---|---|
| Normal | 39 | 0.011 | 0.005–0.027 | 0.0003–0.237 |
| LSIL | 27 | 0.008 | 0.004–0.025 | 0.000–0.157 |
| HSIL | 11 | 0.050 | 0.004–0.103 | 0.001–0.122 |
| ASCUS | 15 | 0.013 | 0.006–0.021 | 0.003–0.053 |
LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; and ASCUS, atypical squamous cells of undetermined significance.
One-way analysis of variance found no statistical difference among the 4 groups (p = 0.325).
Association Between Telomerase Analysis and Colposcopy Findings
Colposcopy results were available for 40 patients (Table 2). These results were classified as follows: 25, CIN 1; 5, CIN 2, and 10, CIN 3 or higher, with only 1 of these 10 representing a microinvasive squamous cell carcinoma. With normal samples included, the test for overall group differences yielded a p value of .097, indicating a possible but not statistically significant difference among the four groups. When CIN 2 or higher was grouped together (average = 0.056; interquartile range = 0.009–0.122), there was statistical significance (p = .045). Compared with patients with normal Pap smear results, the telomerase levels associated with a colposcopy finding of CIN 2 or higher were significantly higher than levels associated with normal findings (p = .035) (Table 1 and Figure 1). Telomerase activity did not differ significantly between patients with CIN 1 and those with colposcopy findings of CIN 2 or higher (p = .273), and there was no significant difference in telomerase activity between patients with CIN 1 and those with normal Pap smear results (p = .565). Thus, this analysis showed an association between higher telomerase levels and CIN 2 or higher-grade lesions. As a predictor of CIN 2,3 or higher, the receiver-operating characteristic curve with an area under the curve of 0.675 did not yield a cutoff point with both high sensitivity and high specificity (Figure 2). The cutoff point with the highest sensitivity and specificity in which both were >50% had a sensitivity of 53% and a specificity of 89%, with a 50% false-positive rate and an 11% false-negative rate.
Table 2.
Telomerase Analysis and Biopsy Results
| Colposcopy/Biopsy result | No.a | Median | 25th–75th Percentileb | Minimum–maximum |
|---|---|---|---|---|
| Normalc | 40 | 0.012 | 0.005–0.020 | 0.0003–0.237 |
| CIN 1 | 25 | 0.017 | 0.008–0.030 | 0.001–0.157 |
| CIN 2 | 5 | 0.021 | 0.015–0.122 | 0.009–0.137 |
| CIN 2 or higher | 15 | 0.056 | 0.009–0.122 | 0.001–0.179 |
| CIN 3 or higher | 10 | 0.061 | 0.005–0.119 | 0.001–0.179 |
CIN, cervical intraepithelial neoplasia.
Biopsy results were not available for 12 samples.
Overall group difference was not statistically significant but differed between normal and CIN 2 or higher groups (p = 0.035).
Those with a normal Pap smear result with no colposcopy/biopsy done were assumed to be “normal.”
Figure 1.
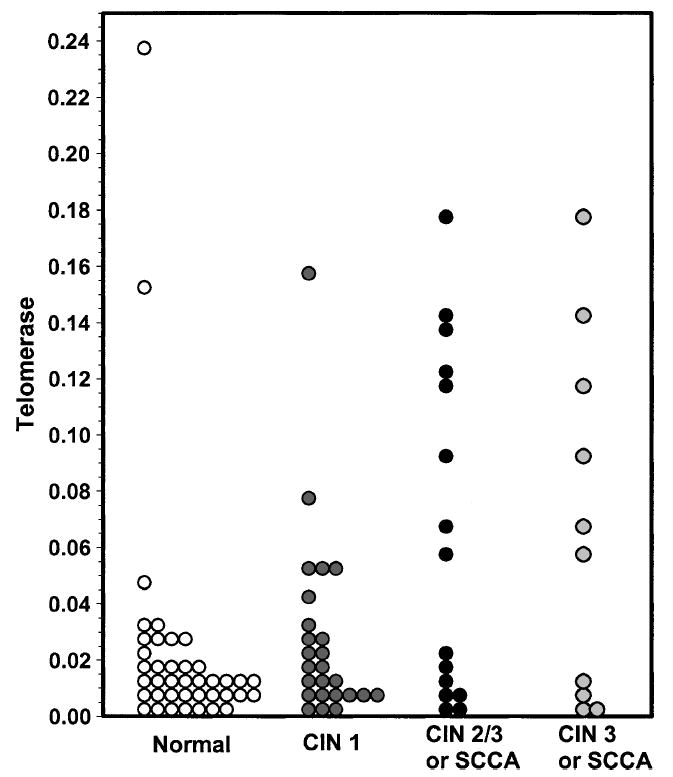
Distribution of telomerase levels according to biopsy results. Telomerase activity was measured in lysates from cytology specimens as described under Materials and Methods. Open circles, normal diagnosis; gray circles, biopsy-proven diagnosis of cervical intraepithelial neoplasia (CIN) 1; and black circles, biopsy-proven diagnosis of CIN 2,3 or squamous cell carcinoma (SCCA).
