Abstract
The term “monoblock” has become a familiar term in the endodontic literature with recent interest in the application of dentin adhesive technology to endodontics. Endodontic “monoblocks” have generated controversial discussions among academicians and clinicians as to whether they are able to improve the quality of seal in root fillings and to strengthen roots. This review attempts to provide a broader meaning to the term “monoblock” and see how this definition may be applied to the materials that have been used in the past and present for rehabilitation of the root canal space. The potential of currently available bondable materials to achieve mechanically homogeneous units with root dentin is then discussed in relation to the classical concept in which the term “monoblock” was first employed in restorative dentistry, and subsequently in endodontics.
Introduction
The term monoblock, literally meaning a single unit, has been employed in dentistry since the turn of the century. In orthodontics, the ‘Monobloc’ was introduced in 1902 by Dr. Pierre Robin by uniting upper and lower acrylic removable appliances for treating patients with the syndrome that was later named after him. This one-piece appliance was subsequently employed to treat patients with Class II division 1 malocclusion and forms the forerunner of contemporary functional appliances by uniting two functional matrices (i.e. the maxilla and the mandible) into a single unit (1). Modified forms have since been used in treating patients with obstructive sleep apnea (2).
Until the day when diseased pulps can be regenerated (3,4), they have to be replaced by some form of restorative materials. With the rigidity of the root weakened by endodontic and restorative instrumentation (5,6), the sealing quality and tooth strengthening potential of endodontic replacement monoblocks become important issues. Strengthening of immature root canals with open apices and reduced circumferential dentin thickness are also issues of concern (7–9). The prolonged use of calcium hydroxide as an apexfication stimulation material results in an increase in the incidence of spontaneous cervical root fracture or fracture after minor impacts (10,11).
Using 3D-speckle interferometry to examine the rigidity of maxillary anterior teeth after different endodontic procedures in response to a load of 3.75 N, root deformation after endodontic access preparation was found to increase significantly from 0.24 ± 0.03 μm in intact roots to 0.36 ± 0.04 μm after preparing an access cavity (6). Presumably, when such root canals are subjected to clinically relevant forces (100 N), their flexures would reach clinically relevant levels. Shaping of the canals manually with stainless steel K-files from ISO #40 to ISO #110 resulted in a gradual but non-significant increase in root deformation. Preparation of a tapered post space resulted in a significant destabilization of the teeth with deformation up to 0.57 ± 0.04 μm. The greatest deformation was observed with a parallel-sided post preparation, with a threefold increase in tooth deformability to 0.73 ± 0.09 μm (6). In another study using strain gauges attached to the cemntoenamel junction to measure root stiffness, it was observed that endodontic procedures such as access preparation, shaping and obturation only reduced the relative stiffness of the roots by 5% (5). By contrast, an occlusal cavity preparation down to the cementoenamel junction level reduced the relative stiffness by 20%. The largest losses in stiffness were related to the loss of marginal ridge integrity, so that an MOD cavity preparation resulted in an average of a 63% loss in relative cuspal stiffness (5).
Primary Monoblocks
Replacement monoblocks created in the root canal spaces may be classified as primary, secondary and tertiary depending on the number of interfaces present between the bonding substrate and the bulk material core (Fig. 1). A primary monoblock has only one interface that extends circumferentially between the material and the root canal wall. In the late seventies when the scientific principles behind dentin bonding were being developed and the concept of unidirectional fiber reinforcement of resins relatively unheard of in dentistry, a 2-hydroxylethyl methacrylate (HEMA) containing root filling material (Hydron; Hydron Technologies, Inc., Pompano Beach, Florida, USA) was marketed commercially for en masse filling of root canals (12). The initial manufacturer-sponsored research was promising. The material stimulated interest as a potential successor for sealer-dependent lateral and vertical gutta-percha obturation techniques. Unlike how poly(HEMA) was used in its optimally polymerized form, Hydron was injected into root canals to be polymerized in-situ (13), often in the presence of residual moisture within the root canals (14,15). HEMA polymerizes in the presence of water to form soft hydrogels that are highly permeable and leachable (16). Subsequent independent studies demonstrated that Hydron-filled root canals exhibited extensive leakages (13, 17–21).
Fig. 1.
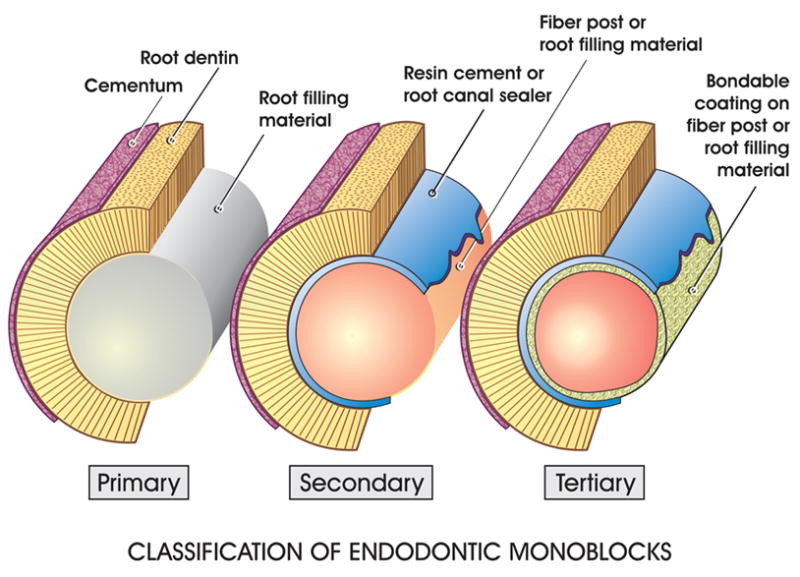
A schematic depicting the classification of endodontic monoblocks. In a primary monoblock, there is only one interface between the root filling material and the root dentin. In a secondary monoblock, there are two interfaces, one between the fiber post/root filling material and the cement/root canal sealer, and the other between the cement/root canal sealer and the root dentin. In a tertiary monoblock, a third interface is created when a bondable coating is present on the surface of the fiber post/root filling material.
Endodontically treated teeth are susceptible to fracture because of extensive restorations and reduced amounts of remaining tooth structure (5,6,22–24). The strength of an endodontically treated tooth is directly proportional to the amount of remaining sound tooth structure. As tooth structure is lost, the potential for tooth fracture increases (25,26). Although the manufacturer of Hydron did not infer that filling root canals with Hydron helps to strengthen roots and prevent root fractures, it is of interest that the moduli of elasticity of porous poly(HEMA) hydrogels such as Hydron ranges from 180–250 MPa (27). In order to reinforce roots, the modulus of elasticity of a root filling material would need to approximate that of dentin (i.e. 14,000 MPa) (28). Thus, with the advantages of hindsight (28), we know that one of the first monoblocks employed in root canals (Hydron) was not stiff enough to strengthen roots even if it could have bonded to root canal surfaces.
