Abstract
Study objectives
Several studies have found associations between diesel exposure, respiratory symptoms, and/or impaired pulmonary function. We hypothesized that prenatal exposure to airborne polycyclic aromatic hydrocarbons (PAH), important components of diesel exhaust and other combustion sources, may be associated with respiratory symptoms in young children. We also hypothesized that exposure to environmental tobacco smoke (ETS) may worsen symptoms beyond that observed to be associated with PAH alone.
Design/participants
To test our hypotheses, we recruited 303 pregnant women from northern Manhattan believed to be at high risk for exposure to both PAH and ETS, collected 48-h personal PAH exposure measurements, and monitored their children prospectively.
Results
By 12 months of age, more cough and wheeze were reported in children exposed to prenatal PAH in concert with ETS postnatally (PAH × ETS interaction odds ratios [ORs], 1.41 [p < 0.01] and 1.29 [p < 0.05], respectively). By 24 months, difficulty breathing and probable asthma were reported more frequently among children exposed to prenatal PAH and ETS postnatally (PAH × ETS ORs, 1.54 and 1.64, respectively [p < 0.05]).
Conclusions
Our results suggest that early exposure to airborne PAH and ETS can lead to increased respiratory symptoms and probable asthma by age 12 to 24 months. Interventions to lower the risk of respiratory disease in young children living in the inner city may need to address the importance of multiple environmental exposures.
Keywords: asthma, environmental tobacco smoke, polycyclic aromatic hydrocarbons
Living in high-traffic areas and/or exposure specifically to diesel exhaust particles (DEP) have been associated with increased respiratory symptoms and impaired lung function in children.1-5 Because of these epidemiologic findings, the concerns over the health risk following exposure to DEP that comprise vehicle exhaust emissions have extended beyond cancer to added concerns of impaired pulmonary health and asthma. In addition, higher DEP exposure has been associated with a greater risk of becoming sensitized to pollen,4 as well as polarization of the local cytokine environment toward the development of T-helper type 2 cytokine responses that are thought to underlie atopy or allergic asthma.6,7
The particulate fraction of DEP is formed mainly of elemental carbon particles to which organic compounds, including polycyclic aromatic hydrocarbons (PAH), are adsorbed. The relatively small size (ie, mass median diameter of 0.05 to 0.3 μm) of DEP facilitates their deep penetration in the respiratory system,8 and multiple mechanisms have been proposed for their effects on airway inflammation. These include increased cytokine production, altered oxidative processes, up-regulated chemokine production (ie, regulated upon activation, normal T-cell expressed and secreted, and monocyte chemotactic protein-1), increased free-radical formation, increased nitric oxide, and direct binding of DEP to allergens.9 PAH that usually comprise DEP specifically have been associated with augmented IgE production.10
We hypothesized that exposure to the PAH, derived from local emissions from diesel and non-diesel motor vehicles, as well as residential heating, power generation, tobacco smoking, and other combustion sources, is associated with adverse respiratory outcomes such as asthma symptoms in young children. We chose to focus on prenatal exposure to PAH because growing evidence suggests that the prenatal period may represent a window when the impact of inhaled environmental agents on respiratory outcomes is heightened. For example, the offspring of mice exposed either to allergens or residual oil fly ash (consisting of heavy metals, PAH) during pregnancy have been found to develop airway hyperreactivity and increased airway eosinophilia.11,12 In light of studies13-15 demonstrating that environmental tobacco smoke (ETS) exposure is associated with abnormal lung function in infancy that persists through adolescence, we also hypothesized that additional exposure to ETS may worsen respiratory outcomes beyond that observed following PAH alone. To test our hypotheses, we recruited a cohort of pregnant women from northern Manhattan, collected 48-h personal PAH exposure measurements in the third trimester of pregnancy, and monitored their children prospectively for the onset of respiratory symptoms. Our results suggest that the interaction of prenatal exposure to PAH and postnatal exposure to ETS leads to increased respiratory symptoms and probable diagnosis of asthma by age 12 to 24 months.
