Abstract
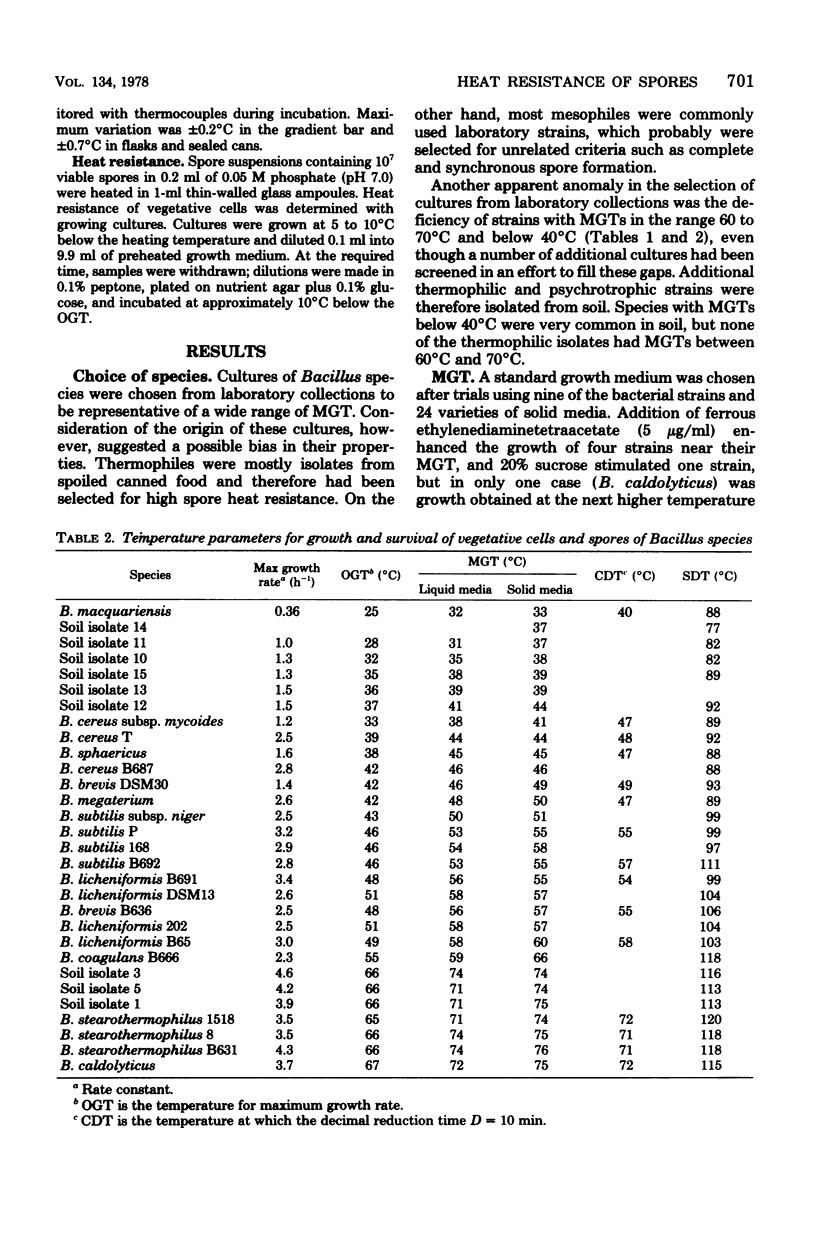
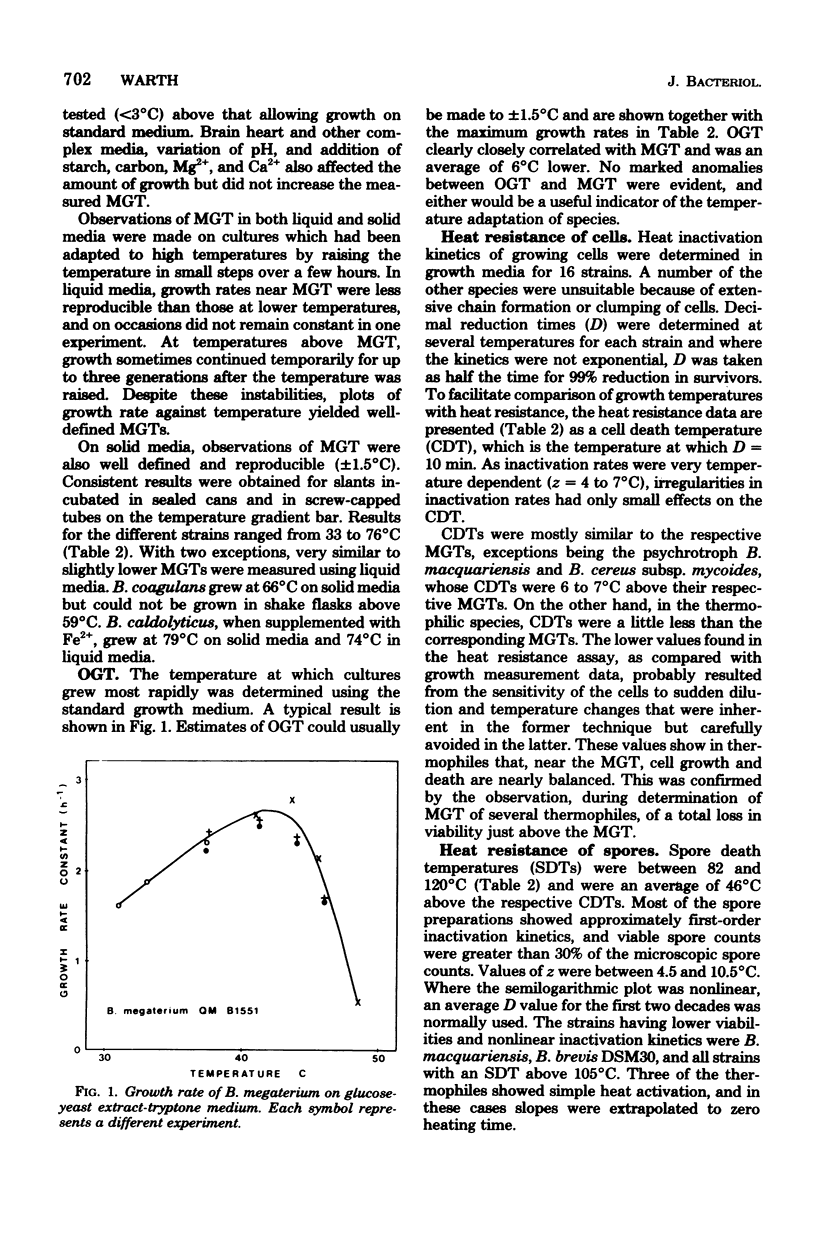
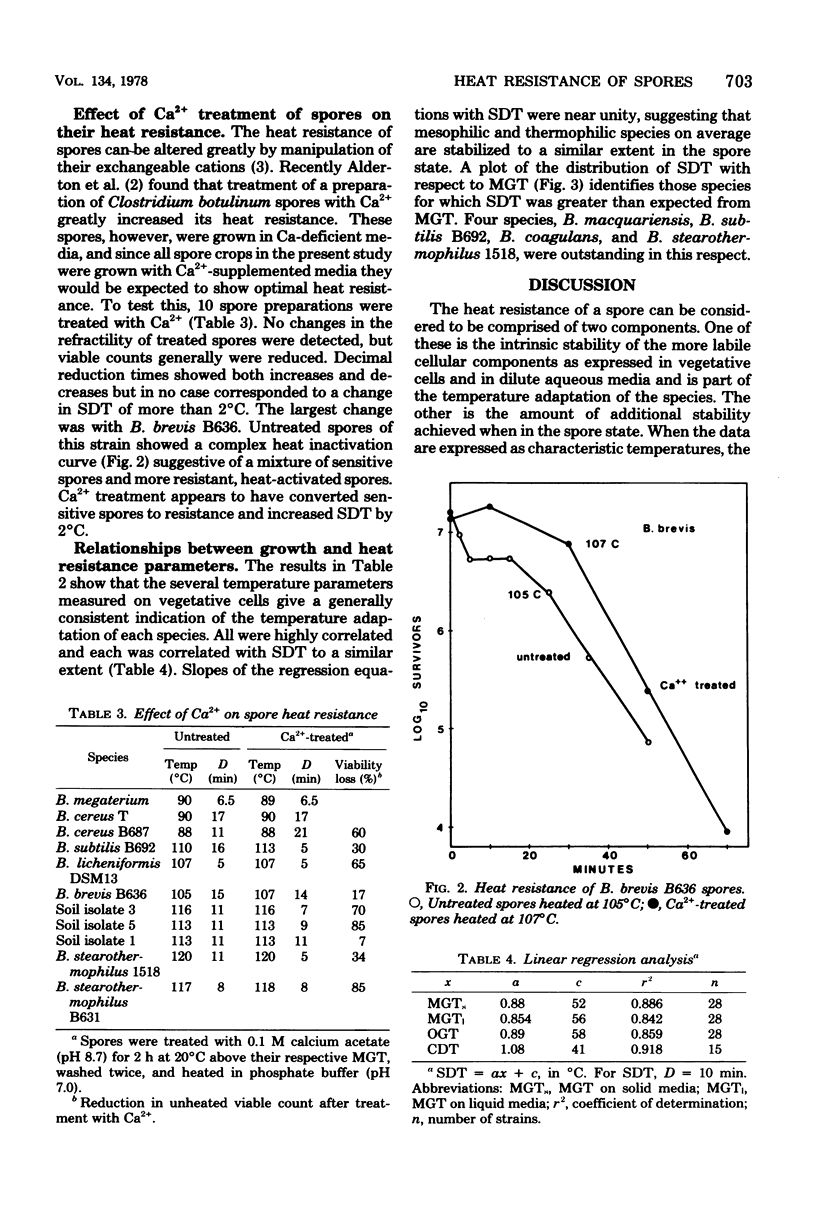
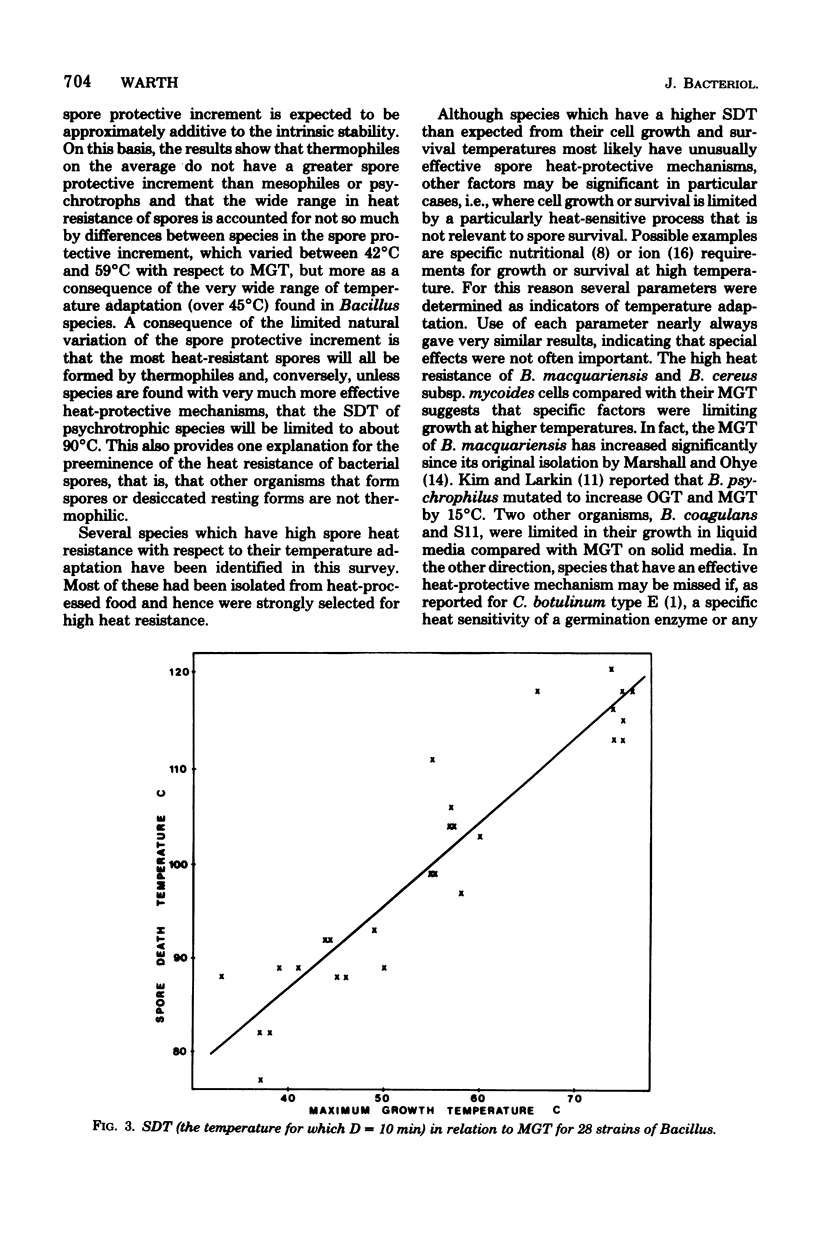
Heat resistance of spores of Bacillus strains was compared with the temperature adaptation of each strain as measured by the optimum and maximum growth temperatures and the heat resistance of vegetative cells. Maximum growth temperatures ranged from 31 to 76 degrees C and were little affected by the nature of the growth medium. The temperature giving maximum growth rate was closely correlated to the maximum temperature for growth, and about 6 degrees C lower. Vetetative-cell heat resistance, determined on exponential-phase cells, was also correlated with maximum growth temperature. The temperature at which spores were inactivated with a decimal reduction time of 10 min was in