Orthograde obturation with mineral trioxide aggregate (MTA; ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK) as an apexification material represents a contemporary version of the primary monoblock in attempts to strengthen immature tooth roots (Fig. 2). MTA is composed principally of Portland cement with the addition of bismuth trioxide to render it radiopaque (29,30). Being an entirely inorganic material, Portland cement undergoes chemical shrinkage following hydration. This volume reduction is associated with the interaction between the cement and water, and is estimated to be 0.001 mL/g of cement (i.e. 0.1%) (31). Thus, a certain amount of volumetric shrinkage should also occur during the setting of MTA. As MTA is not bonded to dentin (Tay and Pashley, unpublished results), this shrinkage does not result in the generation of shrinkage stresses along the cavity walls (32–34). It is prudent to point out that the so called “high bond strength” (ca. 38–40 MPa) reported on the MTA in root sections were produced using a push-out test design (35). This mechanical testing protocol can generate high interfacial strength values that represents the frictional resistance of a material to the cylindrical cavity walls (36). Although MTA does not bond to dentin, interaction of the released calcium and hydroxyl ions of MTA with a phosphate-containing synthetic body fluid results in the formation of apatite-like interfacial deposits (37,38). These deposits fill up any gaps induced during the material shrinkage phase and improves the frictional resistance of MTA to the root canal walls. The formation of these non-bonding, gap-filling apatite deposits probably also accounts for the seal of MTA in orthograde obturations and perforation repair (39,40).
Fig. 2.
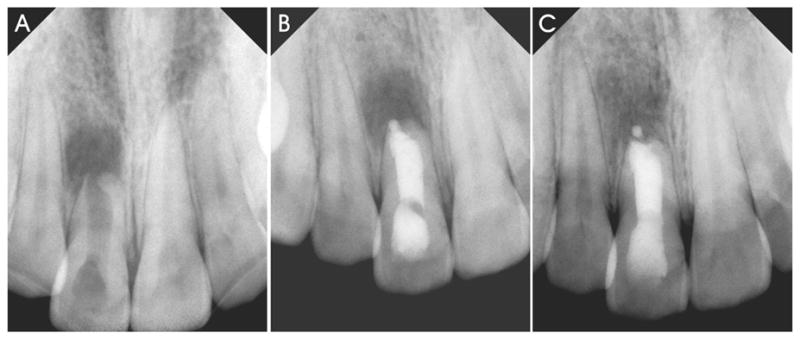
Orthograde filling of root canals with mineral trioxide aggregate (MTA) as an apexification material represents a contemporary version of the primary monoblock in attempts to strengthen immature tooth roots. A. Pre-operative radiograph of a central incisor (tooth 8) with incompletely formed root and open root apex that was necrotic and exhibiting radiographic signs of chronic apical periodontitis. B. Post-operative radiograph of an apexification procedure that was performed with orthograde filling of the entire root with white MTA. C. A 14 month post-treatment radiograph showing gradual bone regeneration along the periapex.
Although the elastic modulus of MTA is not available, studies on Portland cements indicated that the compressive elastic modulus of the latter is approximately 1.7 GPa (i.e. 1,700 MPa) during the early setting stage. The compressive elastic moduli of Portland cement increase after 14 days to 15 GPa (i.e. 15,000 MPa) with a water/cement ratio of 0.6, and around 30 GPa (i.e. 30,000 MPa) with a water cement ratio of 0.33, with minor further increases on further aging (41). Unlike Hydron, MTA should theoretically be able to strengthen roots (elastic modulus 14,000 – 18,600 MPa, according to location and orientation of the dentinal tubules). Nevertheless, in a recent study that examined the fracture resistance of MTA applied to immature sheep roots, it was found that there was no difference in roots that were filled with saline, versus those that were filled with MTA (42). Although the sample size was small, this study illustrates that MTA does not confer any perceivable benefit in root strengthening, apart from its ability to stimulate cementogenesis in apexification and root end fillings (43,44). The inability of MTA to strengthen roots is probably a combination of its lack of bonding to dentin, and that although it has high stiffness in compression, it has little strength in tension (Tay and Pashley, unpublished results).
Secondary Monoblocks
The combined use of a core material and a cement/sealer in contemporary endodontic obturations and fiber post adhesion introduces additional interfaces into a monoblock. Secondary monoblocks are those that have two circumferential interfaces, one between the cement and dentin, the other between the cement and the core material. A secondary monoblock is the type of monoblock that is classically perceived in the restorative and endodontic literature.
It can be seen from the discussion on primary monoblocks that two prerequisites are simultaneously required for a monoblock to function successfully as a mechanically homogenous unit. First, the materials that constitute a monoblock should have the ability to bond strongly and mutually to one another, as well as to the substrate with which the monoblock is intended to reinforce. Second, these materials should have moduli of elasticity that are similar to the substrate. The interaction of these two parameters is nicely illustrated in a recent finite element analysis study of different cements in combination with posts used to restore weakened roots (45). With the increase of the moduli of elasticity of the different cements (Table), the Von Mises stress concentrations in the root dentin decreased from 24.5 MPa to 20.8 MPa. When Panavia F (Kuraray Medical Inc., Tokyo, Japan), a heavily filled resin cement with an elastic modulus 18.3 GPa and a zinc phosphate cement (elastic modulus 9.3–13.4 GPa) were used that were similar to the modulus of elasticity of dentin, the respective Von Mises stress concentrations in the root dentin were lower (20.9 MPa and 20.8 MPa). This is because some of the stresses were redistributed to the cement layer (Von Mises stress 12.3 MPa and 14.0 MPa). On the contrary, when Superbond C&B cement (Sun Medical Co. Ltd., Shiga, Japan; elastic modulus 1.8 GPa) and a glass ionomer cement (elastic modulus 4.0 GPa) were used for cementation, high stress concentrations were found in the root dentin (Von Mises stress 24.5 MPa and 23.6 MPa, respectively). These stresses were directly transferred to the root dentin as the stress concentrations within the cement layers were low (Von Mises stress 2.4 MPa and 4.4 MPa, respectively).
Table.
Comparison of the moduli of elasticity of different materials employed within the root canal space with those of dentin.
| Material | Modulus of elasticity (GPa) | Number of times that of dentin |
|---|---|---|
| Dentin | 14.0 – 18.6 | 1 |
| Gutta-percha | 0.074 – 0.079 | 0.005 |
| Resilon | 0.087 – 0.129 | 0.005 – 0.008 |
| Poly(HEMA) | 0.18 – 0.25 | 0.01 – 0.02 |
| Clearfil SE Bond | 0.56 | 0.034 |
| C&B Metabond | 1.8 | 0.11 |
| Glass ionomer cement | 4.0 | 0.25 |
| Zinc phosphate cement | 9.3 – 13.4 | 0.57 – 0.82 |
| Fiber posts | 17.5 – 21.6 | 1.07 – 1.33 |
| Panava F | 18.3 | 1.12 |
| MTA (Portland cement) | 15 – 30 | 0.92 – 1.84 |
| Ceramic | 96 | 5.9 |
| Titanium alloy | 120 | 7.4 |
| Steel | 200 | 12.3 |
The results excerpted from the above study were derived from the use of a titanium post (45) that has a modulus of elasticity of 120 GPa (120,000 GPa -Table). It would be desirable for the study to be repeated using fiber posts that have moduli of elasticity similar to that of root dentin (46). Nevertheless, important conclusions could be derived with respect to the interaction between dentin adhesion and elastic moduli of the materials employed. For example, although Superbond C&B (C&B Metabond; Parkell Inc., Edgewoord, NY) has been recommended by Bouillaguet et al. (47) for bonding to post spaces due to its adhesive property and its low setting characteristics that permit shrinkage stress relief via resin flow, it would not be the ideal resin cement to restore weakened roots. The deformation of the resin cement was greater than that of the root dentin when occlusion force was passed to the root. As a result, the majority of the stresses were borne by the root. Although zinc phosphate cement demonstrated push out strengths comparable with other resin cements for the cementation of titanium (48,49) or fiber posts (50), (its elastic modulus was close to that of dentin and its stress concentrations in dentin were low), posts cemented with zinc phosphate cements often failed because of the cement’s relatively great elastic modulus, fragility and low bonding potential to the root dentin and the post surfaces (45). This explains why roots reinforced with posts that were cemented with dentin adhesives are more fracture resistant than those cemented with zinc phosphate cements (51). Likewise, the lower modulus of elasticity of glass ionomer cements (45) also explains why they are less efficient than dentin adhesives and composites in strengthening immature roots (7) and roots bonded with quartz-coated carbon fiber posts (52).