Figure 2.
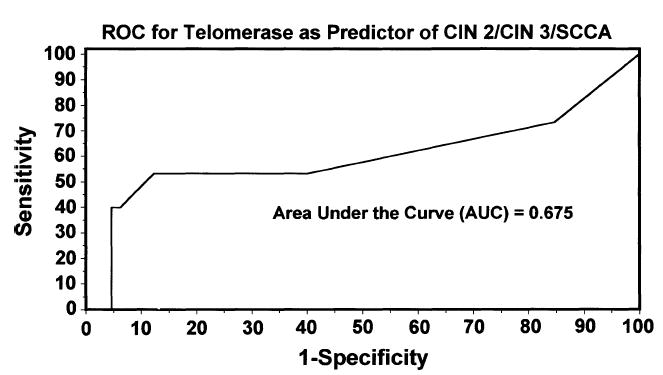
Receiver-operating characteristic (ROC) curve for telomerase activity as a predictor of biopsy result. The cutoff point with the highest sensitivity and specificity in which both were >50% had a sensitivity of 53% and a specificity of 89%. CIN, cervical intraepithelial neoplasia; and SCCA, squamous cell carcinoma.
Association Between Presence of HPV Type 16 or 18 and Pap Smear or Colposcopy Findings
Samples were analyzed for the presence of HPV type 16 or 18 (HPV-16/18) by PCR assay to determine whether the presence of these common high-risk viral subtypes correlated with cytology findings, colposcopy results, or telomerase levels. Eighty-two of the samples contained DNA in which the β-globin control could be amplified by PCR assay. Twenty-nine of these samples were HPV-16/18 positive as measured by specific PCR amplification of the HPV L1 region. Overall, there was no significant difference in telomerase levels between samples that were HPV-16/18 positive and those that were not. When grouped according to Pap smear findings, there was a significantly greater proportion of HPV-16/18 positivity in the HSIL group than in normal (p = .023) and ASCUS (p = .026) groups. Grouping of these samples based on colposcopy findings demonstrated a significantly greater proportion of HPV-16/18 positivity in the CIN 2,3 group than in the CIN 1 group (p = .005). HPV-16/18 positivity was also observed to be greater in the CIN 2,3 group than in the normal group (p = .08). The low rate of HPV-16/18 detection in CIN 1 and LSIL groups is likely due to the fact that the prevalence of low-risk HPV types such 6 and 11, which are common in these categories, was not assessed in this study. In addition, other high-risk HPV types besides 16 and 18 might have been present in these samples. Overall, there was no significant difference in telomerase levels between samples that tested positive for HPV-16/18 and those that did not (p = .111).
DISCUSSION
We present data indicating that higher levels of telomerase activity in cytologic samples are associated with higher-grade cervical lesions. The method of cell collection used in our study was nearly identical to that used for conventional Pap smear analysis, and the protocol for measurement of telomerase relied on a commercially available, nonradioactive, ELISA-based, semiquantitative assay. Thus, the methodology used in our study could be employed in a clinical laboratory.
Our results indicate that telomerase activity is associated with disease progression. Telomerase activity in cells from women with colposcopy-proven CIN 2 or higher-grade lesions was significantly higher than the activity found in cells from women with a normal diagnosis. A statistically significant difference was also found when telomerase levels in Pap smears from women with CIN 2 or higher were compared with those in samples from both women with normal findings and women with CIN 1. This was not the case when CIN 1 was compared with normal findings. Measurement of telomerase activity in cytologic samples may therefore be most useful in detecting higher-grade lesions, although a larger study must be done to more definitively address this issue. We did not observe an association between telomerase levels and cytology results, although higher median telomerase levels were detected in the HSIL group. The lack of a statistically significant association might be explained by the small size of the study population or other factors related to differences in Pap smear and colposcopy results.
Interestingly, we did not observe an association between the presence of HPV-16/18 and telomerase activity. Previous studies also reported mixed results, with some studies indicating an association with HPV positivity and others not reporting a correlation [12, 15, 17, 20, 21, 24-26, 31, 32]. We previously demonstrated that exogenous expression of HPV type 16 E6 could activate telomerase [33]. Our studies using cervical keratinocytes with stably replicating HPV type 16 episomes, however, showed that early-passage cells had low levels of telomerase despite the fact that the cells contained >20 HPV genomes per cell [30]. Higher levels of telomerase were observed at later passage. Thus, telomerase activity may not be present in HPV-containing lesions until malignant progression has occurred. Another possibility is that cytologic samples from early-stage lesions do not contain a high enough ratio of HPV-16/18–infected telomerase-positive cells to normal cells to be detected by the telomerase assay. In addition, some samples with high telomerase levels might contain high-risk HPV types other than 16 or 18.
