Abstract
Reciprocal differentiation of immunosuppressive CD4+CD25+FoxP3+ T regulatory cells (Tregs) and proinflammatory IL-17-producing cells (Th17) from naïve CD4 cells is contingent upon the cytokine environment. Using MACS-purified CD4 cells, we found that rapamycin and cyclosporine A (CsA) potently inhibited the TGFβ and IL-6-induced generation of IL-17-producing cells. Intriguingly, rapamycin promoted, while CsA markedly inhibited, TGFβ-mediated generation of Tregs. The aforementioned effects of rapamycin and CsA were also observed for Flow-sorted CD4+CD25− T cells, indicating that the effect of these two immunosuppressive agents was based on their action on de novo generation of Tregs and Th17 cells from naïve CD4 cells. Our observation suggests a distinct mode of immunosuppressive action and tolerance induction by rapamycin and CsA. The capacity of rapamycin to generate immunosuppressive Tregs and to suppress differentiation of pathogenic Th17 cells furthers our understanding of the basis for the therapeutic immunosuppressive effects of rapamycin in patients with autoimmune diseases and allo-transplantation reactions.
Keywords: rapamycin, CD4+CD25+FoxP3+ T regulatory cells, Th17 cells
Introduction
Recent compelling evidence demonstrated that IL-17-producing T lymphocytes comprise proinflammatory T helper cells, termed Th17 cells, that are major contributors to autoimmune disease (1). In contrast, CD4+CD25+FoxP3+ T regulatory cells (Tregs) actively restrain the inflammatory response, suppress development of autoimmune diseases and dampen a wide spectrum of immune responses (2, 3). Furthermore, Th17 contributes to the transplant rejection while Tregs exhibit protective effects (4). Intriguingly, pathogenic Th17 and immunosuppressive Tregs from naïve CD4 cells are reciprocally induced, contingent upon the presence of either IL-6 or IL-2, respectively, in the presence of transforming growth factor-beta (TGFβ) (5–7). Thus, the impact on the reciprocal differentiation of Tregs and Th17 cells of pharmacological agents may represent an underlying basis of their immunoregulatory actions. Indeed, we reported that pertussis toxin (PTx), an immunological adjuvant commonly used to induce experimental autoimmune diseases, is able to promote the generation of Th17 cells while suppressing differentiation of Tregs (8, 9). On the other hand, it was reported that retinoic acid, an immunosuppressive vitamin A metabolite, has the capacity to promote the generation of Tregs while suppressing differentiation of Th17 cells (10).
Rapamycin is an immunosuppressive drug used to counter autoimmunity and to prevent acute graft rejection in human (11). It is increasingly evident that promotion of Treg activity contributes to the tolerance induced by this compound. For example, Battaglia and colleagues reported that rapamycin selectively expands naturally occurring murine Tregs in vitro (12). Furthermore, Battaglia and Strauss reported rapamycin also selectively promotes expansion of functional human Tregs, while depleting human CD4+CD25− T effector cells (13, 14). Loenen et al found that rapamycin, but not CsA, permits thymic generation and peripheral preservation of mouse Tregs (15). However, the effect of rapamycin on the reciprocal differentiation of Tregs and Th17 cells remains unknown. In this study, we compared the effects of rapamycin with CsA on in vitro TGFβ-mediated differentiation of Tregs and TGFβ/IL-6-induced generation of Th17 cells.
Materials and Methods
Mice and reagents
Female wild type C57BL/6 mice, 8 to 12 wk old, were provided by Animal Production Area of the NCI (Frederick, MD). NCI-Frederick is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.). Antibodies purchased from BD Pharmingen (San Diego, CA) consisted of FITC-anti-CD4 (GK1.5), PE-anti-CD25 (PC61), PE-anti-IL-17 (TC11-18H10), purified anti-CD3 (145-2C11) and purified anti-CD28 (35.71). PE anti-mouse/rat Foxp3 Staining Set (FJK-16s) was purchased from eBioscience (San Diego, CA). Recombinant mouse IL-6 was purchased from PeproTech (Rocky Hill, NJ). RhTGFβ1 was from R&D Systems (Minneapolis, MN). Rapamycin and cyclosporine A were purchased from Sigma (St. Louis, MO).