With an understanding of these principles, it is appropriate to examine the ability of some classic secondary monoblocks described in the restorative and endodontic literature to function as mechanically homogeneous units. The first implied existence of a mechanically homogeneous monoblock in the root canal space was reported in 1996 with the bonding of epoxy resin-based, carbon fiber-reinforced posts (i.e. carbon fiber posts) to root dentin (53). The authors claimed, based on their clinical experience, that carbon-fiber posts, having a modulus of elasticity very similar to the modulus of elasticity of dentin, can achieve a tooth-post-core monoblock instead of an assembly of heterogeneous materials. This should help to distribute masticatory loads homogeneously and reduce stresses during function. Although such a concept is exceedingly appealing in terms of product marketing and advertising, it is too advanced to be realized by the materials available at that time (in the late eighties and early nineties). Although the strongest (ultrahigh modulus) carbon fibers have a tensile modulus (500–1000 GPa) that are 2.5–5 times as strong as that of steel (200 GPa) (54), and that carbon fibers can be made to bond to epoxy resins via a carefully controlled oxidative process in nitric acid, ozone or electrochemical oxidation (55–57), they are no longer surface active once a fiber post is exposed by roughening or with a bur. Furthermore, the stiffness of carbon fiber posts is lowered by the presence of epoxy resin. Epoxy resin is not usually bondable to methacrylates, as ring opening of epoxide groups and chemical grafting of methacrylic groups cannot be achieved under physiological temperatures (58). The beneficial claims of the carbon fiber post-root dentin monoblock could not be validated in independent in vitro and retrospective in vivo studies (59–61). Over the years, the carbon fibers in this type of first generation fiber posts have been replaced by quartz-coated carbon fibers (Fig. 3) and glass fibers that are amendable to silane coupling (62,63). The epoxy resin embedding matrix in older generations of fiber posts is also replaced with highly cross-linked, oxygen inhibition layer-free methacrylate resin matrices that, theoretically, have the potential to bond to methacrylate-based resin cements (64). Different modalities of surface treatments of posts are also available to render these newer generations of fiber posts more conducive to bonding to methacrylate-based resins. Although the use of these newer generations of fiber posts has not yet attained the scientific rigor of an ideal monoblock, they are reported to have performed well in vivo (65). This is probably due to the similarity in the moduli of elasticity between fiber posts and root dentin.
Fig. 3.
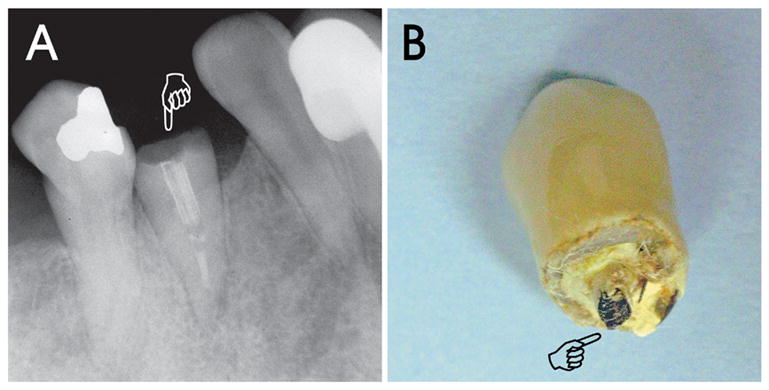
Bonding of a pre-fabricated fiber post and a resin cement to a post space represents the classic secondary monoblock depicted in the restorative and endodontic literature. Although there is ample evidence in the literature to support the use of fiber posts for improving the fracture resistance of endodontically treated teeth, a case is illustrated showing that fiber posts do not necessarily strengthen roots and that crown fracture may still occur after the use of fiber posts. A. A radiograph of a retained root (tooth 21; pointer) with an incomplete root filling, a fractured fiber post containing radiopaque fibers, and horizontal root fracture along the gingival margin. B. A photograph of the fractured crown with a ceramometal crown and the coronal portion of the fractured fiber post (pointer). In this particular case, the presence of eccentric masticatory forces caused by the absence of molar tooth support and the possible pre-existence of a cervical abfraction lesion (see tooth 20) probably accounted for the failure of the fiber post-supported tooth.
By definition, root canal obturations, being indirect fillings of the root canal space created by cleaning and shaping, may be regarded as secondary monoblock systems. However, as conventional root canal sealers do not bond strongly to dentin and gutta-percha (66), they do not behave as mechanically homogenous units with the root dentin. Although glass ionomer cements and resin-modified glass ionomer cements bond to root dentin and have been marketed as root canal sealers (67,68), they do not bond to gutta-percha. Even if they do, the modulus elasticity of gutta-percha points (ca. 80 MPa) (28) is 175–230 times lower than that of dentin (ca. 14,000–18,600 MPa) (28,45,69), making them too plastic (i.e. not stiff enough) to reinforce roots after endodontic therapy. Thus, it is dubious that a glass ionomer-based sealer can be used to prevent root fracture in gutta-percha filled root canals (70).
Interest in utilizing the classical monoblock concept for sealing and reinforcing the root canal space was rekindled in 2004 with the advent of bondable root filling materials that are advocated as alternatives to conventional gutta-percha. To-date, there are three bondable root filling materials available commercially. Of these, Resilon (Resilon Research LLC, Madison, CT) is the only bondable root filling material that may be used for either lateral or warm vertical compaction techniques. As Resilon is applied using a methacrylate-based sealer to self-etching primer treated root dentin, it contains two interfaces, one between the sealer and primed dentin and the other between the sealer and Resilon, and hence may be classified as a type of secondary monoblock. Initial studies on Resilon-filled root canals were highly favorable. Resilon-filled root canals were found to be better than conventionally gutta-percha filled canals in resisting bacterial leakage (71) and improving the fracture resistance of endodontically treated teeth (72). Based on these promising properties, Resilon, together with the Epiphany primer and sealer system (Pentron Clinical Technologies, Wallingford CT) was subsequently referred to as the Resilon Monoblock System (RMS) (73,74) that produces ideal root obturations in terms of coronal sealing and fracture resistance (75). Although Resilon-filled root canals do achieve good apical and coronal seals, it is equivocal from subsequent independent research studies whether such seals are better than those achieved using gutta-percha and conventional root canal sealers (76–79).
