Abstract
The influence of turning and environmental contamination on six spontaneous cocoa bean heap fermentations performed in Ghana was studied through a multiphasic approach, encompassing both microbiological (culture-dependent and culture-independent techniques) and metabolite target analyses. A sensory analysis of chocolate made from the fermented, dried beans was performed as well. Only four clusters were found among the isolates of acetic acid bacteria (AAB) identified: Acetobacter pasteurianus, Acetobacter ghanensis, Acetobacter senegalensis, and a potential new Acetobacter lovaniensis-like species. Two main clusters were identified among the lactic acid bacteria (LAB) isolated, namely, Lactobacillus plantarum and Lactobacillus fermentum. No differences in biodiversity of LAB and AAB were seen for fermentations carried out at the farm and factory sites, indicating the cocoa pod surfaces and not the general environment as the main inoculum for spontaneous cocoa bean heap fermentation. Turning of the heaps enhanced aeration and increased the relative population size of AAB and the production of acetic acid. This in turn gave a more sour taste to chocolate made from these beans. Bitterness was reduced through losses of polyphenols and alkaloids upon fermentation and cocoa bean processing.
Raw cocoa beans have an astringent, unpleasant flavor and have to be fermented, dried, and roasted to obtain the desired characteristic cocoa flavor (27, 30, 41, 45). Fermentation is hence the first step in cocoa powder and chocolate production. It is carried out spontaneously in heaps, boxes, baskets, or trays in cocoa-producing countries in the equatorial zone, led by the Ivory Coast, Brazil, and Ghana (1, 27). During the last decade, knowledge about the spontaneous cocoa bean fermentation process has been increasing (2, 5, 26, 33, 34, 37). The microbiota involved in natural cocoa bean fermentation reflects the environmental factors (temperature, pH, and oxygen tension) and the metabolism of substrates of the cocoa bean pulp. This results in production times of significant amounts of ethanol, lactic acid, and acetic acid, representing a succession of yeasts, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) during the cocoa bean fermentation course (2, 5, 36-38).
In the last 5 years, the microbiology and biochemistry of Ghanaian cocoa bean heap fermentation processes have been studied in detail (5, 18, 26, 33, 34). During early and mid-time spontaneous fermentation of freshly harvested pulp and cocoa beans, piled into a heap, yeasts produce ethanol under anaerobic conditions and cause depectinization of the pulp, enabling the pulp to flow away and air ingress (37, 38). Concerning microaerophilic LAB, citrate-fermenting, acid-tolerant, and ethanol-tolerant Lactobacillus plantarum and Lactobacillus fermentum strains dominate the spontaneous cocoa bean fermentation process (5, 34). Citrate and sugars are converted into acetic acid, lactic acid, and mannitol, enabling a slight increase of the pH of the pulp (5). During the aerobic phase, Acetobacter pasteurianus is the main AAB species involved in spontaneous cocoa bean fermentation; additionally, Acetobacter syzygii, Acetobacter ghanensis, Acetobacter tropicalis, and Acetobacter senegalensis are present (5, 6, 34). AAB oxidize the ethanol of the yeasts into acetic acid. Aerobic, spore-forming bacilli may develop at the end of the fermentation (increased temperature and less acidic and more aerobic conditions), but their role in the process is not clear (2, 37). Also, changes in nature and concentration of peptides, amino acids, pyrazines, alkaloids, and polyphenols occur during cocoa bean fermentation, mainly through endogenous enzymatic activities, microbial consumption and conversion, or physical diffusion (5, 14-16, 19-21, 23, 42).
Acetic acid produced by the aerobic AAB is a key metabolite for the cocoa bean fermentation process. This volatile short-chain fatty acid diffuses into the beans, and this, in combination with heat produced by the exothermic bioconversion of ethanol into acetic acid, causes the death of the seed embryo, the disruption of the internal cellular structure of the beans, and the end of fermentation (5, 34). In turn, biochemical changes in the beans are initiated, leading to the enzymatic formation of precursor molecules that are necessary for the development of the characteristic aroma, flavor, and color of the beans (8, 14, 17). These properties are developed further during drying, roasting, and final processing of well-fermented cocoa beans (41). Hence, factors affecting acetic acid production by aerobic AAB, such as mixing of the beans (heap turning and box fermentations), may lead to an accelerated and altered cocoa bean fermentation process. For instance, on average, Ghanaian cocoa bean heap (100 to 200 kg) fermentations are carried out for 5 to 6 days (as recommended by the authorities), with pods stored for maximally 4 days after harvest. Heaps are not turned or are turned only once instead of the recommended two turns (4, 10, 24). Fermentation is followed by sun drying for 7 to 8 days. It is believed that the heap size, postharvest pod age, fermentation time, drying time, and number and timing of turns during fermentation influence the quality of the fermented cocoa beans. Although this may influence the flavor of the chocolate produced, limited attention is paid to it. Blending of the fermented and dried cocoa beans after processing assures the quality of Ghanaian cocoa (4).
Recently, we have started a multiphasic study of the Ghanaian spontaneous cocoa bean heap fermentation process, encompassing microbiological analysis (culture dependent and culture independent), metabolite target analysis (combining diverse chromatographic and mass spectrometric analyses), and pilot-scale chocolate production (5) instead of microbiological analysis (33, 34) or chocolate production (4) solely. Besides the above-mentioned LAB and AAB species diversity and population dynamics, we have shown that the season and farmer's plantation are of no significant influence on the quality of the fermented and dried cocoa beans (5). The present study aims at unraveling the influence of the external factors aeration (enhanced by turning of the heap) and environmental contamination (by comparison of a fermentation process at a farmer's plantation with a fermentation carried out with beans from cocoa pods moved out of the farmer's environment) on the fermentation course. In particular, the biodiversity and dynamics of LAB and AAB populations during spontaneous cocoa bean heap fermentation and chocolate production made from these beans were studied.
MATERIALS AND METHODS
Cocoa bean fermentation.
The microbiota of six spontaneously fermented cocoa bean heaps (heaps 8 to 13 [Table 1]) was studied in Ghana, as described previously (5). Four fermentations were carried out at the farm site and two at the factory site. The experiments were performed during the main crop of 2005 (October to December 2005). The fermentations at the plantation of farmer 1 in New Tafo were carried out for 6 days with pulp and beans from 2- to 3-day-old cocoa pods (5); the control fermentation was not turned, and the other fermentation was turned twice (after 48 and 72 h). Turning took only a few minutes and involved the transfer of the packed heap, covered with banana leaves and keeping the heat, into a newly formed packed heap covered with banana leaves. These fermentations were carried out simultaneously to exclude the influence of time, weather, and contamination factors (baskets, pods, farmers' hands, banana leaves, knives, etc.). The fermentations at the farm site were performed in duplicate (heaps 8 and 12 and heaps 9 and 13 for turning and no-turning conditions, respectively), except that for heap fermentation 13 the placenta of the cocoa pods was removed to control air penetration as well. Two fermentations were carried out at the facilities of Barry Callebaut Ghana (heaps 10 and 11 for turning and no-turning conditions, respectively) to exclude the factor environmental contamination by the farmer's plantation. Therefore, cocoa pods (1,600 in total) were harvested in New Tafo (at the farmers' plantations) and then immediately transported to the factory in Tema (120 km) by truck. At the factory site, the pods were ripened for two more days and were then opened by four men whose hands and knives were washed with an antiseptic fluid (Dettol). In a subsequent step, the beans were placed into two heaps and fermented for 6 days. One heap (heap 10) was turned after 48 and 72 h of fermentation.
TABLE 1.