Purification of cells
CD4+ cells were purified using Mouse CD4 (L3T4) microbeads and LS column (Miltenyi Biotec Inc., Auburn, CA). CD4+CD25− cells were purified from lymph node (inguinal, axillary and mesenteric regions) and splenic cells using FACSAria cell sorter (BD Biosciences, Mountain View, CA), yielding a purity of ~98% of CD4+CD25− cells.
In vitro cell activation
Purified CD4 cells or CD4+CD25− T cells (2.5×l05 cells/well) were cultured in a 24-well plate, stimulated with plate-bound anti CDS Ab (5 μg/ml) and soluble anti CD28 Ab (2 μg/ml) for 3 days in the presence of TGFβ (2 ng/ml), with or without IL-6 (10 ng/ml). Rapamycin and CsA were added to the wells at the desired concentrations.
Flow Cytometry
After blocking Fc receptors, cells were incubated with appropriately diluted antibodies. For intracellular IL-17 staining, the cells were stimulated with PMA (20 ng/ml, Sigma, St.Louis, MO) and ionomycin (1 μM, Sigma) for 5 h in the presence of GolgiPlug (BD Pharmingen). The cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) and stained with relevant antibodies. Samples were acquired on a FACSort (BD Biosciences, Mountain View, CA) and data analysis was conducted using CellQuest software (BD Biosciences). Expression of IL-17 or FoxP3 was analyzed by gating on homogenous level of CD4+ cells.
Statistical analysis
Comparisons of data were analyzed by two-tailed Student’s t test using Graphpad Prism 4.0.
Results
Unfractionated CD4 cells mimic naïve CD4 cells in the reciprocal generation of Tregs and Th17 cellls
Upon activation, naïve CD4 cells are converted to Foxp3-expressing cells provided TGFβ is present. IL-6 inhibits TGFβ-mediated generation of FoxP3+ cells and instead induces differentiation of IL-17-producing cells (5–7). This in vitro reciprocal differentiation of Treg and Th17 provides a novel means of investigating the impact of pharmacological agents on the polarization of the immune response in immunosuppressive or proinflammatory direction. However, purification of naïve CD4 cells requires special reagents or a flow sorter and it is difficult to obtain large number of naïve CD4 cells. Thus we explored the use of unfractionated CD4 cells instead of purified naïve CD4 cells for the initial study or large-scale screening of the effect of cytokine/pharmacological agents, on the reciprocal differentiation of Tregs and Th17 cells. Consequently, MACS-purified CD4 cells were stimulated with plate-bound anti CD3 Ab, soluble anti CD28 Ab and TGFβ, in the presence or absence of IL-6. FoxP3 expression was analyzed 72 hours later. The remaining cells were re-stimulated with PMA/ionomycin and analyzed for cytoplasmic expression of IL-17. Although TGFβ alone failed to induce the expression of IL-17 (0.4%), TGFβ plus IL-6 resulted in a substantial IL-17 expression by CD4 cells (5.8%, Fig 1A). Freshly isolated CD4 cells only contained ~10% of FoxP3+ cells (data not shown). After 72 hour treatment with TGFβ, the proportion of FoxP3+ cells increased to 51.6% which was decreased to 31.6% by addition of IL-6 (Fig 2A). Therefore, unfractionated CD4 cells exhibited the same response as naïve CD4 cells to TGFβ or TGFβ plus IL-6 in the reciprocal generation of FoxP3+ cells or Th17 cells.