All adhesive restorations create interfacial stresses during polymerization due to the intrinsic volumetric shrinkage associated with converting double bonds to single bonds. Polymerization shrinkage stress can be high enough to debond adhesive interfaces (80,81). The stress increases as the volume to surface area ratio increases. Thus, the configuration of the cavity or “C-factor” is very important. In a box-like class I cavity, there are five bonded cavity walls and only one (i.e. occlusal) unbonded “wall” where polymerization stress can be relieved by resin flow. Such a cavity has a C-factor or 5/1 or 5. In root canals, C-factors can be over 1000 (82). Any polymerizing endodontic sealer will be subjected to large polymerization stresses during their setting that may cause debonding and gap formation along the periphery of the root filling. The extremely high C-factor in root canals has been cited as a possibility for not achieving perfect seals in Resilon-filled root canals (82). According to the manufacturer, Resilon is a polycaprolactone-based, dimethacrylates resin containing thermoplastic composite that contains radio-opaque fillers as well as glass-ionomer filler particles (83–85). The bondability of Resilon to methacrylate resin-based root canal sealers is supposed to be derived from the inclusion of the urethane dimethacrylate resin. However, the concentration of the polymeric components, polycaprolactone and urethane dimethacrylates is probably in the ratio of 10:1 (84), which may not be optimized for optimal adhesion of the root filling material to the methacrylate resin-based sealers. Morphologic studies further revealed that the dimethacrylate in Resilon is not homogeneously dispersed within the polymer blend and appeared as phase separation components within the polycaprolactone (86,87). Both microshear bond testing (87,88) and push-out test (89) showed that the bonding of Resilon to methacrylate resin-based sealers and root dentin is weak. That is, the reported bond strengths are 1–3 MPa, whereas resin-dentin bonds are 25–30 MPa. As Resilon is used commercially as a fully polymerized material that lacks a free radical-containing oxygen inhibition layer, its bondability to resin-based sealers has further been questioned (87). Recently published research further indicated that there is no difference between Resilon and gutta-percha in strengthening and reinforcement of immature roots (90). The modulus of elasticity of Resilon was found to be 86.6 ± 43.2 MPa under dry conditions and 129.2 ± 54.7 MPa after one month of water sorption (28). Thus, similar to gutta-percha, Resilon is not stiff enough to achieve a mechanically homogeneous unit with root dentin (28,45). Root dentin has a modulus of elasticity of 16,000–18,000 MPa. Stiff composites used in restorative dentistry such as Z100 (3M ESPE, St. Paul, MN) have similar high stiffness as root dentin but cannot be removed for retreatments. Unfilled resins only have elastic moduli between 2000–3000 MPa and would not be able to reinforce roots.
Tertiary Monoblocks
Tertiary monoblocks are those in which a third circumferential interface is introduced between the bonding substrate and the abutment material. Fiber posts that contain either an external silicate coating (DT Light SL, VDW GmbH, Munich, Germany), or those that contain unpolymerized resin composite for relining root canals that are too wide or not perfectly round for the fitting of conventional fiber posts (Anatomic Post, RTD, St. Egéve, France) may be considered as tertiary monoblocks. In the latter, the post is adapted to a lubricated post space and photoactivated to partially polymerize the composite (91). The relined assembly is then removed, and optimally polymerized prior to reinsertion for bonding with a resin cement. The efficacies of these systems have not been thoroughly investigated. In the Anatomic Post system, the resin cement layer was significantly reduced except for the apical portion of the post space in which no relining composite was included by the manufacturer (92). Theoretically, a reduction of the resin cement thickness should result in a reduction of volumetric shrinkage. However, it is uncertain whether polymerization shrinkage stresses along the cavity walls are also reduced due to the reduction in resin layer thickness in a low compliance environment. Also, the introduction of a tertiary interface is problematic in that gaps were found to be present between the fiber post and the relining composite (92). These gaps may can act as stress raisers and result in eventual adhesive failure and dislodging of the fiber post from the relining composite.
The two other types of bondable root filling materials previously mentioned also belong to this category, as an additional circumferential interface is introduced by coating the non-bondable gutta-percha points with materials that render them bondable to the root canal sealers. As the tertiary interface exists as an external coating on the surface of the gutta-percha, both systems are designed to be used with either a single-cone technique or a technique that involves the passive placement of accessory cones without lateral compaction, to avoid disruption of these external coatings.
In the EndoRez system (Ultradent, South Jordan, UT), conventional gutta-percha cones are coated with a proprietary resin coating (93). This coating is created by first reacting one of the isocyanato groups of a diisocyanate with the hydroxyl group of a hydroxyl-terminated polybutadiene, as the latter is bondable to the hydrophobic polyisoprene component of the gutta-percha cones. This is followed by the grafting of a hydrophilic methacrylate functional group to the other isocyanato group of the diisocyanate, producing a gutta-percha resin coating that is bondable to a hydrophilic, methacrylate-based dual-cured resin sealer (94). In this system, no dentin adhesive is employed and the generation of an endodontic seal is dependent on the penetration of the hydrophilic sealer into the dentinal tubules and lateral canals following removal of the smear layer. To-date, leakage and morphologic studies showed that the seal of the EndoRez system is mediocre (95–97), although long resin tags could be identified within the dentinal tubules (96,97). This may be attributed to the polymerization shrinkage of the methacrylate-based sealer (97). Also, the sealer bonds weakly to the pre-polymerized proprietary coating, as the latter lacks free radicals for bonding due to the removal of the oxygen inhibition layer for packing purposes (98). Inconsistency of the external proprietary resin coating was also observed in the form of uneven circumferential thickness or partial detachment (94). Although both the tensile bond strength (99) and apical seal (100) of the EndoRez system to intraradicular dentin may be improved using a dual-cured self-etching primer/adhesive such as Clearfil Liner Bond 2V (Kuraray Medical Inc.), there is a potential problem of rapid polymerization of the adhesive in an environment with reduced oxygen concentration. Moreover, even with the adjunctive use of an adhesive, it is unrealistic to expect the establishment of a mechanically homogenous unit with the root canal with the EndoRez system, as the bulk of the material inside the root canal still consists of thermoplastic gutta-percha, an elastomeric polymer that flows when stressed.
In ActiV GP (Brasseler USA, Savannah, GA), the root filling system is marketed as a monoblock system by using conventional gutta-percha cones that are surface-coated with glass ionomer fillers using a proprietary technique (101) (Fig. 4). By doing so, a stiffer gutta-percha cone is achieved that transforms it into a gutta-percha core/cone, enabling the latter to be functioned as both the tapered filling cone and as its own carrier core, thus avoiding the need for a separate interior carrier of plastic or metal (102). The presence of the glass-ionomer filler-coated gutta-percha cone also allows it to be bonded to the root dentin via a glass-ionomer sealer (98). As this system is new, limited information is available. The system produced apical seals to fluid filtration that are comparable to that of gutta-percha and AH Plus sealer (Dentsply Caulk, Milford, DE) (103). However, being a single cone technique, coronal leakage of the ActiV GP system to fluid filtration was worse than that achieved with gutta-percha/AH Plus, probably due to the increase in the volume of the glass ionomer cement sealer (103). The difference between the apical and coronal fluid filtration results could be reflected when the same systems were evaluated using a bacterial leakage technique, where ActiV GP demonstrated more severe bacterial leakage compared with gutta-percha/AH Plus (Monticelli et al., unpublished results). For the reason mentioned previously, it is also unlikely that the use of the ActiV GP system will improve the fracture resistance of endodontically treated teeth.
Fig. 4.
