Abstract
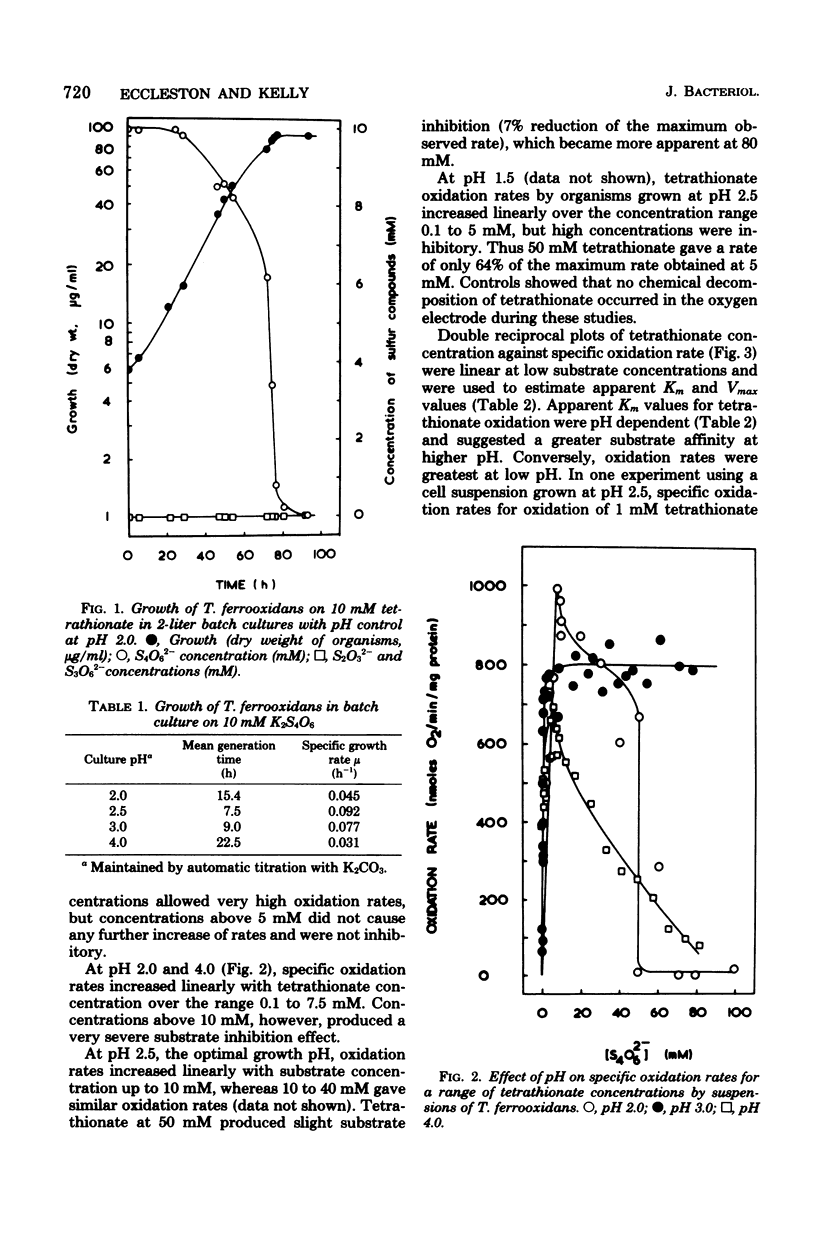
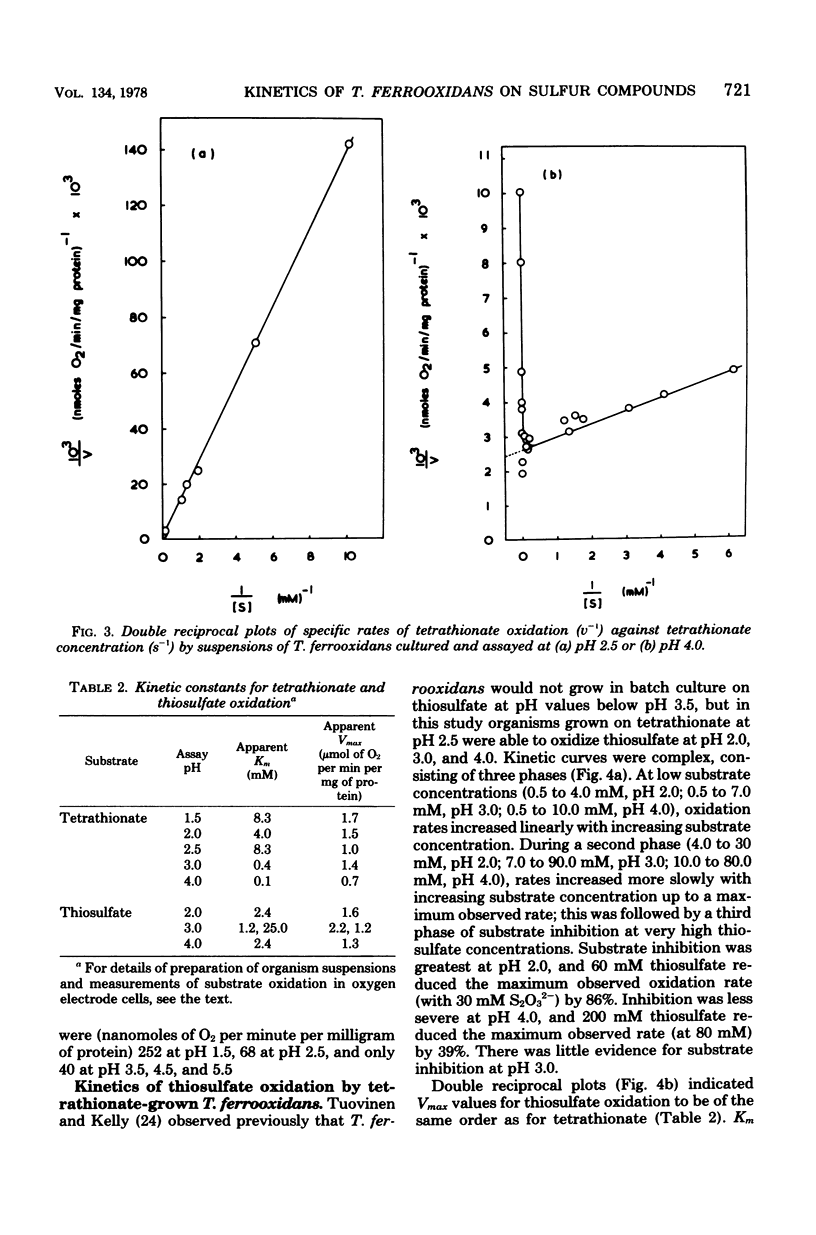
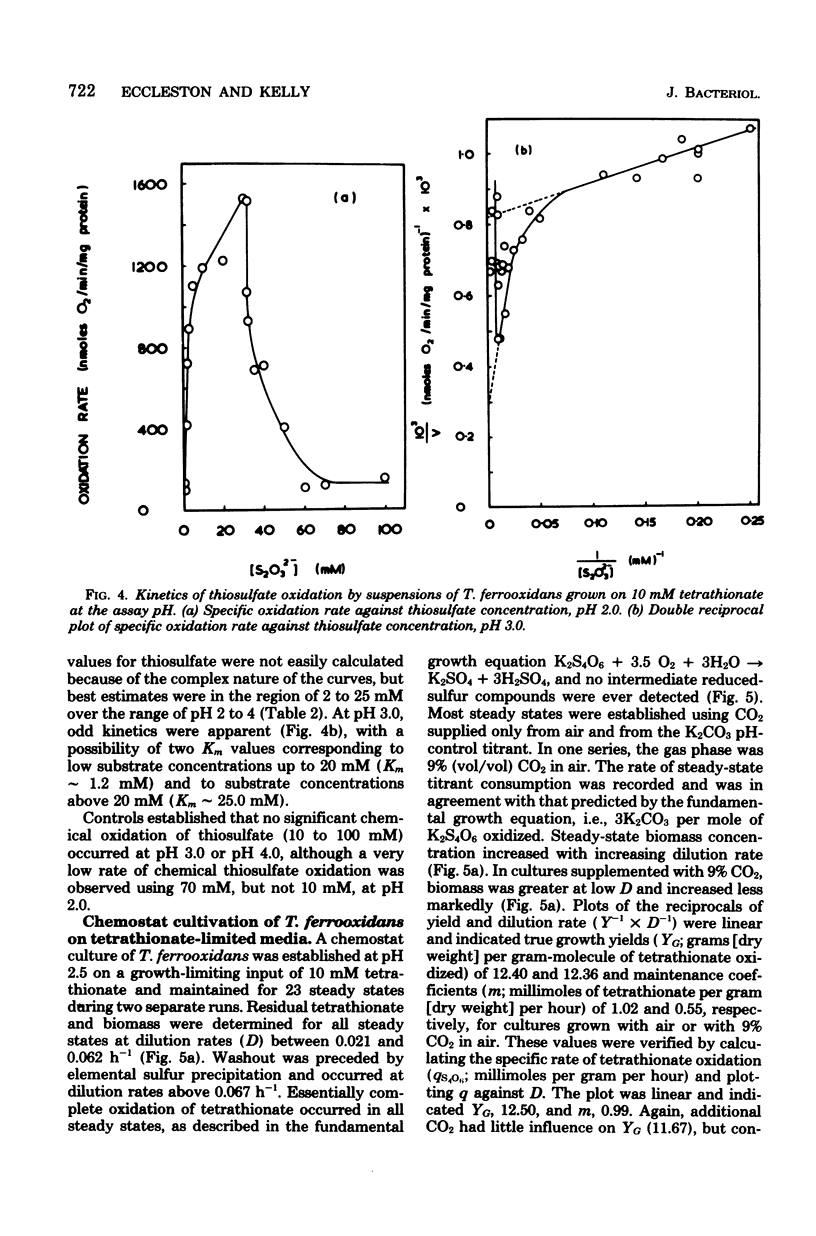
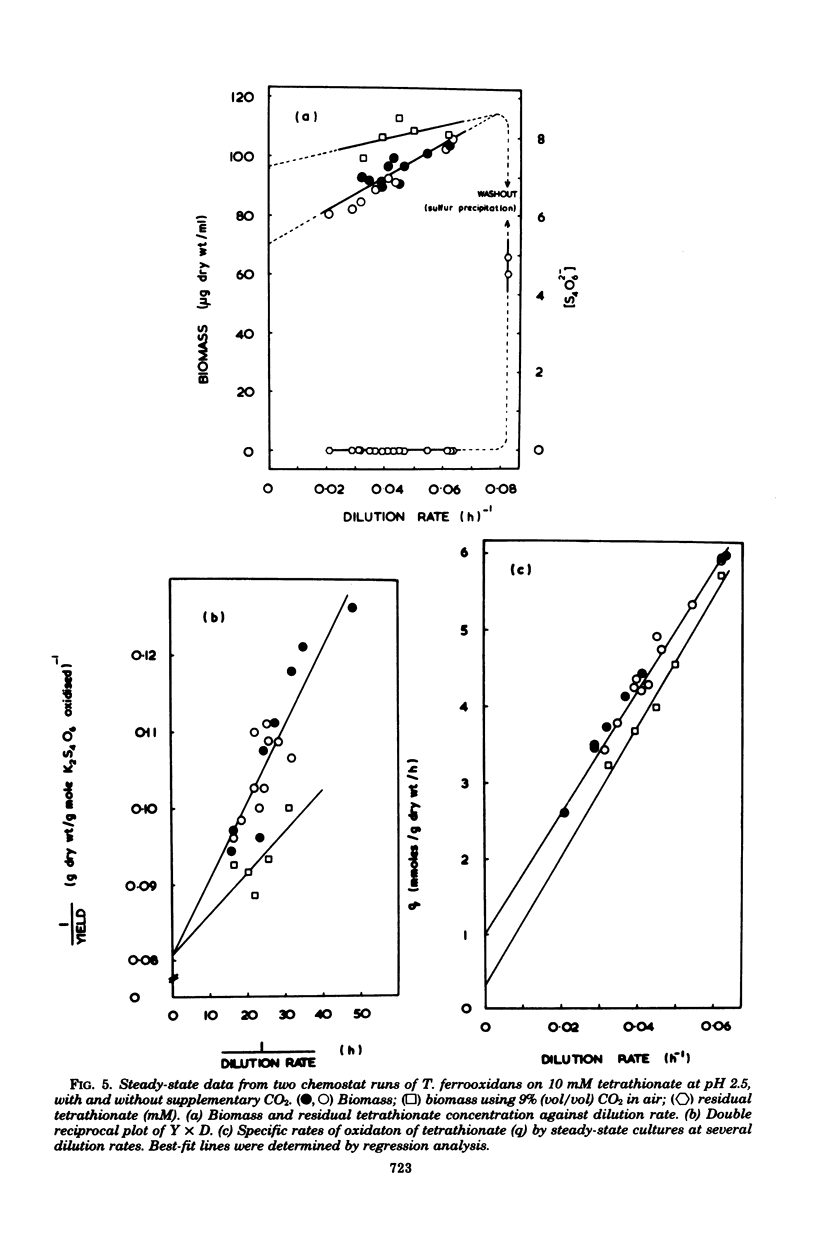
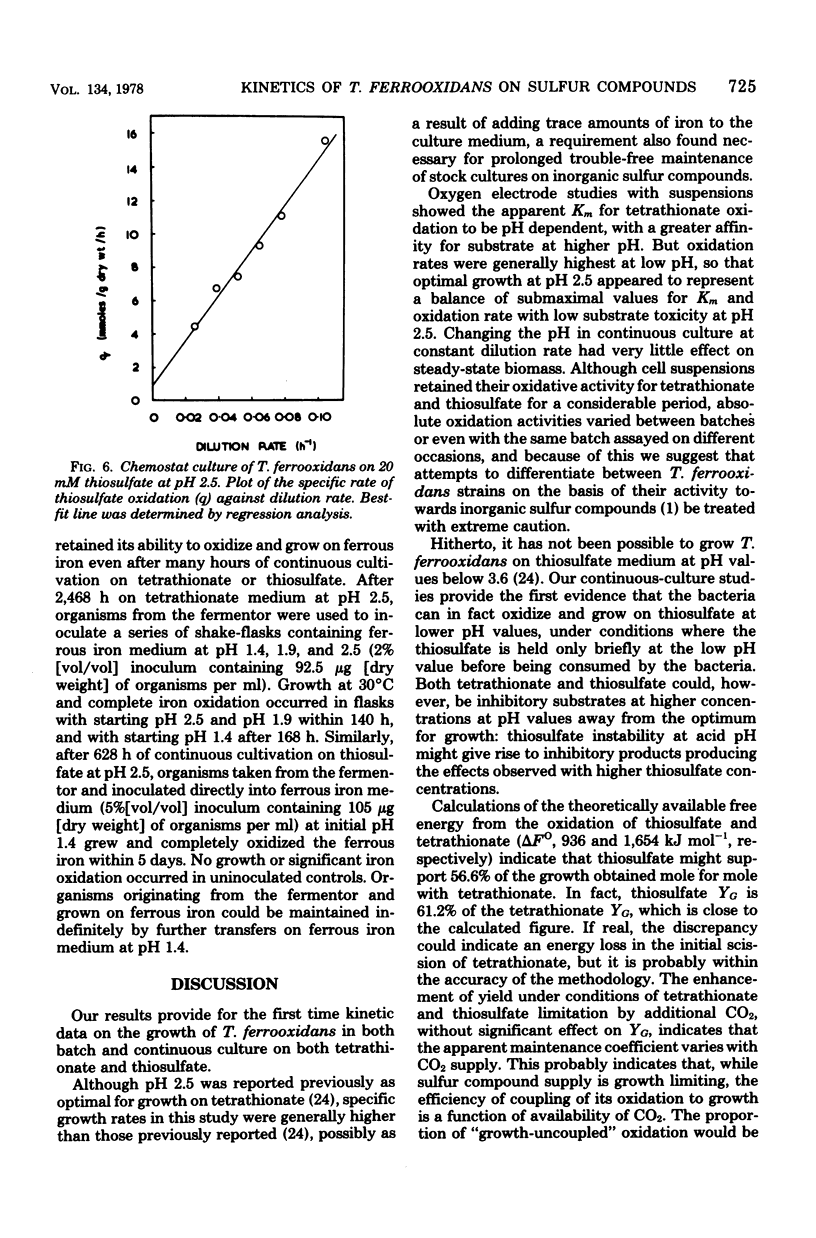
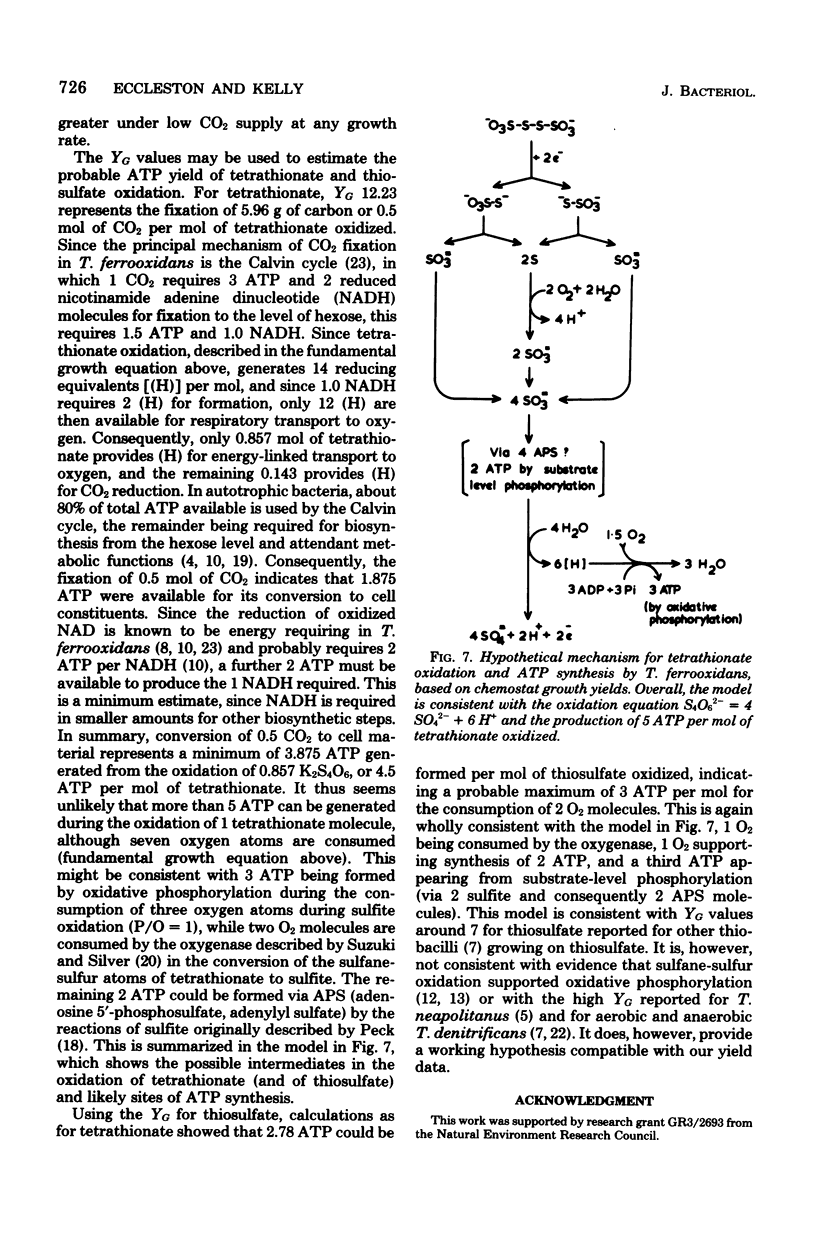
Growth of Thiobacillus ferrooxidans in batch culture on 10 mM potassium tetrathionate was optimal at pH 2.5 (specific growth rate, 0.092 h-1). Oxygen electrode studies on resting cell suspensions showed that the apparent Km for tetrathionate oxidation (0.13 to 8.33 mM) was pH dependent, suggesting higher substrate affinity at higher pH. Conversely, oxidation rates were greatest at low pH. High substrate concentrations (7.7 to 77 mM) did not affect maximum oxidation rates at pH 3.0, but produced substrate inhibition at other pH