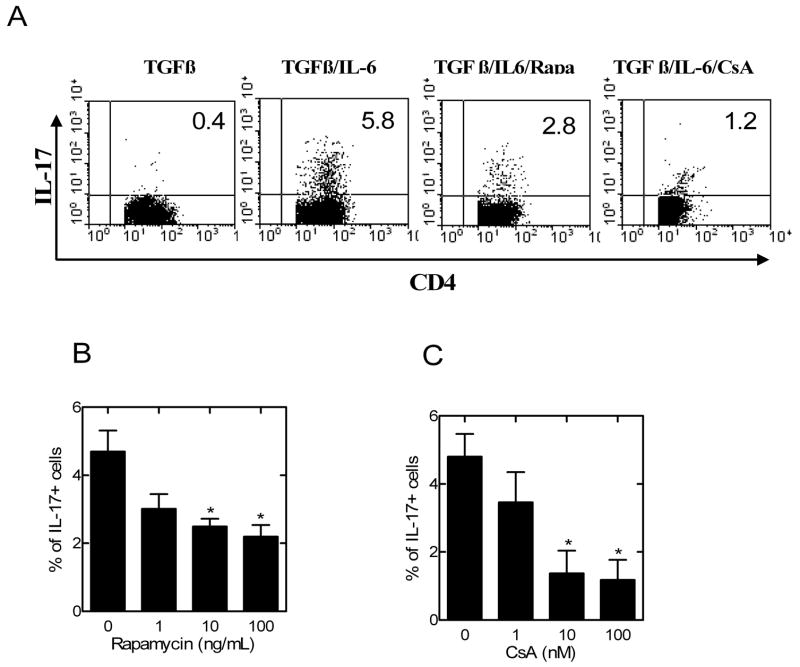
Fig 1.
The effects of rapamycin and CsA on TGFβ/IL-6-mediated generation of IL-17-producing cells from CD4 cells. CD4 cells were isolated from normal C57BL/6 mouse LNs and spleen by MACS. (A) The cells were stimulated with plate-bound anti CD3 Ab and soluble anti CD28 Ab, in the presence of TGFβ, or TGFβ plus IL-6, or TGFβ plus IL-6 plus rapamycin (Rapa, 10 ng/ml), or TGFβ plus IL-6 plus CsA (10 nM). 72 hours later, intracellular expression of IL-17 was analyzed by gating on CD4 cells. The data shown are representative of at least 3 separate experiments with similar results. (B–C) CD4 cells were stimulated as in (A) with TGFβ plus IL-6 and increasing concentration of rapamycin (1~100 ng/ml, B) or CsA (1~100 nM, C). 72 h later the intracellular expression of IL-17 was analyzed by gating on CD4 cells. The data represent the mean percentage of positive cells ± SEM, which are summarized from 3 to 4 separate experiments. * p<0.05, as compared with medium control.
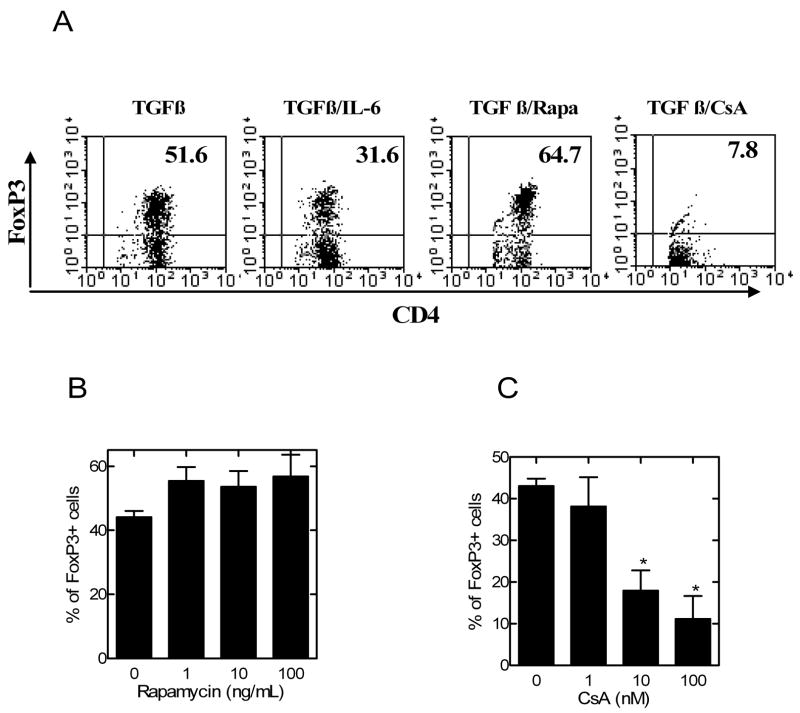
Fig 2.