Materials and Methods
Study Cohort
Informed consent was obtained in accordance with the Columbia University Institutional Review Board. Three hundred ninetynine pregnant nonsmoking Dominican and African-American women residing in Washington Heights, Central Harlem, and the South Bronx were screened. The classification of ethnicity was based on self-definition and group identification. Recruitment and monitoring occurred under the auspices of the Columbia Center for Children’s Environmental Health, which is studying a birth cohort prospectively for the onset of respiratory impairment and asthma in the setting of multiple environmental exposures.16 Subjects were recruited from prenatal clinics associated with New York Presbyterian Medical Center or Harlem Hospital, and had obtained initial prenatal care by the twentieth week of pregnancy. Eligibility was limited to nonsmoking women aged 18 to 35 years who were free from diabetes, hypertension, and known HIV. Self-reported illegal drug users were excluded. A total of 96 women were excluded from the current study if they reported active smoking during the current pregnancy (n = 9), had a missing cotinine level (n = 79), or if they or their newborn had a blood cotinine level > 15 ng/mL immediately postpartum (n = 8), leaving a sample size of 303 enrolled into this study. All mothers and their offspring were monitored prospectively at least through 6 months; 281 of the children reached age 12 months, and 235 of them reached age 24 months by the completion of these analyses.
Questionnaires
During the last trimester of pregnancy, information was elicited on demographic information, residential history, history of active and passive smoking, medical history, occupational history, home characteristics including heating and cooking sources and ventilation, and consumption of PAH-containing foods (ie, fried, broiled, and barbecued meat). The questionnaire also addressed the psychosocial environment and typical daily activities, including usual routes and methods of community travel. Following delivery, biomedical information was abstracted from the mothers’ and infants’ medical records, including measures of fetal growth and any medical complications or events that were not recorded during the pregnancy. Additional questionnaires were administered to the mothers by telephone every 3 months after delivery, and in person at 6 months, 12 months, and 24 months. These included questions addressing the child’s medical history, respiratory symptoms, and health-care utilization (including doctor and emergency department visits and hospitalizations). These questionnaires were used to determine postnatal respiratory outcomes.
PAH Exposure
During the third trimester of pregnancy, each woman wore a personal air monitor for 48 h to determine exposure levels to PAH, as described.17 The personal air-sampling pumps operated continuously over this period, collecting vapors and particles up to 2.5 μm in aerodynamic diameter on a quartz microfiber filter and a polyurethane foam cartridge backup. The samples were analyzed at Southwest Research Institute for eight PAH compounds: benz(a)anthracence, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(ghi)perylene, benzo(a)pyrene, chrysene/isochrysene, dibenz(a,h)anthracene, and indeno(1,2,3)pyrene.
During this 48-h period, information also was gathered on day-to-day activities and environmental exposures. To validate whether subjects complied with carrying the personal air-monitoring devices, motion detectors were installed in the devices of 113 randomly selected women. These data were analyzed to determine the total number of motions detected during typical waking (6 am to 10 pm) and nonwaking hours (10 pm to 6 am), and whether they reached a specific threshold. On average, nearly 95% of the total number of motion detections occurred during waking hours, suggesting that subjects complied with wearing the personal air monitors. For quality control, each personal monitoring result was coded as to accuracy in flow rate, time, and completeness of documentation. Data of unacceptable quality (n = 8) were excluded.
ETS Exposure
ETS was determined by questionnaires administered prenatally and at 6 months, 12 months, and 24 months after birth and addressed exposure to ETS from either the mother or any other household member. Plasma cotinine was collected from maternal and cord blood at birth and analyzed by the Centers for Disease Control and Prevention using high-performance liquid chromatography atmospheric-pressure ionization tandem mass spectrometry.18
Statistical Analysis
PAH exposure was determined from an average concentration over 48 h. Because the eight PAH air concentration measures were significantly intercorrelated (r values ranging from 0.45 to 0.94; all p values < 0.001 by Spearman rank),17 total PAH was computed as the sum of the eight PAH air concentrations. Prenatal ETS was defined as the presence of ETS exposure at home or at work during the entire course of the pregnancy, as revealed on questionnaire data. Postnatal ETS was defined as any reported ETS at home from either the mother or another household member at 6 months, 12 months, or 24 months of age.