There are several issues that must be addressed before measurement of telomerase activity can be used for assessment of disease state in the clinic. The receiver-operating characteristic curve generated from the data in our study did not yield a cutoff point with both a high sensitivity and a high specificity (53% and 89%, respectively). Our results are comparable with those reported in a recent study using a radioactive-based TRAP assay in which telomerase activity had a sensitivity of 29.9% and a specificity of 94.0% for biopsy-confirmed CIN 2,3 [26]. Thus, sensitivity of telomerase detection may be an important concern. Several factors can inhibit telomerase activity. We have found, for example, that the presence of red blood cells is inhibitory to the telomerase enzyme (data not shown). In the present study, care was taken to clean up the samples with PBS washes; however, this does not efficiently remove red blood cells, and many samples with high telomerase activity may have been missed because of inhibitors in the preparation. We recently showed that Dynabeads (Dynal, Oslo, Norway) coated with an epithelial cell–specific antibody can effectively remove telomerase-positive epithelial cells from blood–epithelial cell mixtures [34]. This result suggests that a methodology could be developed to remove contaminants (e.g., other cell types or blood) that might influence the telomerase assay.
Some normal samples in our study also contained very high levels of telomerase. This could be due to contaminating lymphocytes or endometrial cells, both of which have been shown to have endogenous telomerase in the normal state [22]. In the case of endometrial cells, telomerase levels have been shown to fluctuate with menstrual cycle [35]. Better separation techniques might help to eliminate false-positive results. Progress is also being made on the development of antibodies that specifically recognize telomerase-positive cells by immunohistochemistry [36, 37]. These could be used in conjunction with epithelial-specific antibodies and/or conventional Pap analysis to identify cervical cells with high telomerase activity.
In summary, our results indicate that the measurement of telomerase activity in cytologic samples obtained simultaneously with collection of cells for Pap analysis may provide a useful diagnostic tool to triage abnormal Pap smears. Further studies will be necessary to refine methods to improve sample purity and accurate quantitation of telomerase levels.
Acknowledgments
The authors thank Susan Long and the staff at the University of Iowa Gynecology and Obstetrics Clinic for assistance in recruiting patients and procuring samples, the patients who consented to be part of this study, and Erin Collins for technical assistance. This work was supported in part by a seed grant from the University of Iowa Holden Comprehensive Cancer Center. HKA was supported by undergraduate fellowships from the Howard Hughes Foundation and the National Science Foundation. AJK was supported in part by a grant from the National Institutes of Aging.
References
- 1.Noller KL. Incident and demographic trends in cervical neoplasia. Am J Obstet Gynecol. 1996;175:1088–90. doi: 10.1016/s0002-9378(96)70009-1. [DOI] [PubMed] [Google Scholar]
- 2.Nyirjesy I, Billingsley FS, Forman MR. Evaluation of atypical and low-grade cervical cytology in private practice. Obstet Gynecol. 1998;92:601–7. doi: 10.1016/s0029-7844(98)00274-9. [DOI] [PubMed] [Google Scholar]
- 3.Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605–10. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin P, Laskey R, Coleman N. Translational approaches to improving cervical screening. Natl Rev Cancer. 2003;3:217–26. doi: 10.1038/nrc1010. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–73. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3:444–51. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 8.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allsopp RC, Chang E, Kashefiaazam M, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 10.Collins K. Structure and function of telomerase. Curr Opin Cell Biol. 1996;8:374–80. doi: 10.1016/s0955-0674(96)80013-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 12.Wisman GB, Hollema H, de Jong S, et al. Telomerase activity as a biomarker for (pre)neoplastic cervical disease in scrapings and frozen sections from patients with abnormal cervical smear. J Clin Oncol. 1998;16:2238–45. doi: 10.1200/JCO.1998.16.6.2238. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaka T, Zheng PS, Yokoyama M, Fukuda K, Nakao Y, Sugimori H. Telomerase activation in cervical neoplasia. Obstet Gynecol. 1998;91:260–2. doi: 10.1016/s0029-7844(97)00595-4. [DOI] [PubMed] [Google Scholar]