The effects of rapamycin and CsA on TGFβ-mediated generation of FoxP3+ cells from CD4 cells. CD4 cells were isolated from normal C57BL/6 mouse LNs and spleen by MACS. (A) The cells were stimulated with plate-bound anti CD3 Ab and soluble anti CD28 Ab, in the presence of TGFβ, or TGFβ plus IL-6, or TGFβ plus rapamycin (Rapa, 10 ng/ml), or TGFβ plus CsA (10 nM). The data shown are representative of at least 3 separate experiments with similar results. (B–C) CD4 cells were stimulated as in (A) with TGFβ and increasing concentration of rapamycin (1~100 ng/ml, B) or CsA (1~100 nM, C). 72 hours later, intracellular expression of FoxP3 was analyzed by gating on CD4 cells. The data shows the mean percentage of positive cells ± SEM, which are summarized from 3 to 4 separate experiments. * p<0.05, as compared with medium control.
Both rapamycin and CsA are potent inhibitors of Th17 cell generation
We next used unfractionated CD4 cells to examine the effect of rapamycin, by comparison with CsA, on the differentiation of Th17 and Tregs. At a similar concentration usually used by other in vitro studies (12, 13, 15, 16), rapamycin at 10 ng/ml resulted in the reduction of TGFβ/IL-6-stimulated CD4+ IL-17-producing cells from 5.8% to 2.8%. Similarly, CsA (10 nM) also markedly inhibited the generation of IL-17-producing cells to 1.2%. The inhibitory action of rapamycin and CsA was dose-dependent in a concentration range of 1~100 ng/ml and 1~100 nM, respectively (p<0.05, Fig 1B–C). Thus, both rapamycin and CsA potently inhibited the generation of Th17 cells.
Rapamycin, but not CsA, promotes the generation of FoxP3+ cells
Although rapamycin potently inhibited the generation of IL-17 producing cells, this compound (10 ng/ml) concomitantly also enhanced TGFβ-induced generation of FoxP3+ cells by 25% (p>0.05, Fig 2A). This effect of rapamycin was not markedly enhanced by increasing the dose, possibly the dose range of rapamycin (1~100 ng/ml) used in this study had already reached the maximal Treg inducing effect. On the contrary, CsA at 10 nM completely inhibited TGFβ-induced generation of FoxP3+ cells, resulting in even lower than the background levels (~10%) of FoxP3+ cells in CD4 cells (Fig 2A). The inhibitory action of CsA was dose-dependent in a concentration range of 1~100 nM (p<0.05, Fig 2C).
The effects of rapamycin and CsA on the reciprocal differentiation of Tregs and Th17 cells from naïve CD4 cells
Unfractionated CD4 cells contain ~10% of FoxP3+ cells which have the potential to be expanded by rapamycin (13). To further clarify that the effects of rapamycin was based on its action on de novo generation of Treg and Th17 cells from naïve CD4 cells, flow-sorted CD4+CD25− T cells were used.
In the presence of TGFβ and IL-6, 7.1% of CD4+CD25− cells produced IL-17 which were reduced by rapamycin (10 ng/ml) to 4.8% and reduced by CsA (10 nM) to 1.9%. Freshly isolated CD4+CD25− T cells contained less than 2% FoxP3+ cells (data not shown). After treatment with TGFβ, the proportion of FoxP3+ cells was increased to 42.8%. TGFβ-induced expression of FoxP3+ cells was further enhanced by rapamycin (10 ng/ml) to 54.5% and was markedly inhibited by CsA (10 nM) to 5.3% (Fig 3B). Thus, rapamycin and CsA directly affected the de novo generation of Th17 and Tregs from naïve CD4 cells.
Fig 3.