Respiratory outcomes were examined as individual symptoms (cough, difficulty breathing, wheeze, or probable asthma) at three time points (6 months, 12 months, and 24 months) [Fig 1]. Probable asthma was defined as the mother’s report of physician diagnosis of asthma or probable asthma. The associations between exposure to PAH or recent postnatal ETS and individual respiratory symptoms were analyzed by logistic regression, controlling for exposure to prenatal ETS, and repeated controlling for maternal history of asthma, heating season, and birth weight. The interaction effect of PAH and ETS on individual symptoms also was tested using logistic regression. Because almost all of the subjects exposed to postnatal ETS were also exposed to prenatal ETS, the interaction effect was examined only between prenatal PAH and postnatal ETS. Total respiratory score represented the sum of individual respiratory symptoms, and was scored as 0 for no respiratory symptoms, 1 for one respiratory symptom, 2 for two respiratory symptoms, 3 for three respiratory symptoms, and 4 for the presence of cough, difficulty breathing, wheeze, and probable asthma. Responses needed to be provided for all four questions to be considered valid. Scores of 3 and 4 were collapsed into one group and analyzed using general linear model procedures. A Cronbach α reliability coefficient was computed for the total respiratory score, yielding α = 0.68 at 12 months and α = 0.65 at 24 months. Most data were analyzed with SPSS software (SPSS; Chicago, IL); means ± 1 SD are shown. The exceptions were the exposure response curves (Fig 2, 3) that were analyzed using STATA software (STAT v. 7; StataCorp; College Station, TX); p < 0.05 was considered statistically significant. Missing data occurred when the mother was unavailable or unable to complete a questionnaire after repeated attempts at contact or did not answer a specific question at age 12 to 24 months. Data were missing in 17 cases at 12 months, and in 66 cases at 24 months.
Figure 1.

Prevalence of respiratory symptoms at age 6 to 24 months. Respiratory symptoms were determined by questionnaire administered to the mother every 3 months about symptoms in the last 3 months.
Figure 2.
Interaction between PAH and postnatal ETS exposure on cough at 12 months. Data were analyzed using STATA graph software, (StataCorp).
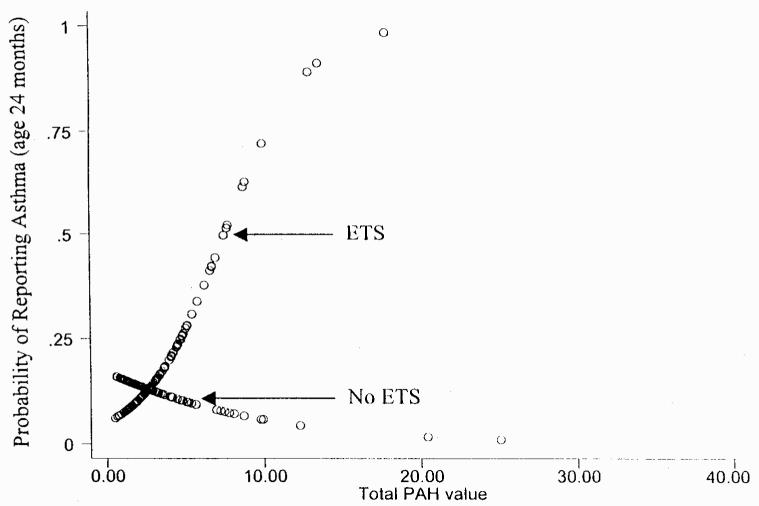
Figure 3.
Interaction between PAH and postnatal ETS exposure on probable asthma at 24 months. Data were analyzed using STATA graph software (StataCorp).
Results
Cohort Characteristics
Characteristics of the study population are shown in Table 1. Our cohort consisted of more Dominicans than African Americans. Seventeen percent of the women had a medical history of asthma, and the majority had at least a twelfth-grade education. The mothers’ age averaged 24.5 ± 5.0 years. In addition, > 51% of the newborns were female. The birth weight of the newborns ranged from 1,875 to 5,110 g (mean weight, 3,387 ± 487 g). There were no significant differences in any of the charted demographics among those participants whose child reached age 24 months, and for those whose child only reached age 6 months or 12 months on the completion of the analysis.
Table 1.