values. Tetrathionate-grown cell suspensions also oxidized thiosulfate at pH 2.0 to 4.0. Apparent Km values (1.2 to 25 mM) were of the same order as for tetrathionate, but kinetics were complex. Continuous culture on growth-limiting tetrathionate at pH 2.5, followed by continuous culture on growth-limiting thiosulfate at pH 2.5, indicated true growth yield values (grams [dry weight] per gram-molecule of substrate) of 12.2 and 7.5, and maintenance coefficient values (millimoles of substrate per gram [dry weight) of organisms per hour) of 1.01 and 0.97 for tetrathionate and thiosulfate, respectively. Yield was increased on both media at low dilution rates by increase in CO2 supply. The apparent maintenance coefficient was lowered without affecting YG, suggesting better energy coupling in CO2-rich environments. Prolonged continuous cultivation on tetrathionate or thiosulfate did not affect the ability of the organism to grow subsequently in ferrous iron medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bounds H. C., Colmer A. R. Comparison of the kinetics of thiosulfate oxidation by three iron-sulfur oxidizers. Can J Microbiol. 1972 Jun;18(6):735–740. doi: 10.1139/m72-117. [DOI] [PubMed] [Google Scholar]
- COLMER A. R. Relation of the iron oxidizer, Thiobacillus ferrooxidans, to thiosulfate. J Bacteriol. 1962 Apr;83:761–765. doi: 10.1128/jb.83.4.761-765.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMER A. R., TEMPLE K. L., HINKLE M. E. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J Bacteriol. 1950 Mar;59(3):317–328. doi: 10.1128/jb.59.3.317-328.