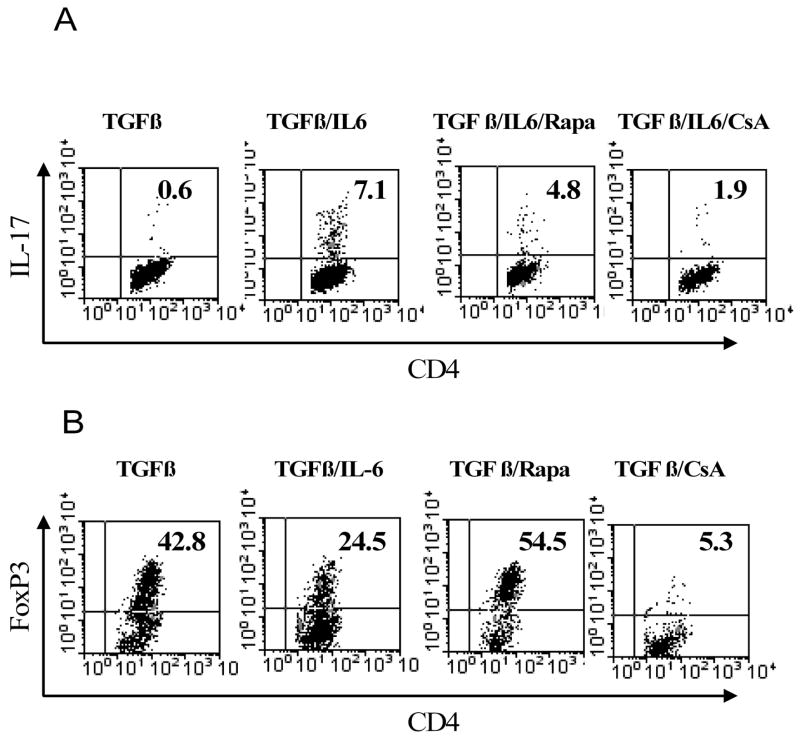
The effects of rapamycin and CsA on generation of FoxP3+ cells and IL-17-producing cells from CD4+CD25− cells. CD4+CD25− cells were isolated from normal C57BL/6 mouse LNs and spleen by flow cytometry. (A) The cells were stimulated with plate-bound anti CD3 Ab and soluble anti CD28 Ab, in the presence of TGFβ, or TGFβ plus IL-6, or TGFβ plus IL-6 plus rapamycin (Rapa, 10 ng/ml), or TGFβ plus IL-6 plus CsA (10 nM). (B) in the parallel experiments, the cells were stimulated with plate-bound anti CD3 Ab and soluble anti CD28 Ab, in the presence of TGFβ, or TGFβ plus IL-6, or TGFβ plus IL-6 plus rapamycin (Rapa, 10 ng/ml), or TGFβ plus IL-6 plus CsA (10 nM). 72 hours later, intracellular expression of FoxP3 or IL-17 was determined by FACS. Analysis was gated on CD4 cells. The data shown are representative of at least 3 separate experiments with similar results.
Discussion
In this study, we demonstrate that unfractionated CD4 cells can be used instead of naïve CD4 cells for initial study or large-scale screening of pharmacological intervention on reciprocal differentiation of Tregs and Th17 cells. Furthermore, we found that rapamycin, but not CsA, promoted de novo generation of Tregs while potently inhibiting the differentiation of Th17 cells. During the preparation of this manuscript, Gao and colleagues reported that rapamycin, but not CsA, had the capacity to promote de novo generation of alloantigen-specific Tregs as demonstrated by a series of elegant in vitro as well as in vivo studies (16). Our data thus support and extend this report.