Characteristics of the Cohort
| Characteristics | No./Total (%) |
|---|---|
| Mother’s ethnicity | |
| Dominican | 177/303 (58.4) |
| African American | 126/303 (41.6) |
| Father’s ethnicity | |
| Dominican | 147/302 (48.7) |
| African American | 95/302 (31.5) |
| Other | 60/302 (19.9) |
| Mothers with history of asthma | 49/282 (17.4) |
| Maternal education (with 12th grade education) | 192/302 (63.6) |
| Mothers with any history of cigarette smoking | |
| Prior to pregnancy | 57/270 (21.1) |
| Child age 6 mo | 36/301 (12.0) |
| Child age 12 mo | 32/276 (11.6) |
| Child age 24 mo | 22/189 (11.6) |
| Any time child age 0-24 mo | 47/303 (15.5) |
| Mother currently receiving public assistance | 117/300 (39.0) |
| Mother currently receiving Medicaid | 270/302 (89.4) |
| Child’s gender | |
| Male | 144/295 (48.8) |
| Female | 151/295 (51.2) |
PAH and ETS Exposure
Total PAH exposures averaged 3.65 ± 3.71 ng/m3 (range, 0.27 to 36.47 ng/m3), similar to levels reported earlier from our cohort.17 While levels varied substantially, all subjects had detectable inhalation levels of one or more PAH. PAH levels were significantly greater during the heating season (October 1 to May 31; 4.09 ± 3.98 ng/m3 vs 2.79 ± 2.95 ng/m3, p < 0.01) than during times when landlords turn off apartment heat. In addition, exposure to ETS was relatively common in the cohort, occurring in 33% of the children at age 6 months, 43% at 12 months, and 42% at 24 months. ETS during pregnancy correlated significantly with ETS reported at 12 months (Cramer v = 0.439, p < 0.01) and 24 months (Cramer v = 0.380, p < 0.01). ETS reported during pregnancy during questionnaire administration correlated with cotinine levels in cord blood (r = 0.345, p < 0.0005, Spearman correlation).
The averaged total PAH levels for those participants who reported ETS exposure during pregnancy (mean PAH level, 3.53 ± 2.81 ng/m3) did not differ significantly from those who did not report ETS exposure during pregnancy (mean PAH level, 3.64 ± 4.14 ng/m3). We also did not find a significant correlation between total PAH levels and cotinine levels at birth, regardless of the history of ETS exposure during pregnancy (data not shown). These observations suggest that PAH contained in sidestream cigarette smoke do not contribute greatly to the prenatal total PAH levels we recorded.
PAH, ETS Exposure, and Respiratory Symptoms at Age 6 Months, 12 Months, and 24 Months
Overall, respiratory symptoms (cough, difficulty breathing, wheeze, and probable asthma) were relatively frequent in our cohort (Fig 1). Children born to mothers exposed to higher levels of PAH during pregnancy did not experience significantly more respiratory symptoms (Tables 2, 3). Recent postnatal ETS exposure (ie, during 6 months preceding questionnaire administration) was independently associated with cough (odds ratio [OR], 2.06; 95% confidence interval [CI], 1.16 to 3.65; p < 0.05) and probable asthma (OR, 3.3; 95% CI, 1.00 to 10.8; p = 0.05) at age 6 months and probable asthma at 12 months (p < 0.05; Table 2) when adjusted for prenatal ETS and PAH exposure.
Table 2.
Prenatal PAH Exposure, Postnatal ETS, and Postnatal Respiratory Symptoms at 12 Months (n = 264)*
| Analysis for Main Effects |
Analysis for Interactions |
|||
|---|---|---|---|---|
| Symptom/Exposure | OR | 95% CI | OR | 95% CI |
| Cough | ||||
| Prenatal ETS | 1.36 | 0.78-2.38 | 1.36 | 0.77-2.41 |
| PAH | 1.01 | 0.94-1.08 | 0.95 | 0.87-1.04 |
| ETS | 1.12 | 0.65-1.96 | 0.35 | 0.13-0.94† |
| PAH × ETS | 1.41 | 1.10-1.82‡ | ||
| Difficulty breathing | ||||
| Prenatal ETS | 0.75 | 0.37-1.53 | 0.75 | 0.37-1.53 |
| PAH | 0.99 | 0.90-1.08 | 0.96 | 0.84-1.09 |
| ETS | 0.99 | 0.49-1.99 | 0.65 | 0.23-1.86 |
| PAH × ETS | 1.12 | 0.91-1.39 | ||
| Wheeze | ||||
| Prenatal ETS | 0.83 | 0.42-1.66 | 0.84 | 0.42-1.69 |
| PAH | 1.05 | 0.97-1.13 | 1.00 | 0.90-1.10 |
| ETS | 1.46 | 0.74-2.86 | 0.57 | 0.19-1.66 |
| PAH × ETS | 1.29 | 1.03-1.61† | ||
| Probable asthma | ||||
| Prenatal ETS | 0.53 | 0.21-1.29 | 0.53 | 0.21-1.29 |
| PAH | 0.92 | 0.77-1.10 | 0.93 | 0.74-1.18 |
| ETS | 2.89 | 1.19-7.00† | 3.14 | 0.78-12.55 |
| PAH × ETS | 0.97 | 0.69-1.38 | ||
PAH refers to measurements collected from personal air monitor during the third trimester of pregnancy; ETS refers to ETS at 6 months or 12 months.
p < 0.05.
p < 0.01.