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Vishniac W. Yield coefficients of Thiobacillus neapolitanus in continuous culture. J Bacteriol. 1967 Mar;93(3):874–878. doi: 10.1128/jb.93.3.874-878.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoor A. T. Energetic aspects of the metabolism of reduced sulphur compounds in Thiobacillus dentrificans. Antonie Van Leeuwenhoek. 1976;42(4):483–492. doi: 10.1007/BF00410179. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Johnstone K. I., White D. Taxonomy of the acidophilic thiobacilli. J Gen Microbiol. 1966 Sep;44(3):373–381. doi: 10.1099/00221287-44-3-373. [DOI] [PubMed] [Google Scholar]
- Kelly D. P., Syrett P. J. Energy coupling during sulphur compound oxidation by Thiobacillus sp. strain C. J Gen Microbiol. 1966 Apr;43(1):109–118. doi: 10.1099/00221287-43-1-109. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landesman J., Duncan D. W., Walden C. C. Oxidation of inorganic sulfur compounds by washed cell suspensions of Thiobacillus ferrooxidans. Can J Microbiol. 1966 Oct;12(5):957–964. doi: 10.1139/m66-129. [DOI] [PubMed] [Google Scholar]
- Margalith P., Silver M., Lundgren D. G. Sulfur oxidation by the iron bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1966 Dec;92(6):1706–1709. doi: 10.1128/jb.92.6.1706-1709.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Silver M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. Biochim Biophys Acta. 1966 Jul 6;122(1):22–33. doi: 10.1016/0926-6593(66)90088-9. [DOI] [PubMed] [Google Scholar]
- TEMPLE K. L., COLMER A. R. The autotrophic oxidation of iron by a new bacterium, thiobacillus ferrooxidans. J Bacteriol. 1951 Nov;62(5):605–611. doi: 10.1128/jb.62.5.605-611.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ores. Z Allg Mikrobiol. 1972;12(4):311–346. doi: 10.1002/jobm.3630120406. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Studies on the growth of Thiobacillus ferrooxidans. V. Factors affecting growth in liquid culture and development of colonies on solid media containing inorganic sulphur compounds. Arch Microbiol. 1974 Jul 22;98(4):351–364. doi: 10.1007/BF00425295. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Niemelä S. I., Gyllenberg H. G. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie Van Leeuwenhoek. 1971;37(4):489–496. doi: 10.1007/BF02218519. [DOI] [PubMed] [Google Scholar]