Characteristic parameters of the six spontaneous cocoa bean heap fermentations carried out over 6 daysa
| Heap no.b | Heap size (kg) | Start pH | End pH | Postharvest pod age (days) | Final wt (kg) | Initial temp (°C) | Maximum fermentation temp (°C) |
|---|---|---|---|---|---|---|---|
| 8 (T, farm) | ±150 | 3.8 | 4.3 | 2 | 45 | 24.9 | 44.6 |
| 9 (NT, farm) | ±150 | 3.7 | 4.2 | 2 | 45 | 26.1 | 43.3 |
| 10 (T, factory) | ±150 | 3.8 | 4.4 | 3 | 32 | 27.6 | 44.3 |
| 11 (NT, factory) | ±150 | 3.8 | 4.3 | 3 | 40 | 28.8 | 44.7 |
| 12 (T, farm) | ±150 | 3.5 | 4.4 | 2-3 | 35 | 26.3 | 45.9 |
| 13 (NT, farm) | ±150 | 3.7 | 4.2 | 2-3 | 38 | 27.0 | 42.8 |
In all cases, there was no rainfall.
T, turned; NT, not turned.
During all fermentations carried out, the outside and inside temperatures of the heaps, pH (inside the heaps), and rainfall (pluviometer) were monitored, as described previously (5). Drying of the fermented beans was carried out for 10 days, in open air in all cases.
Sampling.
Samples were withdrawn from the fermenting cocoa bean mass for immediate microbiological, culture-dependent analysis as well as for culture-independent and metabolite target analyses, as described previously (5).
Plating, enumeration, isolation, and maintenance.
In Ghana, the culture-dependent approach was performed, using different selective agar media that were incubated at the appropriate temperatures for 1 to 4 days in a standard incubator (Jouan, St. Herblain, France), for the monitoring, isolation, and enumeration of yeasts (37°C), LAB (37°C), and AAB (42°C). Yeasts and LAB were plated as described previously (5). As only a limited number of taxa of AAB were found during earlier fermentations studied (5), AAB were monitored and isolated by using four different agar media: deoxycholate-mannitol-sorbitol (DMS) agar (13), as used before; acetic acid medium (AAM) agar (1% d-glucose, 0.5% ethanol, 0.3% acetic acid, 1.5% peptone, 0.8% yeast extract, wt/vol [29]); basal medium with ethanol (BME) agar (0.05% yeast extract, 0.3% vitamin-free Casamino Acids [Difco, Detroit, MI], 0.3% ethanol, wt/vol); and glucose-yeast extract (GY) agar (5% d-glucose, 0.5% yeast extract, wt/vol). The agar concentration was always 1.5% (wt/vol). All media were supplemented with cycloheximide (400 mg liter−1) to inhibit yeast growth. Colony counts were enumerated through 10-fold serial dilution of the samples in saline (0.85% NaCl solution, wt/vol). For comparison, lower and higher values of the microbial counts for the four fermentations performed are included below. The 605 bacterial isolates that were picked up from the different agar media were stored at −80°C in MRS medium (Oxoid, Basingstoke, United Kingdom) (LAB, 234 isolates) or MYP medium (2.5% d-mannitol, 0.5% yeast extract, and 0.3% bacteriological peptone [Oxoid], wt/vol) (AAB, 371 isolates), supplemented with 25% (vol/vol) glycerol as a cryoprotectant.
Identification and cluster analysis.
In Belgium, all bacteria were checked for purity through successive transfers in and plating on the appropriate liquid and agar media, respectively, followed by microscopic observation and a catalase test (5). Subsequently, they were identified through a polyphasic taxonomic approach. Potential LAB and AAB isolates were dereplicated and preliminarily identified by (GTG)5-PCR fingerprinting performed on genomic DNA, as described before (5, 9). Further polyphasic identification was performed through 16S rRNA gene sequence analysis and pheS gene sequence analysis (5, 31).
16S rRNA PCR-DGGE analysis.
Besides culture-dependent cell enumeration, population dynamics were analyzed through a culture-independent approach by means of denaturing gradient gel electrophoresis (DGGE) (35 to 60% denaturant gradient) of amplicons of the V3 region of the 16S rRNA gene, making use of the LAC1-LAC2 primer pair for LAB (43), as described previously (5). Cluster analysis of DGGE profiles was carried out by calculation of similarities in the profiles of bands with the Dice coefficient to provide a qualitative discrimination among the patterns. Bands of interest were sequenced, and the closest known relatives of the partial 16S rRNA gene sequences obtained were evaluated through BLAST analysis (5).
Metabolite target analysis.
Aqueous extracts from fermentation samples for metabolite analysis were obtained as described before (5). Extracts of dried, deshelled, defatted nibs were used to quantify alkaloids and polyphenols. Defatting was performed as follows. Nib samples were milled in a coffee mill to a powder of particles of 2 mm in size; 5 g was suspended in 20 ml of heated petroleum ether (60 to 80°C) for 3 h and centrifuged (1,500 × g, 15 min). After removal of the petroleum ether layer, the previous step was repeated twice. The defatted residue (cocoa powder) was air dried and stored at −20°C before being extracted in boiling water for 1 h at a concentration of 20 mg ml−1. After being cooled to room temperature, the samples were centrifuged, yielding the final extract for analysis.
Concentrations of sugars (sucrose, glucose, and fructose) and mannitol were determined by high-performance anion-exchange chromatography with pulsed amperometric detection. Concentrations of organic acids (lactic acid, acetic acid, and citric acid) were determined by high-performance anion-exchange chromatography with conductivity detection under ion suppression. The concentration of ethanol was determined by gas chromatography coupled to mass spectrometry. The apparatus used, process parameters, sample preparations, and peak identification and quantification were the same as those described previously (5).
Alkaloids and polyphenols on extracts of dried, defatted nibs were determined by high-pressure liquid chromatography (HPLC) with a Nucleosil 100-5 C18 column (250 by 4.6 mm; AIT, Nucleosil, France). Concentrations of theobromine and caffeine were determined using a modified version of the HPLC method described by Kreiser and Martin (25). A mobile phase of water-acetonitrile (80:20, vol/vol) at a flow rate of 1 ml min−1 and UV detection at 254 nm were applied. Quantification was performed by external calibration with standard solutions of theobromine (0.08 mg ml−1) and caffeine (0.02 mg ml−1).
The total polyphenol content was quantified by oxidation of the polyphenols (0.1 ml of extract) with the Folin-Ciocalteu reagent (11). The absorbance of the solution was measured at 760 nm against a reference sample. Results are expressed in milligrams of epicatechin equivalents per gram of dry mass of defatted material, as linear standard curves were obtained for a solution of epicatechin in the concentration range of 5 to 20 mg liter−1. Concentrations of epicatechin and catechin were determined by HPLC and fluorescence detection (excitation at 274 nm and emission at 322 nm). Before analysis, defatted cocoa (4 g liter−1) was dissolved in 90% (vol/vol) water plus 2% (vol/vol) acetic acid (pH 2.5) and 10% (vol/vol) acetonitrile, placed in an ultrasonic bath for 10 min, and filtered with a 0.45-μm cellulose filter. The standards were treated under the same conditions at 200 and 400 mg liter−1. The mobile phase, at a flow rate of 1.0 ml min−1, consisted of water plus acetic acid (pH 2.5, eluent A) and acetonitrile (eluent B) with the following gradient: 0.0 min, 90% A and 10% B; 20.0 min, 85% A and 15% B. Quantification was performed by external calibration with standard solutions of epicatechin (52 mg liter−1) and catechin (22 mg liter−1). Results are expressed in milligram components per gram of cocoa product.
All determinations were performed in triplicate. Standard deviations are indicated below.
Quality assessment of fermented, dried cocoa beans.
Fermented, dried cocoa beans were checked for appearance (bean count per 300 g of beans, bean size, physical damage, insect penetration) and quality in Ghana (Quality Division of the cocoa board; COCOBOD, Accra, Ghana) and by Barry Callebaut Belgium (Wieze, Belgium), making use of the cut test. A total of 300 beans were cut lengthwise through the middle to expose the maximum cut surface of the cotyledons. Both halves were examined in full daylight and placed in one of the following categories: fully brown (fermented); partly brown, partly purple (partly fermented); purple (underfermented); slaty (not fermented); insect damaged; moldy; or germinated.