Table 3.
Prenatal PAH Exposure, Postnatal ETS, and Postnatal Respiratory Symptoms at 24 Months (n = 169)*
| Analysis for Main Effects |
Analysis for Interactions |
|||
|---|---|---|---|---|
| Symptom/Exposure | OR | 95% CI | OR | 95% CI |
| Cough | ||||
| Prenatal ETS | 1.36 | 0.68-2.72 | 1.36 | 0.68-2.72 |
| PAH | 1.06 | 0.96-1.18 | 1.03 | 0.91-1.16 |
| ETS | 0.59 | 0.30-1.18 | 0.40 | 0.14-1.15 |
| PAH × ETS | 1.12 | 0.89-1.40 | ||
| Difficulty breathing | ||||
| Prenatal ETS | 0.58 | 0.21-1.55 | 0.59 | 0.22-1.62 |
| PAH | 1.02 | 0.91-1.15 | 0.88 | 0.68-1.12 |
| ETS | 0.54 | 0.20-1.44 | 0.10 | 0.02-0.58† |
| PAH × ETS | 1.54 | 1.06-2.22† | ||
| Wheeze | ||||
| Prenatal ETS | 0.62 | 0.23-1.65 | 0.63 | 0.23-1.70 |
| PAH | 1.03 | 0.91-1.16 | 0.84 | 0.59-1.20 |
| ETS | 1.66 | 0.63-4.37 | 0.46 | 0.09-2.36 |
| PAH × ETS | 1.44 | 0.95-2.18 | ||
| Probable asthma | ||||
| Prenatal ETS | 0.52 | 0.20-1.34 | 0.48 | 0.17-1.30 |
| PAH | 1.08 | 0.97-1.20 | 0.89 | 0.67-1.20 |
| ETS | 2.36 | 0.91-6.10 | 0.38 | 0.07-1.99 |
| PAH × ETS | 1.64 | 1.10-2.45† | ||
See Table 2 for definition of terms.
p < 0.05.
However, at 12 months of age, more cough and wheeze were reported among children jointly exposed to prenatal PAH and postnatal ETS (Table 2, Fig 2). Likewise, at 24 months of age, more difficulty breathing and probable asthma was reported among children jointly exposed to prenatal PAH and postnatal ETS (Table 3, Fig 3). Neither history of asthma in the mother, birth weight, nor PAH measurement during the heating season altered the results significantly. History of asthma in the father was not incorporated into the logistic analyses because the data were considered unreliable. Despite the correlation between ETS during pregnancy and ETS reported at 12 months and 24 months, no differences were found when the logistic regression analyses summarized in Tables 2, 3 were repeated without controlling for prenatal ETS exposure. This analysis suggests that when postnatal ETS is in the model, prenatal ETS does not contribute significantly to the interaction. Thus, the early postnatal period appears to be the important time window for respiratory outcomes related to ETS exposure. Furthermore, when the individual symptoms were combined into a single score, interactions between prenatal PAH and postnatal ETS are apparent at both 12 months and 24 months (Table 4). Interactions between PAH and ETS exposure were not found for respiratory symptoms at age 6 months.
Table 4.
Prenatal PAH, Postnatal ETS, and Respiratory Score Age 12 Months and 24 Months*
| Analysis for Main Effects |
Analysis for Interactions |
|||
|---|---|---|---|---|
| Time | B | p Value | B | p Value |
| 12 mo (n = 263) | ||||
| Intercept | 1.04 | 0.73 | ||
| Prenatal ETS | - 0.02 | 0.90 | - 0.01 | 0.92 |
| PAH | 0.01 | 0.72 | 0.09 | 0.018† |
| ETS | 0.15 | 0.29 | - 0.23 | 0.27 |
| PAH × ETS | 0.11 | 0.014† | ||
| 24 mo (n = 169) | ||||
| Intercept | 0.68 | 0.30 | ||
| Prenatal ETS | - 0.07 | 0.67 | - 0.08 | 0.65 |
| PAH | 0.03 | 0.28 | 0.13 | 0.002† |
| ETS | - 0.09 | 0.60 | - 0.63 | 0.011† |
| PAH × ETS | 0.15 | 0.003† | ||
See Table 2 for definition of terms.