- 14.Shroyer KR, Thompson LC, Enomoto T, Eskens JL, Shroyer AL, McGregor JA. Telomerase expression in normal epithelium, reactive atypia, squamous dysplasia, and squamous cell carcinoma of the uterine cervix. Am J Clin Pathol. 1998;109:153–62. doi: 10.1093/ajcp/109.2.153. [DOI] [PubMed] [Google Scholar]
- 15.Snijders PJ, van Duin M, Walboomers JM, et al. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human papillomavirus DNA. Cancer Res. 1998;58:3812–8. [PubMed] [Google Scholar]
- 16.Kyo S, Takakura M, Tanaka M, Kanaya T, Inoue M. Telomerase activity in cervical cancer is quantitatively distinct from that in its precursor lesions. Int J Cancer. 1998;79:66–70. doi: 10.1002/(sici)1097-0215(19980220)79:1<66::aid-ijc13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Nair P, Jayaprakash PG, Nair MK, Pillai MR. Telomerase, p53 and human papillomavirus infection in the uterine cervix. Acta Oncol. 2000;39:65–70. doi: 10.1080/028418600430996. [DOI] [PubMed] [Google Scholar]
- 18.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558–61. [PubMed] [Google Scholar]
- 19.Wisman GB, Knol AJ, Helder MN, et al. Telomerase in relation to clinicopathologic prognostic factors and survival in cervical cancer. Int J Cancer. 2001;91:658–64. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1099>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Yashima K, Ashfaq R, Nowak J, et al. Telomerase activity and expression of its RNA component in cervical lesions. Cancer. 1998;82:1319–27. [PubMed] [Google Scholar]
- 21.Anderson S, Shera K, Ihle J, et al. Telomerase activation in cervical cancer. Am J Pathol. 1997;151:25–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Jarboe EA, Liaw KL, Thompson LC, et al. Analysis of telomerase as a diagnostic biomarker of cervical dysplasia and carcinoma. Oncogene. 2002;21:664–73. doi: 10.1038/sj.onc.1205073. [DOI] [PubMed] [Google Scholar]
- 23.Nowak JA. Telomerase, cervical cancer, and human papillomavirus. Clin Lab Med. 2000;20:369–82. [PubMed] [Google Scholar]
- 24.Reesink-Peters N, Helder MN, Wisman GB, et al. Detection of telomerase, its components, and human papillomavirus in cervical scrapings as a tool for triage in women with cervical dysplasia. J Clin Pathol. 2003;56:31–5. doi: 10.1136/jcp.56.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai K, Yaginuma Y, Tsuruoka H, Griffin M, Hayashi H, Ishikawa M. Telomerase activity and human papillomavirus (HPV) infection in human uterine cervical cancers and cervical smears. Eur J Cancer. 1998;34:2082–6. doi: 10.1016/s0959-8049(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 26.Jarboe EA, Thompson LC, Heinz D, McGregor JA, Shroyer KR. Telomerase and human papillomavirus as diagnostic adjuncts for cervical dysplasia and carcinoma. Hum Pathol. 2004;35:396–402. doi: 10.1016/j.humpath.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 27.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 28.Bauer HM, Ting Y, Greer CE, et al. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–7. [PubMed] [Google Scholar]
- 29.Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82:1477–84. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 30.Sprague D, Phillips S, Mitchell C, et al. Telomerase activation in cervical keratinocytes containing stably replicating human papillomavirus type 16 episomes. Virology. 2002;301:247–54. doi: 10.1006/viro.2002.1542. [DOI] [PubMed] [Google Scholar]
- 31.Zheng PS, Iwasaka T, Zhang ZM, Pater A, Sugimori H. Telomerase activity in Pap smear-negative exfoliated cervical cells and its association with lesions and oncogenic human papillomaviruses. Gynecol Oncol. 2000;77:394–8. doi: 10.1006/gyno.2000.5779. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DK, Ngan HY, Cheng RY, Cheung AN, Liu SS, Tsao SW. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer. 1999;35:154–60. doi: 10.1016/s0959-8049(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 33.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 34.Allen HK, Klingelhutz AJ. Papillomavirus infection and telomerase activation. Papillomavirus Report. 2003;14:155–62. [Google Scholar]
- 35.Saito T, Schneider A, Martel N, et al. Proliferation-associated regulation of telomerase activity in human endometrium and its potential implication in early cancer diagnosis. Biochem Biophys Res Commun. 1997;231:610–4. doi: 10.1006/bbrc.1997.6164. [DOI] [PubMed] [Google Scholar]
- 36.Masutomi K, Yu EY, Khurts S, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–53. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 37.Kyo S, Masutomi K, Maida Y, et al. Significance of immunological detection of human telomerase reverse transcriptase: re-evaluation of expression and localization of human telomerase reverse transcriptase. Am J Pathol. 2003;163:859–67. doi: 10.1016/S0002-9440(10)63446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