Chocolate production and sample analysis.
Chocolate was made in the pilot plant of Barry Callebaut France (Louviers, France). Thirty kilograms of fermented, dried beans was roasted for 30 min in a pilot roaster using an air temperature of 130 to 140°C. The beans were deshelled by breaking and winnowing the beans, and the nibs were ground into cocoa liquor using a ball mill and passed through a 20-mesh-sized screen. The cocoa liquor was then mixed with cane sugar and passed through a three-roll refiner. Deodorized cocoa butter was melted at 60°C and partly mixed for 15 min with the sugar-cocoa mixture before passing the refiner. The recipe used for chocolate production consisted of 42% (wt/wt) cocoa liquor, 46% (wt/wt) sugar, and 11.4% (wt/wt) cocoa butter, with the addition of 0.6% (wt/wt) lecithin and 0.03% (wt/wt) vanillin. The chocolate mix was conched at 50°C for 2 h with addition of cocoa butter, lecithin, and vanillin, subsequently tempered, and finally stored at room temperature until further analysis.
For cocoa liquor analysis, 20 g was defatted by extraction in a Soxhlet apparatus with 500 ml petroleum ether (60 to 80°C) over 4 h, followed by a second extraction with the same volume of petroleum ether for 3 h. The defatted residue (cocoa powder) was air dried and stored at −20°C before being extracted in triplicate in boiling water for 1 h at a concentration of 20 mg ml−1. After being cooled to room temperature, the samples were centrifuged in Eppendorf vials (10,000 × g, 10 min). The supernatant was used to quantify alkaloids, as described above. For the determination of polyphenols, 1 g of cocoa powder was extracted in 60 ml of boiling water under reflux for 2 h, according to a standard method (11). Samples were centrifuged in 50-ml Falcon tubes (1,500 × g, 15 min), and the supernatant was filtered (Schleicher & Schuell, Dassel, Germany). Extracts were analyzed for alkaloids and polyphenols as described above.
Chocolate sensory analysis.
Sensory analysis of the chocolate products was performed by a trained panel of eight members of Barry Callebaut Belgium. Characteristic flavor notes of the samples were recorded and discussed during the sessions. The flavor descriptors were sour, fruity, cocoa, bitter, flowery, taste intensity, and aftertaste intensity, expressed as numerical values between 0 and 100. As a final parameter, the aftertaste was described. Before analysis, chocolate samples were brought to a temperature of 45°C to be melted before sensory evaluation. A maximum of four chocolate samples were evaluated during each session to reduce perception fatigue. Water was used for rinsing the mouth between different samples. The panel members compared each sample tasted with an internal reference sample. This reference sample has certain scores for the flavor descriptors mentioned above.
RESULTS
Culture-dependent population dynamics of the spontaneous cocoa bean heap fermentations.
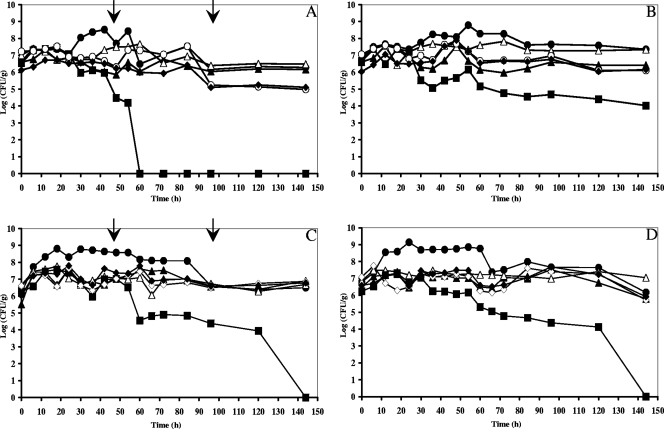
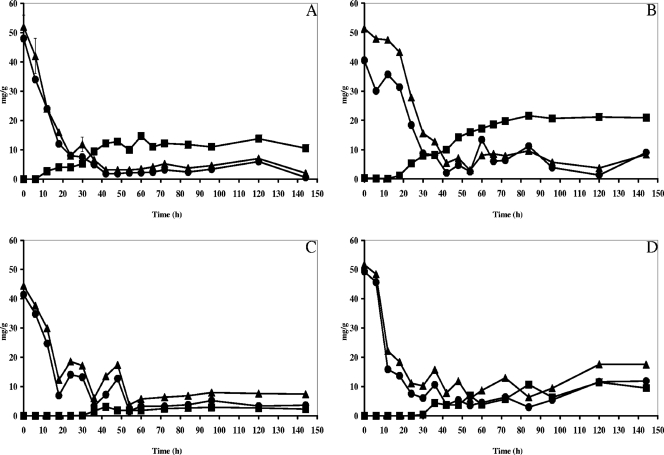
The population dynamics of yeasts, LAB, and AAB were slightly influenced by turning but not by the environmental contamination factor (Fig. 1). In general, simultaneous growth of yeasts, LAB, and AAB took place. As seen before (5), high yeast counts (6.2 to 6.6 log CFU g−1 of pulp and beans) and high LAB counts (6.2 to 6.6 log CFU g−1) were present in the heaps at the beginning of the fermentation, indicating the use of well-ripened pods. The size of the yeast population increased during the first 12 to 24 h and grew to maximum populations of 7.3 to 7.6 log CFU g−1. Upon prolonged fermentation, the yeast population declined. In the case of turned heaps (heaps 8, 10, and 12), the yeast population declined rapidly after turning, resulting in no yeast retrieval after 96, 144, and 60 h of fermentation in heaps 8, 10, and 12, respectively. LAB grew during fermentation to maximum populations of 8.7 to 9.1 log CFU g−1 after 24 to 54 h. Afterwards there was a slight decrease of the LAB population, sometimes stabilizing upon prolonged fermentation (6.1 to 7.3 log CFU g−1). It was remarkable that high counts of AAB were found from the beginning of the heap fermentations (5.4 to 6.5 log CFU g−1). However, only in the fermentations with turning did a considerable increase of AAB and a remarkable stabilization upon prolonged fermentation take place. Every time the heap was turned, an increase in the AAB counts was noticed. In the turned heaps, maximum population densities of 6.8 to 7.6 log CFU g−1 were reached after 30 to 72 h of fermentation.
FIG. 1.
Microbial succession of yeasts (malt extract agar, ▪), LAB (MRS agar, •), AAB (DMS agar, ▴; BME agar, ⋄; AAM agar, ⧫; GY agar, ○), and total aerobic bacteria (plate count agar, ▵) during Ghanaian spontaneous cocoa bean heap fermentations at the farm and factory sites and with the influence of turning (indicated by an arrow). (A) Heap 10 (turning, factory); (B) heap 11 (no turning, factory); (C) heap 12 (turning, farm); (D) heap 13 (no turning, farm).
All fermentations were characterized by an initial pH of approximately 3.8 that increased to approximately pH 4.1 to 4.5 after 120 to 144 h of fermentation. The heaps that were turned reached a slightly higher end value (±4.40 versus ±4.20 for turned and no-turned heaps, respectively).
The ambient temperatures during day and night were 24 to 39°C and 19 to 24°C, respectively. The temperatures inside the heaps ranged from an average of 25°C at the start of the fermentations to a maximum temperature of 45.6°C (average of the range was 43.2 to 47.7°C). The maximum temperature was reached faster when the heap was turned (after 72 h of fermentation), followed by a slight decrease to an end value of ±44.5°C after 144 h of fermentation. A continuous, linear increase of the temperature was noticed when the heaps were not turned, with a maximum temperature at the end of these fermentations of ±43.6°C after 144 h of fermentation.
Identification of the isolates.