Indicates significance.
By age 12 months, a trend toward increased cough (PAH × ETS OR, 1.62; 95% CI, 1.07 to 2.47) and wheeze (PAH × ETS OR, 1.49; 95% CI, 1.00 to 2.22) was reported more often among boys, but not girls, exposed to prenatal PAH in concert with postnatal. By 24 months, an increase in the respiratory score among boys, but not girls, exposed to prenatal PAH in concert with postnatal ETS was found (PAH × ETS B, 0.201; p < 0.005).
Discussion
Growing evidence suggests that exposure to DEP may contribute to respiratory problems in children.1,3,5 Yet, gaps in our understanding include questions regarding the role of various components that comprise DEP, such as PAH, the important time window of exposure, and the importance of the interaction of DEP exposure with other potentially hazardous environmental exposures. Our results suggest that PAH exposure is associated with adverse respiratory outcomes by age 12 to 24 months when occurring in concert with postnatal ETS exposure. Strengths of this study include the prospective design beginning prenatally, close follow-up of participants in an effort to reduce recall bias, careful exclusion of the confounder of prenatal ETS exposure,19 and assessment of the effects of multiple exposures found in an inner-city environment.
It has been hypothesized that intrauterine environmental exposures may increase the risk for subsequent diseases, such as ischemic heart disease (ie, Barker hypothesis20). In the case of PAH, we and others17,21 have linked fetal exposure to the nonrespiratory outcomes of reduced head circumference and birth weight. The results reported here are the first evidence implicating prenatal PAH exposure, in the setting of other environmental exposures, to asthma symptoms. We recognize that because personal air monitoring for PAH was not repeated postnatally, it is uncertain whether our findings represent delayed effects of prenatal PAH exposure on children, or more immediate effects of postnatal PAH exposure during the first 2 years of life that are similar to those recorded prenatally. Nonetheless, the risk for increased respiratory problems following exposure to PAH appears to begin very early.
Potentially hazardous environmental exposures usually do not occur in isolation, particularly in high-risk communities that are disproportionately exposed to diesel traffic, ETS, poor housing, and increased social stress.5,16,22,23 Hence, it is of particular interest that two environmental exposures may act in synergy to worsen respiratory outcomes at age 12 to 24 months. PAH readily reach the fetus and frequently damage DNA, forming PAH-DNA adducts,17 and these or other mediators could increase risk for later disease by modulating developmental programming through altered gene expression or chromatin remodeling. Our results suggest that prenatal PAH exposure interacted with ETS exposure that occurred postnatally instead of prenatally. One possibility is that the apparent inhibitory effects of PAH on birth weight in turn affected airway caliber in the young child, making the airways more susceptible to future insults. Other mechanisms by which PAH could increase airway inflammation include their ability to up-regulate allergic or proinflammatory cytokines such as granulocyte macrophagecolony stimulating factor, interleukin (IL)-4, IL-2, IL-8,24-27 augment antigen-specific IgE isotype switching,10,28,29 or induce oxidative stress.30 PAH or DEP could also act as adjuvants or allergen carriers.31 DEP exposure has been shown to reduce host defense to viruses.32 In addition, ETS has been shown to increase airway inflammation in multiple studies.32-35
Based on the high correlation between our questionnaire data and cotinine levels at birth, we believe the ETS histories are relatively reliable. Others36 as well have validated questionnaire data when estimating the presence of ETS exposure. Certainly some discrepancies can be attributed to the short half-life of cotinine that could underestimate true ETS exposure over time.37 We also doubt that significant levels of side-stream emissions from nearby cigarettes contributed to our total PAH levels. This conclusion is based in part on our finding that personal total PAH levels were not higher among pregnant women reporting ETS. Nonetheless, we recognize that a noteworthy contribution to our analyses of PAH derived from ETS (eg, chrysene) instead of from diesel emission cannot be completely ruled out.