Both rapamycin and CsA are potent immunosuppressive agents and are currently used to improve the outcome of transplantation (11, 17). CsA inhibits the activity of calcineurin, which leads to a decreased phosphorylation of the nuclear factor of activated T cells (NFTA) and thus impairs translocation of NFTA from cytoplasm to the nucleus, resulting in the inhibition of IL-2 transcription (17). IL-2 plays a non-redundant role in TGFβ-induced FoxP3 expression from naïve CD4 cells (2, 18). However, rapamycin binds to a protein kinase, called mammalian target of rapamycin, and blocks cell growth and proliferation in response to IL-2 (11). Therefore, deprivation of IL-2 production by CsA can be proposed to explain its capacity to suppress FoxP3 induction. In Tregs, the effects of IL-2 are mediated by activation of the Janus kinase/STAT pathway, whereas rapamycin-sensitive downstream targets of phosphatidyl-inositol 3-kinase are not activated (19). Therefore, Coenen et al suggested that (15) rapamycin does not affect the IL-2-dependent peripheral survival of Tregs. Our data also suggest that rapamycin does not block IL-2 signaling leading to the de novo generation of Tregs from naïve CD4 cells because TGFβ-induced expression of FoxP3 was not inhibited by this compound. It must be pointed out that even though rapamycin has the capacity to permit TGFβ-mediated generation of Tregs as shown in this report and others (16), the absolute number of Tregs produced may be compromised by rapamycin (1~100 ng/ml). This is supported by FACS analysis showing scatter properties of the cells treated with rapamycin suggestive of their lesser activation in response to plate-bound CD3 and soluble CD28 Abs (data not shown). In addition, CD4 expression by some cells was reduced by rapamycin treatment. It has been reported that rapamycin has the capacity to preserve activation-induced cell death (AICS) (16). Those cells may represent dead or dying cells (and thus were gated out in the analysis) resulting from anti CD3 and anti CD28 Abs stimulation. Thus, determining the optimal regimen whereby rapamycin promotes the conversion of CD4 cells to Tregs without concomitant cytotoxicity will be important.
Although promoting TGFβ-mediated generation of Tregs, rapamycin simultaneously inhibited TGFβ/IL-6-mediated differentiation of Th17 cells. IL-6 is a crucial cytokine for the generation of Th17 cells (5–7) and rapamycin has been reported to inhibit IL-6 signal transduction (20). Indeed, we also observed that rapamycin was able, at least partially, to abrogate the usual reduction of TGFβ-mediated FoxPS expression by IL-6 (data not shown). Thus, blockade of IL-6 biological activity may contribute to the inhibitory effect of rapamycin on the generation of Th17 cells.
Our observation that rapamycin promoted differentiation of Tregs and inhibited generation of Th17 cells, while CsA inhibited differentiation of both Tregs and Th17 cells, suggests a distinct mode of immunosuppressive action by rapamycin and CsA. Our data provides an additional mechanistic basis for the reported capacity of rapamycin to induce tolerance (21). The capacity of rapamycin to promote the generation of immunosuppressive Tregs and to suppress the differentiation of pathogenic Th17 cells furthers our understanding of the basis for the therapeutic immunosuppressive effects of rapamycin on patients with autoimmune diseases and allo-transplantation reactions.
Acknowledgments
We cordially appreciate Dr. Joost J Oppenheim (LMI, CCR, NCI-Frederick, NIH) for his critical review, comments and discussions.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 4.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 6.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 7.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Howard OM, Oppenheim JJ. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J Immunol. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–680. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 11.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 14.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 15.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, Joosten I. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–545. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 16.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 19.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahan BD, Gibbons S, Tejpal N, Stepkowski SM, Chou TC. Synergistic interactions of cyclosporine and rapamycin to inhibit immune performances of normal human peripheral blood lymphocytes in vitro. Transplantation. 1991;51:232–239. doi: 10.1097/00007890-199101000-00038. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 22.Wu DY, Goldschneider I. Cyclosporin A-induced autologous graft-versus-host disease: a prototypical model of autoimmunity and active (dominant) tolerance coordinately induced by recent thymic emigrants. J Immunol. 1999;162:6926–6933. [PubMed] [Google Scholar]
- 23.Damoiseaux JG, van Breda Vriesman PJ. Cyclosporin A-induced autoimmunity: the result of defective de novo T-cell development. Folia Biol (Praha) 1998;44:1–9. [PubMed] [Google Scholar]