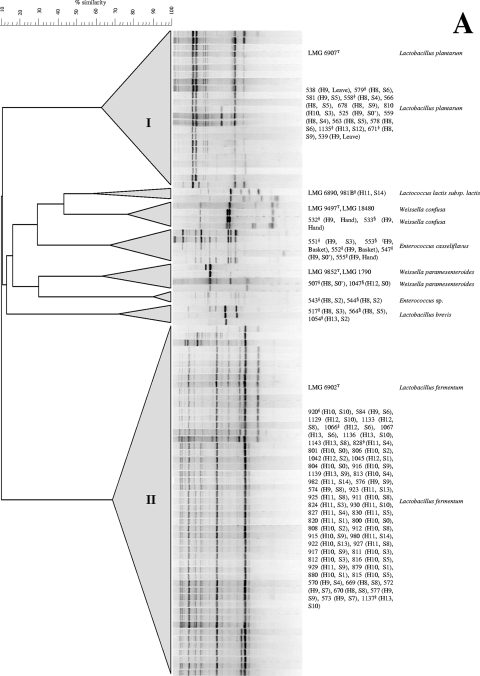
Out of 234 isolates from MRS agar, 108 isolates could be recovered and purified and were catalase-negative cocci or rods. After (GTG)5-PCR fingerprinting and validation with 16S rRNA and pheS sequencing, these LAB isolates were grouped into two large clusters, which were identified as either L. plantarum or L. fermentum (Fig. 2A). L. plantarum was found in heaps 8, 9, 10, and 13; L. fermentum was picked up from all heaps. Occasionally, isolates belonging to Enterococcus casseliflavus, Lactococcus lactis subsp. lactis, Lactobacillus brevis, Weissella confusa, and Weissella paramesenteroides were found, but only in the beginning of the fermentations, on the surfaces of the baskets, and/or on the hands of the farmer (Fig. 2A).
FIG. 2.
Dendrogram based on the numerical analysis of generated, digitized (GTG)5-PCR fingerprints from fermented cocoa bean isolates identified as LAB (A) and AAB (B). Banding patterns were clustered together with reference strains by the unweighted-pair group method using average linkages, with correlation levels expressed as percent values of the Pearson correlation coefficient. Species validation of representative strains of each cluster was carried out by 16S rRNA (indicated by *) and pheS (indicated by §) gene sequence analysis.
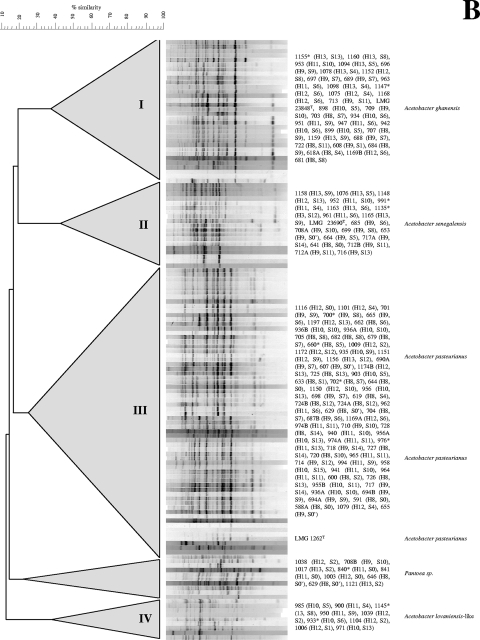
To avoid selective isolation of A. pasteurianus from DMS agar, as hypothesized before (5), several cultivation media were used for the isolation of AAB during this study. Three hundred seventy-one colonies were recovered (200 from DMS agar, 55 from BME agar, 46 from AAM agar, and 70 from GY agar); 209 isolates (56.3%) were potential AAB, as revealed by phenotypic analyses. Numerical (GTG)5-PCR analysis and validation with 16S rRNA gene sequencing revealed the presence of three major clusters, belonging to the species A. pasteurianus, A. ghanensis, and A. senegalensis, except for BME agar, on which no A. ghanensis strains were found (Fig. 2B; Table 2). A fourth cluster of nine strains was found on AAM agar, representing 15.3% of the isolates analyzed, which corresponded with an A. lovaniensis-like AAB species (Fig. 2B; Table 2). The 16S rRNA gene sequence similarity with the reference strain of A. lovaniensis was 99.71%. On DMS agar, the major cluster was represented by A. pasteurianus. On AAM agar, the major cluster was represented by A. ghanensis (37% of the isolates), followed by A. pasteurianus and A. lovaniensis-like (15% of the isolates each), which may indicate a selection based on acetic acid tolerance (22). The results for GY agar were very similar to those for DMS agar, yielding equal percentages of isolates in each of the main clusters, with A. pasteurianus as the major cluster (34% of the isolates). On BME agar, 72% of the isolates did not match with any of the AAB species mentioned above and did not form a separate cluster. Dispersed isolates belonged to enterobacterial species, as revealed by 16S rRNA gene sequence analysis. Moreover, 8% of these isolates belonged to Pantoea sp., as revealed by 16S rRNA gene sequence similarity (100% identity).
TABLE 2.
Diversity of AAB in Ghanaian cocoa bean heap fermentations in different isolation media used
| Species | % of total no. of isolates (n) obtained from agar medium
|
|||
|---|---|---|---|---|
| DMS (n = 200) | BME (n = 55) | AAM (n = 46) | GY (n = 70) | |
| A. senegalensis | 11 | 8 | 3.8 | 17 |
| A. pasteurianus | 34.5 | 4 | 15.3 | 34 |
| A. ghanensis | 13.6 | 0 | 36.6 | 17 |
| A. lovaniensis-like | 0.9 | 8 | 15.3 | 2 |
| Pantoea sp. | 0.9 | 8 | 0 | 0 |
| Other spp. | 39 | 72 | 7.7 | 30 |
The major AAB species found in the heaps that were turned (heaps 8, 10, and 12) belonged to A. pasteurianus, representing 60% of the total species found, whereas in the nonturned heaps (heaps 9, 11, and 13) only 37% of the species were A. pasteurianus (Fig. 2B). No differences in LAB and AAB species were seen between heap fermentations carried out at the farm (plantation) and factory (out of the plantation) sites, indicating that the cocoa pods were the actual source of the microorganisms.
Culture-independent population dynamics of the spontaneous cocoa bean heap fermentations.
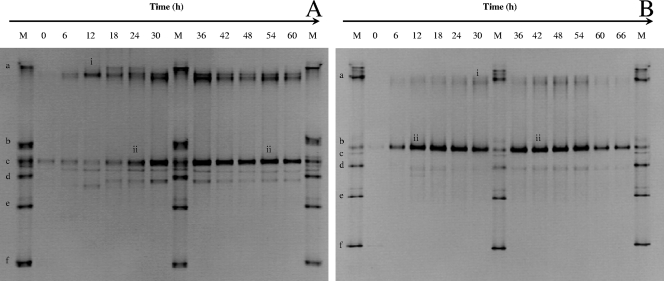
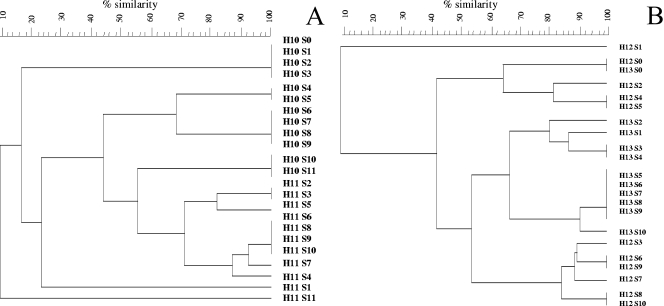
The 16S rRNA PCR-DGGE results show that L. plantarum and L. fermentum were present during spontaneous cocoa bean heap fermentation (Fig. 3). Concerning the fermentations at the farm, L. plantarum and L. fermentum were present from the start of the fermentation in heap 13 (not turned) and both species dominated the first 3 days of fermentation (Fig. 3A). In heap 12 (turned), “W. ghanensis” (5) and L. fermentum were present, the latter species dominating the fermentation process (data not shown). Concerning the fermentations at the factory site, L. fermentum was present from the start of the fermentation and dominated the whole fermentation process, being less dominant in heap 11 (not turned) than in heap 10 (turned) (Fig. 3B), except for a later stage during fermentation. Cluster analysis of the PCR-DGGE profiles of heap fermentations 10 and 11 as well as of heap fermentations 12 and 13 revealed not only a shift in the bacterial ecology from day 1 to day 2, whereby the starting microbiota was dependent on the handling conditions, but also different evolutions of the bacterial ecology between turning and no turning of the heaps (Fig. 4).