Our data also may suggest that by age 24 months, boys become more susceptible to the respiratory effects of combined PAH and ETS exposure. While gender differences in the early response to PAH exposure have not been reported previously, boys have been found to be more susceptible to prenatal ETS-induced wheeze by age 18 to 30 months.13 The largest percentage deficits in response to in utero exposure to ETS occurred in measures related to small airway flow, such as maximal midexpiratory flow and forced expiratory flow at 75% of vital capacity.14 Hence, we propose that the relatively smaller airways reported in young boys38 may increase the risk for inflammation-induced respiratory symptoms or airway hyperreactivity. It is important to recognize, however, that given the large number of statistical tests we performed, we should be cautious not to overinterpret the pattern of gender specific results.
In conclusion, prenatal exposure to PAH and early exposure to ETS in an urban cohort was associated with increased respiratory symptoms and probable asthma by age 12 to 24 months. Our data suggest that interventions to lower the risk of respiratory disease in young children living in the inner city may need to address the importance of multiple environmental exposures.
ACKNOWLEDGMENT
The authors thank Yair Hazi, PhD, for his assistance in the PAH measurements, and the subjects of this study for taking the time and effort to participate in the research.
Grant support was provided by the National Institute of Environmental Health Sciences (grants P501 ES09600 and 5 RO1 ES08977, RO1ES111158, RO1 ES012468), National Institute of Environmental Health Sciences Center for Environmental Health in Northern Manhattan (P30 ES09089), Environmental Protection Agency (grants R827027, 8260901), Irving General Clinical Research Center (grant RR00645), Bauman Family Foundation, Gladys & Roland Harriman Foundation, W. Alton Jones Foundation, New York Community Trust, Educational Foundation of America, Rockefeller Financial Services, Horace W. Smith Foundation, Beldon Fund, John Merck Fund, and V. Kann Rasmussen Foundation.
Abbreviations
- CI
confidence interval
- DEP
diesel exhaust particles
- ETS
environmental tobacco smoke
- IL
interleukin
- OR
odds ratio
- PAH
polycyclic aromatic hydrocarbons
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
References
- 1.Dockery DW, Speizer FE, Stram DO, et al. Effects of inhalable particles on respiratory health of children. Am Rev Respir Dis. 1989;139:587–594. doi: 10.1164/ajrccm/139.3.587. [DOI] [PubMed] [Google Scholar]
- 2.Rudell B, Ledin MC, Hammarstron U, et al. Effects on symptoms and lung functions in humans experimentally exposed to diesel exhaust. Occup Environ Med. 1996;53:658–662. doi: 10.1136/oem.53.10.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehring U, Cyrys J, Sedlimeir G, et al. Traffic-related air pollution and respiratory health during the first 2 years of life. Eur Respir J. 2002;19:690–698. doi: 10.1183/09031936.02.01182001. [DOI] [PubMed] [Google Scholar]
- 4.Wyler C, Braun-Fahrlander C, Kunzli N, et al. Exposure to motor vehicle traffic and allergic sensitization. Epidemiology. 2000;11:450–456. doi: 10.1097/00001648-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Northridge ME, Yankura J, Kinney PL, et al. Diesel exhaust exposure among adolescents in Harlem: a community-driven study. Am J Public Health. 1999;89:998–1002. doi: 10.2105/ajph.89.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Sanchez D, Tsien A, Fleming J, et al. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–2413. [PubMed] [Google Scholar]
- 7.Romagnani The Th1/Th2 paradigm and allergic disorders. Allergy. 1998;46(suppl):12–15. doi: 10.1111/j.1398-9995.1998.tb04951.x. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Sanchez D. The role of diesel exhaust particles and their associated polycyclic aromatic hydrocarbons in the induction of allergic airway disease. Allergy. 1997;52:52–56. doi: 10.1111/j.1398-9995.1997.tb04871.x. [DOI] [PubMed] [Google Scholar]
- 9.Pandya RJ, Solomon G, Kinner A, et al. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ Health Perspect. 2002;101:103–112. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanoh T, Suzuki T, Ishimori M, et al. Adjuvant activities of pyrene, anthracene, fluoranthene and benzo(a)pyrene in production of anti-IgE antibody to Japanese cedar pollen allergen in mice. J Clin Lab Immunol. 1996;48:133–147. [PubMed] [Google Scholar]
- 11.Hamada K, Suzaki Y, Goldman A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–1689. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 12.Hamada K, Suzaki Y, Itoh T, et al. Exposure of pregnancy mice to air pollutant aerosol increases asthma susceptibility in offspring [abstract] Am J Respir Crit Care Med. 2003;167:A942. [Google Scholar]