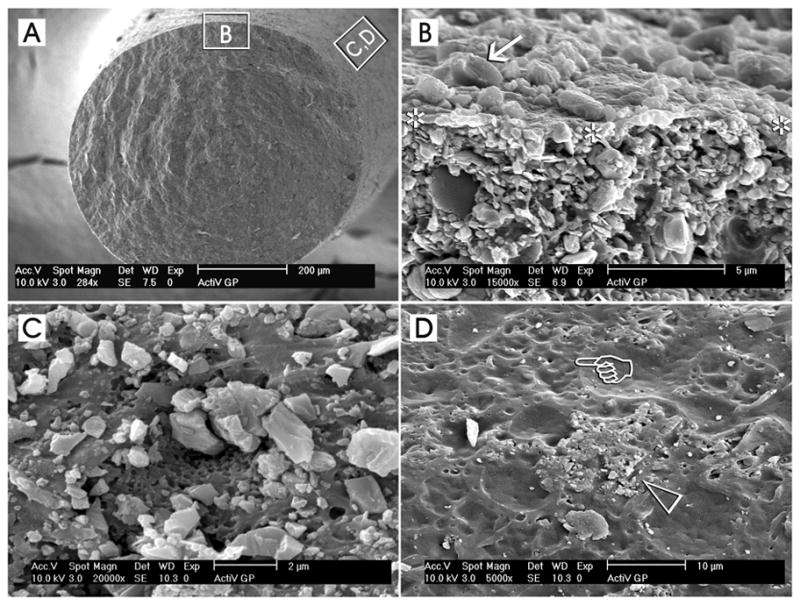
Surface coating of conventional gutta-percha cones with glass ionomer fillers (ActiV GP, Brasseler USA, Savannah, GA) represents an example of a part of the components of a tertiary endodontic monoblock, in which these filler-coated gutta-percha cones are bonded to intraradicular dentin with the use of a glass ionomer root canal sealer. A. A low magnification scanning electron micrograph of a cryofractured ActiV GP gutta-percha cone depicting the representative locations from which the higher magnification micrographs were derived. B. A high magnification interfacial view showing the surface of the fractured gutta-percha cone (between asterisks) with the glass ionomer fillers (arrow) on top of the surface and the filler-dense gutta-percha cone below. C. A high magnification surface view showing a region that is heavily-coated with glass ionomer fillers. The dimensions of these angular fillers ranged from submicrometer to 2 μm in diameter. D. Incomplete or uneven coating of the gutta-percha cone surface could often be observed along different regions of the same coated gutta-percha cone. In this micrograph, the glass ionomer fillers were sparse (open arrowhead) and numerous dimpled, filler-free areas (pointer) could be identified.
Conclusion
Although the concept of creating mechanically homogenous units with root dentin is excellent in theory, accomplishing these “ideal monoblocks” in the root canal space is easier said than done. Beginning with dentin adhesive application, removing thick smear layers or attempts to infiltrate these smear layers with mild self-etching adhesives is not as predictably achieved inside a long narrow channel even with improved vision from a surgical microscope. Evaporating adhesive solvents and hydrogen-bonded water from hydrophilic adhesives is difficult even for crown dentin (104,105). To date, there is no data on how this may be performed efficaciously inside root canals without avoiding over-thinning of the adhesive (106,107) or inadvertently introducing air forcefully beyond the root apex that may result in subcutaneous emphysema (108,109). Even when the effect of dentin permeability in endodontically treated teeth is minimal (110), entrapment of residual moisture within the root canal can result in the permeation of this unbound water through hydrophilic adhesive layers (111), and its expression in the form of water droplets on the adhesive surface along the post space (112) or root canal. Entrapment of these water droplets between the adhesive and resin cements/sealer is analogous to introducing crack tips in fracture toughness testing (113). They can act as stress raisers that promote crack growth and propagation during loading along the interface. The highly unfavorable cavity geometry within the root canal space is detrimental to the relief of shrinkage stresses during the polymerization of the resin cements or sealers. Thus, until non-shrinking composites are available (114), the pursuit of an ideal monoblock for reinforcing the root canal may be viewed as an ideal goal. Moreover, the moduli of elasticity of the post, root filling material and the accompany resin cements or sealers have to match that of root dentin in order that loading stresses are evenly distributed and borne by all the monoblock components. These issues become increasing more complex as additional interfaces are incorporated from the primary to the tertiary monoblocks.
Acknowledgments
This work was funded by grants R01 DE014911 and R01 DE015306 from the National Institute of Dental and Craniofacial Research, Bethesda, MD (P.I. D. Pashley). The authors are grateful to Michelle Barnes for secretarial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wahl N. Orthodontics in 3 millennia. Chapter 9: functional appliances to midcentury . Am J Orthod Dentofacial Orthop. 2006;129:829–33. doi: 10.1016/j.ajodo.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Cozza P, Ballanti F, Prete L. A modified monobloc for treatment of young children with obstructive sleep apnea. J Clin Orthod. 2004;38:241–7. [PubMed] [Google Scholar]
- 3.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg M, Lacerda-Pinheiro S, Jegat N, Six N, Septier D, Priam F, Bonnefoix M, Tompkins K, Chardin H, Denbesten P, Veis A, Poliard A. The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin North Am. 2006;50:277–98. doi: 10.1016/j.cden.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15:512–6. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- 6.Lang H, Korkmaz Y, Schneider K, Raab WH. Impact of endodontic treatments on the rigidity of the root. J Dent Res. 2006;85:364–8. doi: 10.1177/154405910608500416. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Chan AT, Chen YM, Yip KH, Smales RJ. Effectiveness and dentin bond strengths of two materials for reinforcing thin-walled roots. Dent Mater. 2006 doi: 10.1016/j.dental.2006.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg F, Kaplan A, Roitman M, Manfre S, Picca M. Reinforcing effect of a resin glass ionomer in the restoration of immature roots in vitro. Dent Traumatol. 2002;18:70–2. doi: 10.1034/j.1600-9657.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho CA, Valera MC, Oliveira LD, Camargo CH. Structural resistance in immature teeth using root reinforcements in vitro. Dent Traumatol. 2005;21:155–9. doi: 10.1111/j.1600-9657.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 10.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 11.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 12.Benkel BH, Rising DW, Goldman LB, Rosen H, Goldman M, Kronman JH. Use of a hydrophilic plastic as a root canal filling material. J Endod. 1976;2:196–202. doi: 10.1016/S0099-2399(76)80133-1. [DOI] [PubMed] [Google Scholar]
- 13.Yesilsoy C. Radiographic evidence of absorption of Hydron from an obturated root canal. J Endod. 1984;10:321–3. doi: 10.1016/S0099-2399(84)80187-9. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya N, Nomura M, Yoshikubo A, Arai T, Nakamura J, Cox CF. Effect of canal drying methods on the apical seal. J Endod. 2000;26:292–94. doi: 10.1097/00004770-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Petschelt A. Drying of root canals. Dtsch Zahnarztl Z. 1990;45:222–6. [PubMed] [Google Scholar]
- 16.Chirila TV, Chen YC, Griffin BJ, et al. Hydrophilic sponges based on 2 hydroxyethyl methacrylate, I: effect of monomer mixture composition on the pore size. Polym Int. 1993;32:221–32. [Google Scholar]
- 17.Rhome BH, Solomon EA, Rabinowitz JL. Isotopic evaluation of the sealing properties of lateral condensation, vertical condensation, and Hydron. J Endod. 1981;7:458–61. doi: 10.1016/S0099-2399(81)80306-8. [DOI] [PubMed] [Google Scholar]