FIG. 3.
PCR-DGGE profiles (35 to 60% denaturant gradient) of LAB representing 16S rRNA gene fragments of heap 13 (A) and heap 10 (B), sampled during a 6-day cocoa bean fermentation. All 16S rRNA gene fragments were amplified by the LAC1-LAC2 primer pair. The ladder (M) consisted of (a) Lactobacillus plantarum LMG 6907T, (b) Lactobacillus acidophilus LMG 9433T, (c) Lactobacillus fermentum LMG 6902T, (d) Leuconostoc mesenteroides subsp. mesenteroides LMG 6893T, (e) Pediococcus acidilactici LMG 11384T, and (f) Lactobacillus casei LMG 6904T. The closest relatives of the fragments sequenced (the percentages of identical nucleotides compared to sequences retrieved from the GenBank database are shown in parentheses) were as follows: L. plantarum (i, 100%) and L. fermentum (ii, 100%).
FIG. 4.
Cluster analysis of the PCR-DGGE profiles of spontaneous cocoa bean heap fermentations. Dendrograms were based on the Dice coefficient of similarity (weighted) and obtained with the unweighted-pair group method using average linkages clustering algorithm. Samples are indicated by fermentation heap (H), sample number (S), and farmer. (A) Bacterial shift in and effect of turning on cocoa bean heap fermentations 10 and 11 at the factory. (B) Bacterial shift in and effect of turning on cocoa bean heap fermentations 12 and 13 at the farm.
Metabolite target analyses of the spontaneous cocoa bean heap fermentations.
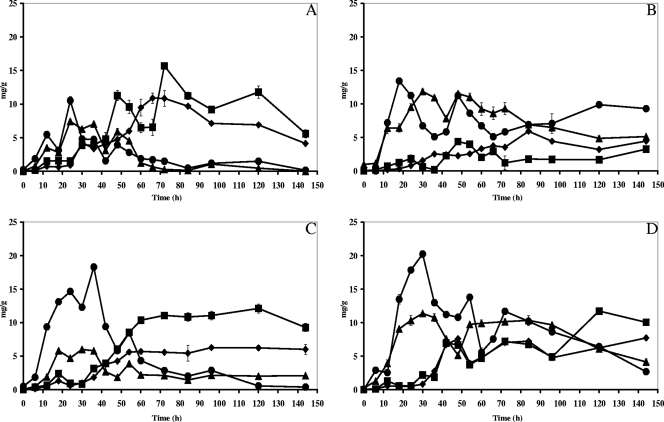
Courses of sugar consumption and metabolite production were similar for fermentations performed at the farm and factory sites for turning and no-turning conditions (Fig. 5). Almost all sucrose in the pulp was metabolized at the start of the fermentations, due to yeast growth and pod ripening before fermentation. Fructose and glucose disappeared simultaneously in the pulp and were almost completely depleted at the end of the fermentations (Fig. 5). The sucrose present in the beans was continuously converted into fructose and glucose; citric acid was slowly and not completely consumed in the case of heap 12, whereas for heaps 10, 11, and 13 there was a normal early consumption of citric acid during fermentation (data not shown).
FIG. 5.
Course of sugar consumption during Ghanaian spontaneous cocoa bean heap fermentations at the farm and factory sites and with the influence of turning. (A) Residual glucose (•), fructose (▴), and mannitol (▪) produced in the pulp for heap 10 (turning, factory). (B) Residual glucose (•), fructose (▴), and mannitol (▪) produced in the pulp for heap 11 (no turning, factory). (C) Residual glucose (•), fructose (▴), and mannitol (▪) produced in the pulp for heap 12 (turning, farm). (D) Residual glucose (•), fructose (▴), and mannitol (▪) produced in the pulp for heap 13 (no turning, farm).
Mannitol was produced from the beginning of the fermentation process and increased over time. There was an influence of turning and environment on the production of mannitol in the fermenting mass. The fermentations performed at the farm reached end values for mannitol of 2.87 ± 0.02 and 9.49 ± 0.03 mg g−1 of pulp for heaps 12 (turned) and 13 (not turned), respectively (Fig. 5C and D). The fermentations performed at the factory achieved mannitol levels of 13.80 ± 0.03 and 21.60 ± 0.04 mg g−1 of pulp for heaps 10 (turned) and 11 (not turned), respectively (Fig. 5A and B).
Ethanol increased upon fermentation, almost simultaneously in pulp and beans (Fig. 6). The fermentations performed at the farm reached a high maximum value of 20.23 ± 0.53 mg g−1 in the pulp after 30 h of fermentation, followed by a decline of ethanol levels. A faster decrease in ethanol was noticed in the fermentation that was turned, and all ethanol was almost completely gone at the end of the fermentation (0.37 ± 0.10 mg g−1) (Fig. 6C and D). The ethanol present in the beans, as a result of inside diffusion, reached a maximum value of 6.00 ± 0.11 and 11.36 ± 0.16 mg g−1 after 36 and 30 h of fermentation, respectively, for heaps 12 and 13, respectively, declined from this moment on, and remained constant for heap 12 after 60 h of fermentation (2.06 ± 0.11 mg g−1). The concentration of ethanol in the beans from heap 13 started to decline after 84 h of fermentation and reached an end value of 4.12 ± 0.16 mg g−1 (Fig. 6C and D). The fermentations performed at the factory reached a lower maximum value after 24 h of fermentation (10.00 ± 0.16 mg g−1 in the pulp and 7.02 ± 0.12 mg g−1 in the beans) (Fig. 6A and B). A faster decrease of the ethanol was again noticed in the fermentation that was turned, and ethanol was almost completely gone at the end of the fermentation (0.37 ± 0.10 mg g−1 in the pulp) (Fig. 6A and B).
FIG. 6.
Course of acetic acid and ethanol during Ghanaian spontaneous cocoa bean heap fermentations at the farm and factory sites and with the influence of turning. (A) Course of acetic acid produced in the pulp (▪) and in the beans (⧫) and course of ethanol produced in the pulp (•) and in the beans (▴) for heap 10 (turning, factory). (B) Course of acetic acid produced in the pulp (•) and in the beans (⧫) and course of ethanol produced in the pulp (•) and in the beans (▴) for heap 11 (no turning, factory). (C) Course of acetic acid produced in the pulp (▪) and in the beans (⧫) and course of ethanol produced in the pulp (•) and in the beans (▴) for heap 12 (turning, farm). (D) Course of acetic acid produced in the pulp (▪) and in the beans (⧫) and course of ethanol produced in the pulp (•) and in the beans (▴) for heap 13 (no turning, farm).
As for ethanol, the time course of acetic acid was heap and fermentation practice dependent. A clear difference was noticed between the fermentations that were turned every 2 days and the ones that were not turned (Fig. 6). A high level of acetic acid was reached in heaps 10 and 12 (16.00 ± 0.46 mg g−1 in the pulp and 12.00 ± 0.25 mg g−1 in the beans of heap 10 versus 5.00 ± 0.23 mg g−1 in the pulp and 5.00 ± 0.06 mg g−1 in the beans of heap 11) (Fig. 6), in accordance with the high maximal fermentation temperature compared to that for previous fermentations (for instance, 47.6°C versus 45°C in heaps 10 and 11, respectively). The amount of lactic acid was considerably smaller in the turned fermentations than in the ones that were not turned (2.00 ± 0.02 mg g−1 in the pulp and 0.67 ± 0.01 mg g−1 in the beans of heap 12 and 4.60 ± 0.18 mg g−1 in the pulp and 1.40 ± 0.03 mg g−1 in the beans of heap 13) (data not shown). In heap 13, removal of the placenta may be responsible for the lower maximum fermentation temperature of 42°C, as this promoted more anaerobic conditions due to a better packing of the heap and hence a pronounced growth of LAB and production of lactic acid compared to AAB growth and acetic acid production.