- 13.Lux AL, Henderson AJ, Pocock SJ. Wheeze associated with prenatal tobacco smoke exposure: a prospective, longitudinal study. Arch Dis Child. 2000;83:307–312. doi: 10.1136/adc.83.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliland FD, Berhne K, McConnell R, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YF, Gilliland FD, Berhane K, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 16.Perera FP, Illman SM, Kinney PL, et al. The challenge of preventing environmentally-related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera F, Rauh V, Tsai W-Y, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–206. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernert JT, McGuffey JE, Morrison MA, et al. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24:333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 19.Georgiadis P, Topinka J, Stoikidou M, et al. Biomarkers of genotoxicity of air pollution (the AULIS project): bulky DNA adducts in subjects with moderate to low exposures to airborne polycyclic aromatic hydrocarbons and their relationship to environmental tobacco smoke and other parameters. Carcinogenesis. 2001;22:1447–1457. doi: 10.1093/carcin/22.9.1447. [DOI] [PubMed] [Google Scholar]
- 20.Barker DJ, Winter PD, Osmond C, et al. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 21.Perera FP, Whyatt RM, Jedrychowski W, et al. Recent developments in molecular epidemiology: a study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 22.United Church of Christ Commission for Racial Justice . Toxic waste and race in the United States: a national study of the racial and socioeconomic characteristics of communities with hazardous waste sites. United Church of Christ; New York, NY: 1987. [Google Scholar]
- 23.Rauh V, Chew G, Garfinkel R. Deteriorated housing contributes to high cockroach allergen levels in inner city house-holds. Environ Health Perspect. 2002;10:323–327. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takano H, Yoshikawa T, Ichinose T, et al. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression. Am J Respir Crit Care Med. 1997;156:36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 25.Ohta K, Yamashita N, Tajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104:1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 26.Bommel H, Li-Weber M, Serfling E, et al. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol. 2000;105:796–802. doi: 10.1067/mai.2000.105124. [DOI] [PubMed] [Google Scholar]
- 27.Salvi SS, Nordenhall C, Blomberg A, et al. Acute exposure to diesel exhaust increases Il-8 and GRO-a production in healthy human airways. Am J Respir Crit Care Med. 2000;161:550–557. doi: 10.1164/ajrccm.161.2.9905052. [DOI] [PubMed] [Google Scholar]
- 28.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces in vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998;19:507–512. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 29.Heo Y, Saxon A, Hankinson O. Effect of diesel exhaust particles and their components on the allergen-specific IgE and IgG1 response in mice. Toxicology. 2001;159:143–158. doi: 10.1016/s0300-483x(00)00418-2. [DOI] [PubMed] [Google Scholar]
- 30.Whitekus MJ, Li N, Zhang M, et al. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin. The J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 31.Ormstad H. Suspended particulate matter in indoor air: adjuvants and allergen carriers. Toxicology. 2000;152:53–68. doi: 10.1016/s0300-483x(00)00292-4. [DOI] [PubMed] [Google Scholar]
- 32.Harrod KS, Jaramillo RJ, Rosenberger CL, et al. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol. 2003;28:451–463. doi: 10.1165/rcmb.2002-0100OC. [DOI] [PubMed] [Google Scholar]
- 33.Yu M, Pinkerton EU, Witschi H. Short-term exposure to aged and diluted sidestream cigarette smoke enhances ozone-induced lung injury in B6C3F1 mice. Toxicol Sci. 2002;65:99–106. doi: 10.1093/toxsci/65.1.99. [DOI] [PubMed] [Google Scholar]
- 34.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menon P, Rando RJ, Stankus RP, et al. Passive cigarette smoke-challenge studies: increase in bronchial hyperreactivity. J Allergy Clin Immunol. 1992;89:560–566. doi: 10.1016/0091-6749(92)90323-t. [DOI] [PubMed] [Google Scholar]
- 36.Coultas DB, Peake GT, Samet JM. Questionnaire assessment of lifetime and recent exposure to environmental tobacco smoke. Am J Epidemiol. 1989;130:338–347. doi: 10.1093/oxfordjournals.aje.a115340. [DOI] [PubMed] [Google Scholar]
- 37.Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 38.Lum S, Hoo AF, Dezateux C, et al. The association between birth weight, sex, and airway function in infants of nonsmoking mothers. Am J Respir Crit Care Med. 2001;164:2078–2084. doi: 10.1164/ajrccm.164.11.2104053. [DOI] [PubMed] [Google Scholar]