- 18.Osins BA, Carter JM, Shih-Levine M. Microleakage of four root canal sealer cements as determined by an electrochemical technique. Oral Surg Oral Med Oral Pathol. 1983;56:80–8. doi: 10.1016/0030-4220(83)90060-9. [DOI] [PubMed] [Google Scholar]
- 19.Murrin JR, Reader A, Foreman DW, Beck M, Meyers WJ. Hydron versus gutta-percha and sealer: a study of endodontic leakage using the scanning electron microscope and energy-dispersive analysis. J Endod. 1985;11:101–9. doi: 10.1016/s0099-2399(85)80227-2. [DOI] [PubMed] [Google Scholar]
- 20.Langeland K, Olsson B, Pascon EA. Biological evaluation of Hydron. J Endod. 1981;7:196–204. doi: 10.1016/S0099-2399(81)80176-8. [DOI] [PubMed] [Google Scholar]
- 21.Tanzilli JP, Nevins AJ, Borden BG. A histologic study comparing Hydron and gutta-percha as root canal filling materials in monkeys. J Endod. 1981;7:396–401. doi: 10.1016/S0099-2399(81)80037-4. [DOI] [PubMed] [Google Scholar]
- 22.Lertchirakarn V, Palamara JE, Messer HH. Finite element analysis and strain-gauge studies of vertical root fracture. J Endod. 2003;29:529–34. doi: 10.1097/00004770-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wu MK, van der Sluis LW, Wesselink PR. Comparison of mandibular premolars and canines with respect to their resistance to vertical root fracture. J Dent. 2004;32:265–8. doi: 10.1016/j.jdent.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Rundquist BD, Versluis A. How does canal taper affect root stresses? Int Endod J. 2006;39:226–37. doi: 10.1111/j.1365-2591.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 25.Sornkul E, Stannard JG. Strength of roots before and after endodontic treatment and restoration. J Endod. 1992;18:440–3. doi: 10.1016/S0099-2399(06)80845-9. [DOI] [PubMed] [Google Scholar]
- 26.Trabert KC, Caputo AA, Abou-Rass M. Tooth fracture: a comparison of endodontics and restorative treatments. J Endod. 1978;4:341–5. doi: 10.1016/s0099-2399(78)80232-5. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Hedberg EL, Liu Z, Bahulekar R, Meszlenyi RK, Mikos AG. Preparation of macroporous poly(2-hydroxyethyl methacrylate) hydrogels by enhanced phase separation. Biomaterials. 2000;21:2163–9. doi: 10.1016/s0142-9612(00)00137-x. [DOI] [PubMed] [Google Scholar]
- 28.Williams C, Loushine RJ, Weller RN, Pashley DH, Tay FR. A comparison of cohesive strength and stiffness of Resilon and gutta-percha. J Endod. 2006;32:553–5. doi: 10.1016/j.joen.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Islam I, Chng HK, Yap AU. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006;39:220–5. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 31.Bentz DP. Three-dimensional computer simulation of Portland cement hydration and microstructure development. J Am Ceram Soc. 1997;80:3–21. [Google Scholar]
- 32.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25:435–40. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 33.Francis LF, McCormick AV, Vaessen DM, Payne JA. Development and measurement of stress in polymer coatings. J Mater Sci. 2002;37:4717–31. [Google Scholar]
- 34.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21:962–70. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Yan P, Peng B, Fan B, Fan M, Bian Z. The effects of sodium hypochlorite (5.25%), chlorhexidine (2%), and Glyde File Prep on the bond strength of MTA-dentin. J Endod. 2006;32:58–60. doi: 10.1016/j.joen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Goracci C, Fabianelli A, Sadek FT, Papacchini F, Tay FR, Ferrari M. The contribution of friction to the dislocation resistance of bonded fiber posts. J Endod. 2005;31:608–12. doi: 10.1097/01.don.0000153841.23594.91. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 38.Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006;32:425–8. doi: 10.1016/j.joen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Human saliva penetration of root canals obturated with two types of mineral trioxide aggregate cements. J Endod. 2005;31:453–6. doi: 10.1097/01.don.0000145429.04231.e2. [DOI] [PubMed] [Google Scholar]
- 40.De-Deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T. MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage model. Int Endod J. 2006;39:293–8. doi: 10.1111/j.1365-2591.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 41.Haecker C-J, Garboczi EJ, Bullard JWm Bohn RB, Sun Z, Shah SP, Voigt T. Modeling the linear elastic properties of Portland cement paste. Cement Concr Res. 2005;35:1948–60. [Google Scholar]
- 42.Andreasen JO, Munksgaard EC, Bakland LK. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent Traumatol. 2006;22:154–6. doi: 10.1111/j.1600-9657.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 43.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endo. 1995;21:603–8. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 44.Baek SH, Plenk H, Jr, Kim S. Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root-end filling materials. J Endod. 2005;31:444–9. doi: 10.1097/01.don.0000148145.81366.a5. [DOI] [PubMed] [Google Scholar]
- 45.Li LL, Wang ZY, Bai ZC, Mao Y, Gao B, Xin HT, Zhou B, Zhang Y, Liu B. Three-dimensional finite element analysis of weakened roots restored with different cements in combination with titanium alloy posts. Chin Med J (Engl) 2006;119:305–11. [PubMed] [Google Scholar]
- 46.Barjau-Escribano A, Sancho-Bru JL, Forner-Navarro L, Rodriguez-Cervantes PJ, Perez-Gonzalez A, Sanchez-Marin FT. Influence of prefabricated post material on restored teeth: fracture strength and stress distribution. Oper Dent. 2006;31:47–54. doi: 10.2341/04-169. [DOI] [PubMed] [Google Scholar]
- 47.Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater. 2003;19:199–205. doi: 10.1016/s0109-5641(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 48.Schmage P, Sohn J, Nergiz I, Ozcan M. Various conditioning methods for root canals influencing the tensile strength of titanium posts. J Oral Rehabil. 2004;31:890–4. doi: 10.1111/j.1365-2842.2004.01372.x. [DOI] [PubMed] [Google Scholar]
- 49.Schmage P, Sohn J, Ozcan M, Nergiz I. Effect of surface treatment of titanium posts on the tensile bond strength. Dent Mater. 2006;22:189–94. doi: 10.1016/j.dental.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Sadek FT, Goracci C, Monticelli F, Grandini S, Cury AH, Tay FR, Ferrari M. Immediate and 24-hour evaluation of the interfacial strengths of fiber posts. J Endod. doi: 10.1016/j.joen.2006.07.005. in press. [DOI] [PubMed] [Google Scholar]
- 51.Mendoza DB, Eakle WS, Kahl EA, Ho R. Root reinforcement with a resin-bonded preformed post. J Prosthet Dent. 1997;78:10–4. doi: 10.1016/s0022-3913(97)70081-7. [DOI] [PubMed] [Google Scholar]