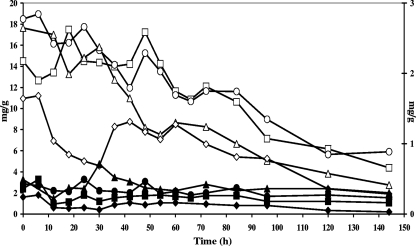
Through the entire fermentation process, the concentrations of caffeine remained approximately the same (±0.3% on dry, fat-free cocoa); the amounts of theobromine and total polyphenols decreased over fermentation time (data not shown). The influences of fermentation time are, however, different for epicatechin and catechin (Fig. 7). Concentrations of catechin barely changed during the course of the fermentation, whereas epicatechin concentrations started from ±15 mg g−1 in the beans and showed a strong decrease after 48 h of fermentation, when the fermenting bean mass reached a temperature of 40°C (data not shown). The concentrations ranged between 1.9 and 5.9 mg g−1 in the beans after 144 h of fermentation (Fig. 7).
FIG. 7.
Course of epicatechin (heap 10 [H10], □; H11, ○; H12, ▵; H13, ⋄) (primary axis) and catechin (H10, ▪; H11, •; H12, ▴; H13, ⧫) (secondary axis) during Ghanaian spontaneous cocoa bean heap fermentations at the farm and factory sites and with the influence of turning.
At the end of the 6-day fermentation period, there was a clear difference in the color of the fermented beans derived from turned heaps (8, 10, and 12) and the color of the ones that were not turned (9, 11, and 13). The fermented beans that were turned showed a uniform, dark brown, fully fermented color. The heaps that were not turned showed a higher percentage of purple beans, and the browning intensity was less (data not shown).
Quality assessment of fermented, dried cocoa beans.
The quality of the fully fermented sun-dried beans was assessed visually by using the cut test. Cocoa beans of good quality were produced in all cases, as the contents of slaty and violet beans were lower than 1% and 20%, respectively, with the remaining beans being judged as brown (data not shown). The cut test further revealed that a turned heap was fermented better after 6 days of fermentation than one that was not turned.
Chocolate production and sensory analysis.
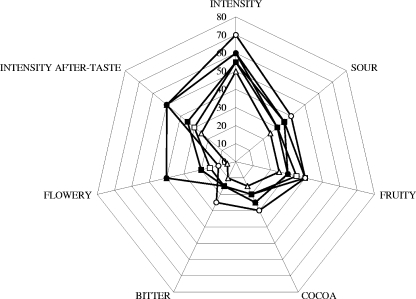
The results obtained showed that the chocolates prepared from beans of heaps 8 to 13 differed in flavor but were not bitter (Fig. 8). The content of polyphenols decreased upon further treatment of the beans. Only one-third of the epicatechin was present after the beans were transformed into cocoa mass. Again, ±50% of the polyphenols present in the cocoa mass was lost by transforming the cocoa mass into chocolate. The catechin concentration was below the detection limit (Table 3).
FIG. 8.
Flavor profiles of the chocolates produced from fermented, dried beans derived from the different heaps: heap 8, □; heap 9, ▪; heap 10, ○; heap 11, •; heap 12, ▵; and heap 13, ▴.
TABLE 3.
Concentrations of total polyphenol content, epicatechin, and catechin found in cocoa liquor and chocolate
| Sample and heap (H) no. from which the beans were derived | Fat (%, wt/wt) | Total polyphenols (%, wt/wt) | Epicatechin (mg g−1) | Catechin (mg g−1) |
|---|---|---|---|---|
| Cocoa liquor | ||||
| H8 | 58.17 | 1.80 | 0.83 | 0.16 |
| H9 | 57.24 | 2.07 | 1.47 | 0.20 |
| H10 | 58.98 | 2.47 | 0.80 | 0.18 |
| H11 | 57.50 | 2.60 | 1.96 | 0.29 |
| H12 | 59.08 | 1.91 | 0.55 | 0.12 |
| H13 | 57.50 | 2.08 | 1.43 | 0.19 |
| Chocolate | ||||
| H8 | 37.53 | 0.53 | 0.35 | <0.09 |
| H9 | 38.21 | 0.59 | 0.61 | <0.09 |
| H10 | 35.10 | 0.26 | 0.46 | <0.09 |
| H11 | 35.25 | 0.75 | 0.80 | 0.13 |
| H12 | 37.87 | 0.36 | 0.21 | <0.09 |
| H13 | 36.09 | 0.42 | 0.32 | <0.09 |
Chocolate produced from beans of heap 10 had a very strong, sour flavor, whereas chocolate from beans of heap 11 did not (Fig. 8). The latter chocolate had a strong flowery taste and aftertaste, while the former did not, indicating the influence of turning. The aftertaste of the chocolate of beans from heap 10 was unpleasant and comparable with that of poorly fermented beans. Chocolate produced from beans of heaps 8, 9, and 12 gave very similar results, except that the aftertastes were different (common, ripe fruit, and woody oak, respectively). The sensory scores for chocolate made from beans of heap 13 were higher for all parameters than those for heaps 8, 9, and 12. The aftertaste of this chocolate could be described as fresh flowers.
DISCUSSION
The spontaneous cocoa bean fermentation process is a very heterogeneous process, with great variations in both microbial counts and metabolite concentrations (2, 5, 37; this study). Variations seem to depend on the type of cocoa, country, farm, pod ripeness, postharvest pod storage and pod diseases, variations in pulp/bean ratio, fermentation method, size of the batch, season and weather conditions, turning frequency or no turning, and fermentation time (37, 45). However, it has recently been shown that fermentation of cocoa beans does not depend on the season or farm but more likely depends on production method, batch size, pod ripeness and storage, and fermentation conditions (5). The present study revealed a slight influence of turning and almost no influence of environmental contamination on the population dynamics of yeasts, LAB, and AAB during spontaneous heap fermentation of cocoa beans, as revealed by culture-dependent and -independent methods for LAB and culture-dependent analysis for yeasts and AAB. The influence of turning was reflected mainly by a boost of AAB growth (cell counts, acetic acid concentration, and maximum fermentation temperature). As most Ghanaian farmers ferment beans for only 3 to 5 days and turn the heap once or not at all and as Ghanaian fermented cocoa is among the best in the world to produce standard chocolate, turning might influence the final quality of the fermented beans.
Bacterial species analysis revealed the presence of mainly L. plantarum and L. fermentum as LAB species and A. pasteurianus, A. ghanensis, and A. senegalensis as AAB species, confirming the restricted biodiversity of both LAB and AAB during Ghanaian spontaneous cocoa bean heap fermentation (5). As it is very well known that isolation of AAB from natural environments is difficult, mainly due to problematic cultivation and preservation (39), four different isolation media (AAM, BME, DMS, and GY agars) were compared during this study. Whereas A. pasteurianus was the major species found on DMS and GY agars, A. ghanensis was the major cluster represented on AAM agar. These data indicate isolation based on acetic acid tolerance (22). Also, Lisdiyanti et al. (29) found a different and wider diversity among AAB from tropical flowers and fruits when using various isolation media. All of these results confirm the importance of culture media used for isolation of AAB (28, 29, 40), probably explaining why a limited number of taxa of AAB have been described up to now, not only in the particular case of spontaneous cocoa bean fermentation but also in the case of (fermented) foods and fruits in general. Therefore, as evidenced by the present study, the combination of at least two different media (for instance, DMS and AAM agars) for the isolation of AAB is recommended for biodiversity studies of AAB. Also, the use of different culture media could lead to an increased isolation of new AAB species (7, 29). Recently, A. ghanensis (5, 6) and A. senegalensis (5, 32) as well as an A. lovaniensis-like species (this study) were isolated from AAM and BME agar media, which were never used before for the isolation of AAB from fermented cocoa bean samples. As 72% of the isolates from BME agar belonged to enterobacterial species, as revealed by partial sequencing of the 16S rRNA gene, this medium is not sufficiently selective for the isolation of AAB. The preferential isolation of Pantoea sp. from this agar medium is reflected by its ability to use ethanol as the sole energy source. Its presence may be ascribed to its ubiquitous occurrence in the environment, in particular, in soil and fruits (12).