the range of 75 to 121 degrees C. This temperature was 46 +/- 7 degrees C higher than the maximum growth temperature and correlated with it and the other cell parameters. Spore heat resistance can be considered to have two components, the temperature adaptation characteristic of the species and the stabilization conferred by the spore state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton G., Chen J. K., Ito K. A. Effect of lysozyne on the recovery of heated Clostridium botulinum spores. Appl Microbiol. 1974 Mar;27(3):613–615. doi: 10.1128/am.27.3.613-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey D. H. Thermophilic Bacteria. J Bacteriol. 1919 Jul;4(4):301–306. doi: 10.1128/jb.4.4.301-306.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J. G., Peeler J. T., Twedt R. M. Heat resistance of ileal loop reactive Bacillus cereus strains isolated from commercially canned food. Appl Microbiol. 1975 Dec;30(6):943–945. doi: 10.1128/am.30.6.943-945.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross T. Thermophilic actinomycetes. J Appl Bacteriol. 1968 Mar;31(1):36–53. doi: 10.1111/j.1365-2672.1968.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Garibaldi J. A. Influence of temperature on the iron metabolism of a fluorescent pseudomonad. J Bacteriol. 1971 Mar;105(3):1036–1038. doi: 10.1128/jb.105.3.1036-1038.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Larkin J. M. Production of mesophilic mutants from a psychrophilic Bacillus. Can J Microbiol. 1973 Nov;19(11):1452–1454. doi: 10.1139/m73-235. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Ohye D. F. Bacillus macquariensis n.sp., a psychrotrophic bacterium from sub-antarctic soil. J Gen Microbiol. 1966 Jul;44(1):41–46. doi: 10.1099/00221287-44-1-41. [DOI] [PubMed] [Google Scholar]
- Michels M. J., Visser F. M. Occurrence and thermoresistance of spores of psychrophilic and psychrotrophic aerobic sporeformers in soil and foods. J Appl Bacteriol. 1976 Aug;41(1):1–11. doi: 10.1111/j.1365-2672.1976.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Mosley G. A., Card G. L., Koostra W. L. Effect of calcium and anaerobiosis on the thermostability of Bacillus stearothermophilus. Can J Microbiol. 1976 Apr;22(4):468–474. doi: 10.1139/m76-073. [DOI] [PubMed] [Google Scholar]
- Roberts T. A., Derrick C. M. Sporulation of Clostridium putrefaciens and the resistance of the spores to heat, gamma-radiation and curing salts. J Appl Bacteriol. 1975 Feb;38(1):33–37. doi: 10.1111/j.1365-2672.1975.tb00497.x. [DOI] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