- 52.Bolhuis P, de Gee A, Feilzer A. The influence of fatigue loading on the quality of the cement layer and retention strength of carbon fiber post-resin composite core restorations. Oper Dent. 2005;30:220–7. [PubMed] [Google Scholar]
- 53.Dallari A, Rovatti L. Six years of in vitro/in vivo experience with Composipost. Compend Contin Educ Dent Suppl. 1996:S57–S63. [PubMed] [Google Scholar]
- 54.Brady GS, Clauser HR, Vaccari JA. Materials handbook. McGraw-Hill; 1997. [Google Scholar]
- 55.Wu Z, Pittman CU, Gardner SD. Nitric acid oxidation of carbon fibers and the effects of subsequent treatment in refluxing aqueous NaOH. Carbon. 1995;33:597–605. [Google Scholar]
- 56.Fu X, Lu W, Chung DDL. Ozone treatment of carbon fiber for reinforcing cement. Carbon. 1998;36:1337–45. [Google Scholar]
- 57.Yue ZR, Jiang W, Wang L, Gardner SD, Pittman CU. Surface characterization of electrochemically oxidized carbon fibers. Carbon. 1999;11:1785–96. [Google Scholar]
- 58.Kammer S, Keomara K, Sandner B, Schreiber R. Synthesis and polymerization of epoxy methacrylates. 2. Acylated epoxy methacrylates and their copolymers. Macro Mater Eng. 2001;286:276–84. [Google Scholar]
- 59.Purton DG, Payne JA. Comparison of carbon fiber and stainless steel root canal posts. Qunitessence Int. 1996;27:93–7. [PubMed] [Google Scholar]
- 60.Sidoli GE, King PA, Setchell DJ. An in vitro evaluation of a carbon fiber-based post and core system. J Prosthet Dent. 1997;78:5–9. doi: 10.1016/s0022-3913(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 61.Segerström S, Astback J, Ekstrand KD. A retrospective long term study of teeth restored with prefabricated carbon fiber reinforced epoxy resin posts. Swed Dent J. 2006;30:1–8. [PubMed] [Google Scholar]
- 62.Dibenedetto AT, Lex PJ. Evaluation of surface treatments for glass fibers in composite materials. Polym Eng Sci. 2004;29:543–55. [Google Scholar]
- 63.Perdigão J, Gomes G, Lee IK. The effect of silane on the bond strengths of fiber posts. Dent Mater. 2006 doi: 10.1016/j.dental.2005.11.002. in press. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari M, Goracci C, Sadek FT, Monticelli F, Tay FR. An investigation of the interfacial strengths of methacrylate resin-based glass fiber post-core build-ups by their components. J Adhes Dent. in press. [PubMed] [Google Scholar]
- 65.Mannocci F, Qualtrough AJ, Worthington HV, Watson TF, Pitt Ford TR. Randomized clinical comparison of endodontically treated teeth restored with amalgam or with fiber posts and resin composite: five-year results. Oper Dent. 2005;30:9–15. [PubMed] [Google Scholar]
- 66.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002;28:684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Koch K, Min PS, Stewart GG. Comparison of apical leakage between Ketac Endo sealer and Grossman sealer. Oral Surg Oral Med Oral Pathol. 1994;78:784–7. doi: 10.1016/0030-4220(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 68.Saunders WP, Saunders EM, Herd D, Stephens E. The use of glass ionomer as a root canal sealer - a pilot study. Int Endod J. 1992;25:238–44. doi: 10.1111/j.1365-2591.1992.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe T, Miyazaki M, Inage H, Kurokawa H. Determination of elastic modulus of the components at dentin-resin interface using the ultrasonic device. Dent Mater J. 2004;23:361–7. doi: 10.4012/dmj.23.361. [DOI] [PubMed] [Google Scholar]
- 70.Lertchirakarn V, Timyam A, Messer HH. Effects of root canal sealers on vertical root fracture resistance of endodontically treated teeth. J Endod. 2002;28:217–9. doi: 10.1097/00004770-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 71.Shipper G, Ørstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon) J Endod. 2004;30:342–7. doi: 10.1097/00004770-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Teixeira FB, Teixeira EC, Thompson JY, Trope M. Fracture resistance of roots endodontically treated with a new resin filling material. J Am Dent Assoc. 2004;135:646–52. doi: 10.14219/jada.archive.2004.0255. [DOI] [PubMed] [Google Scholar]
- 73.Shipper G, Teixeira FB, Arnold RR, Trope M. Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or Resilon. J Endod. 2005;31:91–6. doi: 10.1097/01.don.0000140569.33867.bf. [DOI] [PubMed] [Google Scholar]
- 74.Teixeira FB, Teixeira EC, Thompson J, Leinfelder KF, Trope M. Dentinal bonding reaches the root canal system. J Esthet Restor Dent. 2004;16:348–54. doi: 10.1111/j.1708-8240.2004.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 75.Teixeira FB. Ideal obturation using synthetic root-filling systems: coronal sealing and fracture resistance. Prac Proced Aesthet Dent. 2006;18:S7–S11. [PubMed] [Google Scholar]
- 76.Tay FR, Loushine RJ, Weller RN, Kimbrough WF, Pashley DH, Mak YF, Lai CN, Raina R, Williams MC. Ultrastructural evaluation of the apical seal in roots filled with a polycaprolactone-based root canal filling material. J Endod. 2005;31:514–9. doi: 10.1097/01.don.0000152298.81097.b7. [DOI] [PubMed] [Google Scholar]
- 77.Stratton RK, Apicella MJ, Mines P. A fluid filtration comparison of gutta-percha versus Resilon, a new soft resin endodontic obturation system. J Endod. 2006;32:642–5. doi: 10.1016/j.joen.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Aptekar A, Ginnan K. Comparative analysis of microleakage and seal for 2 obturation materials: Resilon/Epiphany and gutta-percha. J Can Dent Assoc. 2006;72:245a–d. [PubMed] [Google Scholar]
- 79.Raina R, Loushine RJ, Weller RN, Tay FR, Pashley DH. Evaluation of the quality of seal in Resilon/Epiphany and gutta-percha/AH Plus filled root canals using a fluid filtration approach. J Endod. doi: 10.1016/j.joen.2007.05.003. in press. [DOI] [PubMed] [Google Scholar]
- 80.Feilzer AJ, de Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dent Res. 1987;66:1636–9. doi: 10.1177/00220345870660110601. [DOI] [PubMed] [Google Scholar]
- 81.Carvalho RM, Pereira JC, Yoshiyama M, Pashley DH. A review of polymerization contraction: the influence of stress development versus stress relief. Oper Dent. 1996;21:17–24. [PubMed] [Google Scholar]
- 82.Tay FR, Loushine RJ, Lambrechts P, Weller RN, Pashley DH. Geometric factors affecting dentin bonding in root canals: a theoretical modeling approach. J Endod. 2005;31:584–9. doi: 10.1097/01.don.0000168891.23486.de. [DOI] [PubMed] [Google Scholar]
- 83.Jia WT, Alpert B. Root canal filling material. United States Patent & Trademark Office. United States Patent Application 20030113686, June 19, 2003. [Google Scholar]
- 84.Jia WT. United States Patent & Trademark Office. Dental filling material. United States Patent Application 20050066854, March 31, 2005. [Google Scholar]