The fact that only Acetobacter species were retrieved from the culture media used reflects the availability of only ethanol as the energy source during spontaneous cocoa bean fermentation. Indeed, during cocoa bean fermentation most sugars are depleted by both yeasts and LAB, whereas Gluconobacter sp. prefers glucose as the energy source (22). The latter taxon is isolated mainly from less-fermented Malaysian cocoa bean samples (2). The selective isolation of A. pasteurianus may be explained by its acid and heat resistance and its preferential growth in the presence of lactic acid and mannitol. Moreover, turning of the heaps favored growth of A. pasteurianus, as revealed by culture-dependent analysis. A turned heap reached a higher fermentation temperature than one that was not turned, was fermented faster and more completely, and spread a more pronounced acid smell, and the AAB population (dominated by A. pasteurianus) reached a higher total cell count. This indicates that turning aerates the fermenting mass quite well and favors A. pasteurianus growth and metabolism, this species being more competitive in an acid- and ethanol-rich environment. This may be due to poor or no growth of L. plantarum under these conditions and common yeast activities at the onset of fermentation, i.e., production of ethanol necessary as the energy source for AAB and depectinization to allow aeration of the heap.
L. plantarum and L. fermentum were present in the fermentations carried out at the farm, while L. fermentum was the only LAB present in the factory fermentations. Turning of the heap had no clear influence on the number of LAB present during fermentation, but it seemed to favor growth of L. fermentum compared to L. plantarum, as revealed by 16S rRNA PCR-DGGE. Also, cluster analysis of the PCR-DGGE profiles of LAB showed that early contaminations of the heaps at the factory and farm were different. However, it was interesting to see that the environmental factor had no clear influence on the natural fermentation course, either on the species diversity or on the population dynamics. This indicates that the inoculum for the sterile bean mass depends on the direct environment (the farmers' plantations), i.e., the surfaces of the cocoa pods and the baskets used (5, 35). This means that care has to be taken at the plantations, as a plantation that is not maintained well may lead to bad cocoa fruits (small fruits with less pulp), diseased cocoa pods (black pods), and thus an inappropriate microbial mix present on the pod surface that eventually starts the cocoa bean fermentation, hence leading to a badly fermented end product.
Further, turning and environment influenced eventual mannitol production, which was lower with turning and higher during the factory fermentation than during the farm fermentation. LAB may use alternative external electron acceptors, such as fructose and oxygen, during fermentation (3). Whereas turning favors the use of oxygen as an alternative external electron acceptor by LAB instead of fructose, which is converted into mannitol, the bigger influence of the environmental factor may be ascribed to different L. fermentum strains, which differed in mannitol production capacity, involved in the factory fermentation. Turning and environment displayed a clear influence on the course and amounts of ethanol during fermentation. The influence of environment could be due to different and/or fewer yeasts that were present during the fermentations performed at the factory, as the pod contents were stored transiently in cleaned baskets. The ethanol in the turned fermentations disappeared more rapidly and was almost completely gone at the end of fermentation, due to better aeration of the fermenting mass (faster evaporation of ethanol and rapid ethanol oxidation by AAB). The amount of acetic acid that was produced in a turned heap was much larger, the amount of lactic acid produced was significantly smaller, and the maximum fermentation temperature was higher than those for a heap that was not turned during fermentation. Chocolate made from such beans tasted very sour. Turning of the heaps and the environmental factor had no clear influence on the concentrations of alkaloids and polyphenols in the beans. A general trend of decreasing concentrations over fermentation time was observed. However, polyphenol concentrations decreased faster from the moment the fermentation temperature was higher than 40°C, which is the optimal temperature for polyphenol oxidase activity (44). As losses of polyphenols proceeded upon chocolate production, bitterness of the final chocolates was absent. All of these results show that turning and environment may influence the resulting fermented cocoa beans and hence the taste of chocolate.
To summarize, turning of heaps accelerates fermentation speed and favors AAB growth but does not necessarily favor the flavor of the fermented beans and the chocolate made thereof, at least for small heaps. It is obvious that turning is necessary for large heaps to get a more homogeneous fermentation throughout the heap and to prevent excessive mold growth at the surface of the cocoa bean mass. Finally, whereas environment does not influence the fermentation course of the cocoa beans significantly, the initial nature and size of the inoculum could be of great importance to control the fermentation process and to steer chocolate flavor.
Acknowledgments
This research was funded by the Research Council of the Vrije Universiteit Brussel (OZR, GOA, and IOF projects), the Fund for Scientific Research-Flanders, the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT projects 040043 and 050818), the Federal Research Policy (Action for the Promotion of and Cooperation with the Belgian Coordinated Collections of Microorganisms, BCCM), and Barry Callebaut N.V.
The cooperation of the Ghanaian Cocoa Producers' Alliance (COCOBOD, Accra, Ghana) and the Cocoa Research Institute of Ghana is highly appreciated. Approval was obtained by COCOBOD to cooperate with local farmers.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Anonymous. 2006. ICCO annual report for 2004/2005. International Cocoa Organization, London, United Kingdom. http://www.icco.org.
- 2.Ardhana, M., and G. Fleet. 2003. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 86:87-99. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson, L. 1998. Lactic acid bacteria: classification and physiology, p. 1-72. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria: microbiology and functional aspects. Marcel Dekker, Inc., New York, NY.
- 4.Baker, D. M., K. I. Tomlins, and C. Gray. 1994. Survey of Ghanaian cocoa farmer fermentation practices and their influence on cocoa flavour. Food Chem. 51:425-431. [Google Scholar]
- 5.Camu, N., T. De Winter, K. Verbrugghe, I. Cleenwerck, P. Vandamme, J. S. Takrama, M. Vancanneyt, and L. De Vuyst. 2007. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 73:1809-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleenwerck, I., N. Camu, K. Engelbeen, T. De Winter, K. Vandemeulebroecke, P. De Vos, and L. De Vuyst. 2007. Acetobacter ghanensis sp. nov., a novel acetic acid bacterium isolated from traditional heap fermentations of Ghanaian cocoa beans. Int. J. Syst. Evol. Microbiol. 57:1647-1652. [DOI] [PubMed] [Google Scholar]
- 7.Cleenwerck, I., K. Vandemeulebroecke, D. Janssens, and J. Swings. 2002. Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int. J. Syst. Evol. Microbiol. 52:1551-1558. [DOI] [PubMed] [Google Scholar]
- 8.de Brito, E. S., N. H. Pezoa García, M. I. Gallão, A. L. Cortelazzo, P. S. Fevereiro, and M. R. Braga. 2000. Structural and chemical changes in cocoa (Theobroma cacao L) during fermentation, drying and roasting. J. Sci. Food Agric. 81:281-288. [Google Scholar]
- 9.De Vuyst, L., N. Camu, T. De Winter, K. Vandemeulebroecke, V. Van de Perre, M. Vancanneyt, P. De Vos, and I. Cleenwerck. 4 September 2007. Validation of the (GTG)5-rep-PCR fingerprinting technique for rapid classification and identification of acetic acid bacteria, with a focus on isolates from Ghanaian fermented cocoa beans. Int. J. Food Microbiol. doi: 10.1016/j.ijfoodmicro.2007.02.030. [DOI] [PubMed]
- 10.Duncan, J. 1984. A survey of Ghanaian cocoa farmers fermentation and drying practices and their implication for Malaysian practices. Int. Conf. Cocoa Coconuts. Society of Planters, Kuala Lumpur, Malaysia.
- 11.EEC. 1990. Official journal of the European Communities, p. 178-179. European Commission, Brussels, Belgium.
- 12.Greenwood, D., R. C. B. Slack, and J. F. Reutherer. 1997. Medical microbiology. A guide to microbial infections: pathogenesis, immunity, laboratory diagnosis and control, 15th ed. Churchill Livingstone, London, United Kingdom.
- 13.Guiraud, J. P. 1998. Microbiologie alimentaire. Technique et Ingénierie, Série Agro-Alimentaire. Dunod, Paris, France.
- 14.Hansen, C. E., M. del Olmo, and C. Burri. 1998. Enzyme activities in cocoa beans during fermentation. J. Sci. Food Agric. 77:273-281. [Google Scholar]
- 15.Hashim, P., J. Selamat, S. K. S. Muhammad, and A. Ali. 1998. Changes in free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. J. Sci. Food Agric. 78:535-542. [Google Scholar]
- 16.Hashim, P., J. Selamat, S. K. S. Muhammad, and A. Ali. 1998. Effect of mass and turning time on free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. J. Sci. Food Agric. 78:543-550. [Google Scholar]
- 17.Holm, C. S., J. W. Aston, and K. Douglas. 1993. The effects of the organic acids in cocoa on flavour of chocolate. J. Sci. Food Agric. 61:65-71. [Google Scholar]
- 18.Jespersen, L., D. S. Nielsen, S. Hønholt, and M. Jakobsen. 2005. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res. 5:441-453. [DOI] [PubMed] [Google Scholar]
- 19.Jinap, M. S., B. Jamilah, and S. Nazamid. 2003. Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. Int. J. Food Sci. Technol. 38:285-295. [Google Scholar]
- 20.Jinap, S., and A. Zeslinda. 1995. Influence of organic acids on flavour perception of Malaysian and Ghanian cocoa beans. J. Food Sci. Technol. 32:153-155. [Google Scholar]
- 21.Jinap, S., P. S. Dimick, and R. Hollender. 1995. Flavour evaluation of chocolate formulated from cocoa beans from different countries. Food Control 6:105-110. [Google Scholar]
- 22.Kersters, K., P. Lisdiyanti, K. Komagata, and J. Swings. 2006. The family Acetobacteraceae: the genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter and Kozakia, p. 163-200. In M. Dworkin, S. Falkow, E. Rosenberg, K.-M. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, 3rd ed. Springer, New York, NY.
- 23.Kirchhoff, P. M., B. Biehl, H. Ziegeler-Berghausen, M. Hammoor, and R. Lieberei. 1989. Kinetics of the formation of free amino acids in cocoa seeds during fermentation. Food Chem. 34:161-179. [Google Scholar]
- 24.Knapp, A. W. 1934. Cocoa fermentations in West Africa. J. Soc. Chem. Ind. 53:151. [Google Scholar]
- 25.Kreiser, W. R., and R. A. Martin. 1978. High pressure liquid chromatographic determination of theobromine and caffeine in cocoa and chocolate products. J. Assoc. Off. Anal. Chem. 61:1424-1427. [PubMed] [Google Scholar]
- 26.Lagunes Galvez, S., G. Loiseau, J. L. Paredes, M. Barel, and J.-P. Guiraud. 2007. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 114:124-130. [DOI] [PubMed] [Google Scholar]
- 27.Lehrian, D. W., and G. R. Patterson. 1983. Cocoa fermentation, p. 529-575. In G. Reed (ed.), Biotechnology, a comprehensive treatise, vol. 5. Verlag Chemie, Basel, Switzerland. [Google Scholar]
- 28.Lisdiyanti, P., H. Kawasaki, T. Seki, Y. Yamada, T. Uchimura, and K. Komagata. 2000. Systematic study of the genus Acetobacter with descriptions of Acetobacter indonesiensis sp. nov., Acetobacter tropicalis sp. nov., Acetobacter orleanensis (Henneberg 1906) comb. nov., Acetobacter lovaniensis (Frateur 1950) comb. nov., and Acetobacter estunensis (Carr 1958) comb. nov. J. Gen. Appl. Microbiol. 46:147-165. [DOI] [PubMed] [Google Scholar]
- 29.Lisdiyanti, P., K. Katsura, W. Potacharoen, R. R. Navarro, Y. Yamada, T. Uchimura, and K. Komagata. 2003. Diversity of acetic acid bacteria in Indonesia, Thailand, and the Philippines. Microbiol. Cult. Collect. 19:91-98. [Google Scholar]
- 30.Lopez, A. S., and P. S. Dimick. 1995. Cocoa fermentation, p. 561-577. In G. Reed and T. W. Nagodawithana (ed.), Enzymes, biomass, food and feed, 2nd ed. VCH, Weinheim, Germany.
- 31.Naser, S. M., F. L. Thompson, B. Hoste, D. Gevers, P. Dawyndt, M. Vancanneyt, and J. Swings. 2005. Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 151:2141-2150. [DOI] [PubMed] [Google Scholar]
- 32.Ndoye, B., I. Cleenwerck, K. Engelbeen, R. Dubois-Dauphin, A. T. Guiro, S. Van Trappen, A. Willems, and P. Thonart. 2007. Acetobacter senegalensis sp. nov., a thermotolerant acetic acid bacterium isolated in Senegal (sub-Saharan Africa) from mango fruit (Mangifera indica L). Int. J. Syst. Evol. Microbiol. 57:1576-1581. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen, D. S., S. Hønholt, K. Tano-Debrah, and L. Jespersen. 2005. Yeast populations associated with Ghanaian cocoa fermentations analysed using denaturing gradient gel electrophoresis (DGGE). Yeast 22:271-284. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen, D. S., O. D. Teniola, L. Ban-Koffi, M. Owusu, T. S. Andersson, and W. H. Holzapfel. 2007. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 114:168-186. [DOI] [PubMed] [Google Scholar]
- 35.Ostovar, K., and P. G. Keeney. 1973. Isolation and characterization of microorganisms involved in the fermentation of Trinidad's cacao beans. J. Food Sci. 38:611-617. [Google Scholar]
- 36.Schwan, R. F. 1998. Cocoa fermentations conducted with a defined microbial cocktail inoculum. Appl. Environ. Microbiol. 64:1477-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan, R. F., and A. E. Wheals. 2004. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 44:205-221. [DOI] [PubMed] [Google Scholar]
- 38.Schwan, R. F., A. H. Rose, and R. G. Board. 1995. Microbial fermentation of cocoa beans, with emphasis on enzymatic degradation of the pulp. J. Appl. Bacteriol. Symp. Suppl. 79:96S-107S. [Google Scholar]
- 39.Sievers, M., C. Gaberthüel, C. Boesch, W. Ludwig, and M. Teuber. 1995. Phylogenetic position of Gluconobacter species as a coherent cluster separated from all Acetobacter species on the basis of 16S ribosomal RNA sequences. FEMS Microbiol. Lett. 126:123-126. [DOI] [PubMed] [Google Scholar]
- 40.Sokollek, S. J., and W. P. Hammes. 1997. Description of a starter culture preparation for vinegar fermentation. Syst. Appl. Microbiol. 20:481-491. [Google Scholar]
- 41.Thompson, S. S., K. B. Miller, and A. S. Lopez. 2001. Cocoa and coffee, p. 721-733. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC.
- 42.Voigt, J., H. Heinrichs, G. Voigt, and B. Biehl. 1994. Cocoa-specific aroma precursors are generated by proteolytic digestion of the vicilin-like globulin of cocoa seeds. Food Chem. 50:177-184. [Google Scholar]
- 43.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollgast, J., and E. Anklam. 2000. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 33:423-447. [Google Scholar]
- 45.Wood, G. A. R., and R. A. Lass. 1985. Cocoa, 4th ed. Longman Group Limited, London, United Kingdom.