- 85.Jia WT, Trope M, Alpert B. United States Patent & Trademark Office. Dental filling material. United States Patent Application 20050069836, March 31, 2005. [Google Scholar]
- 86.Tay FR, Pashley DH, Williams MC, Raina R, Loushine RJ, Weller RN, Kimbrough WF, King NM. Susceptibility of a polycaprolactone-based root canal filling material to degradation. I. Alkaline hydrolysis. J Endod. 2005;31:593–8. doi: 10.1097/01.don.0000152301.72828.61. [DOI] [PubMed] [Google Scholar]
- 87.Hiraishi N, Papacchini F, Loushine RJ, Weller RN, Ferrari M, Pashley DH, Tay FR. Shear bond strength of Resilon to a methacrylate-based root canal sealer. Int Endod J. 2005;38:753–63. doi: 10.1111/j.1365-2591.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- 88.Tay FR, Hiraishi N, Pashley DH, Loushine RJ, Weller RN, Gillespie WT, Doyle MD. Bondability of Resilon to a methacrylate-based root canal sealer. J Endod. 2006;32:133–7. doi: 10.1016/j.joen.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 89.Gesi A, Raffaelli O, Goracci C, Pashley DH, Tay FR, Ferrari M. Interfacial strength of Resilon and gutta-percha to intraradicular dentin. J Endod. 2005;31:809–13. doi: 10.1097/01.don.0000158230.15853.b7. [DOI] [PubMed] [Google Scholar]
- 90.Stuart CH, Schwartz SA, Beeson TJ. Reinforcement of immature roots with a new resin filling material. J Endod. 2006;32:350–3. doi: 10.1016/j.joen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Grandini S, Sapio S, Simonetti M. Use of anatomic post and core for reconstructing an endodontically treated tooth: a case report. J Adhes Dent. 2003;5:243–7. [PubMed] [Google Scholar]
- 92.Grandini S, Goracci C, Monticelli F, Borracchini A, Ferrari M. SEM evaluation of the cement layer thickness after luting two different posts. J Adhes Dent. 2005;7:235–40. [PubMed] [Google Scholar]
- 93.Haschke E. Adhesive endodontic cones and related methods. United States Patent Application 20040202986. US Patent & Trademark Office, October 14, 2004. [Google Scholar]
- 94.Jensen SD, Fischer DJ. United States Patent & Trademark Office. Method for filling and sealing a root canal. Patent Number 6,811,400, November 2, 2004. [Google Scholar]
- 95.Sevimay S, Kalayci A. Evaluation of apical sealing ability and adaptation to dentine of two resin-based sealers. J Oral Rehabil. 2005;32:105–10. doi: 10.1111/j.1365-2842.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- 96.Tay FR, Loushine RJ, Monticelli F, Weller RN, Breschi L, Ferrari M, Pashley DH. Effectiveness of resin-coated gutta-percha cones and a dual-cured, hydrophilic methacrylate resin-based sealer in obturating root canals. J Endod. 2005;31:659–64. doi: 10.1097/01.don.0000171942.69081.53. [DOI] [PubMed] [Google Scholar]
- 97.Bergmans L, Moisiadis P, De Munck J, Van Meerbeek B, Lambrechts P. Effect of polymerization shrinkage on the sealing capacity of resin fillers for endodontic use. J Adhes Dent. 2005;7:321–9. [PubMed] [Google Scholar]
- 98.Hiraishi N, Loushine RJ, Vano M, Chieffi N, Weller RN, Ferrari M, Pashley DH, Tay FR. Is an oxygen inhibited layer required for bonding of resin-coated gutta-percha to a methacrylate-based root canal sealer? J Endod. 2006;32:429–33. doi: 10.1016/j.joen.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Doyle MD, Loushine RJ, Agee KA, Gillespie WT, Weller RN, Pashley DH, Tay FR. Improving the performance of EndoRez root canal sealer with a dual-cured two-step self-etch adhesive. I. Adhesive strength to dentin. J Endod. 2006;32:766–70. doi: 10.1016/j.joen.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Gillespie WT, Loushine RJ, Weller RN, Mazzoni A, Doyle MD, Waller JL, Pashley DH, Tay FR. Improving the performance of EndoREZ root canal sealer with a dual-cured two-step self-etch adhesive. II. Apical and coronal seal. J Endod. 2006;32:771–5. doi: 10.1016/j.joen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Koch K, Brave D. A new endodontic obturation technique. Dent Today. 2006;25:102, 104–7. [PubMed] [Google Scholar]
- 102.Koch K, Brave D. Integral gutta percha core/cone obturation technique. United States Patent 7,021,936, 2006. [Google Scholar]
- 103.Monticelli F, Sword J, Martin RL, Schuster GS, Weller RN, Ferrari M, Pashley DH, Tay FR. Sealing properties of two contemporary single-cone obturation systems. Int Endod J. doi: 10.1111/j.1365-2591.2007.01231.x. in press. [DOI] [PubMed] [Google Scholar]
- 104.Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–72. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Hiraishi N, Breschi L, Prati C, Ferrari M, Tagami J, King NM. Technique sensitivity associated with air-drying of HEMA-free, single-bottle, one-step self-etch adhesives. Dent Mater. 2006 doi: 10.1016/j.dental.2006.03.007. in press. [DOI] [PubMed] [Google Scholar]
- 106.Hilton TJ, Schwartz RS. The effect of air thinning on dentin adhesive bond strength. Oper Dent. 1995;20:133–7. [PubMed] [Google Scholar]
- 107.Kim JS, Choi YH, Cho BH, Son HH, Lee IB, Um CM, Kim CK. Effect of light-cure time of adhesive resin on the thickness of the oxygen-inhibited layer and the microtensile bond strength to dentin. J Biomed Mater Res B Appl Biomater. 2006;78:115–23. doi: 10.1002/jbm.b.30463. [DOI] [PubMed] [Google Scholar]
- 108.Battrum DE, Gutmann JL. Implications, prevention and management of subcutaneous emphysema during endodontic treatment. Endod Dentr Traumatol. 1995;11:109–14. doi: 10.1111/j.1600-9657.1995.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 109.Eleazer PD, Eleazer KR. Air pressures developed beyond the apex from drying root canals with pressurized air. J Endod. 1998;24:833–6. doi: 10.1016/S0099-2399(98)80013-7. [DOI] [PubMed] [Google Scholar]
- 110.Fogel HM, Marshall FJ, Pashley DH. Effects of distance from the pulp and thickness on the hydraulic conductance of human radicular dentin. J Dent Res. 1988;67:1381–5. doi: 10.1177/00220345880670110401. [DOI] [PubMed] [Google Scholar]
- 111.King NM, Hiraishi N, Yiu CK, Pashley EL, Loushine RJ, Rueggeberg FA, Pashley DH, Tay FR. Effect of resin hydrophilicity on water-vapour permeability of dental adhesive films. Eur J Oral Sci. 2005;113:436–42. doi: 10.1111/j.1600-0722.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 112.Chersoni S, Acquaviva GL, Prati C, Ferrari M, Grandini S, Pashley DH, Tay FR. In vivo fluid movement through dentin adhesives in endodontically treated teeth. J Dent Res. 2005;84:223–7. doi: 10.1177/154405910508400303. [DOI] [PubMed] [Google Scholar]
- 113.Mecholsky JJ., Jr Fracture mechanics principles. Dent Mater. 1995;11:111–2. doi: 10.1016/0109-5641(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 114.Eick JD, Robinson SJ, Byerley TJ, Chappelow CC. Adhesives and nonshrinking dental resins of the future. Quintessence Int. 1993;24:632–40. [PubMed] [Google Scholar]


