Abstract
Treponema denticola, a periodontal pathogen, has recently been shown to exhibit properties of a facultative anaerobic spirochete, in contrast to its previous recognition as an obligate anaerobic bacterium. In this study, the capacity and possible mechanism of T. denticola survival and growth under aerobic conditions were investigated. Factors detrimental to the growth of T. denticola ATCC 33405, such as oxygen concentration and hydrogen sulfide (H2S) levels as well as the enzyme activities of gamma-glutamyltransferase, cysteinylglycinase, and cystalysin associated with the cells were monitored. The results demonstrated that T. denticola grew only at deeper levels of broth (≥3 ml in a 10-ml tube), high inoculation ratios (≥20% of culture in medium), and short cultivation times (≤4 days for one passage) and in media containing l-cysteine or glutathione as the substrate for H2S production during aerobic growth. The determination of the factors showed that oxygen levels were always lower (0 to 0.6%) with significantly higher concentrations of H2S and higher activities of the three enzymes in all cultures grown aerobically. Further data revealed that H2S production from the T. denticola enzymes plus their substrates resulted in removal of dissolved O2 in the growth cultures in a dose-dependent manner. These results demonstrated that T. denticola was able to generate microanaerobic environments in growth media for its survival and growth under aerobic conditions. Furthermore, the organism can be defined as a true obligate anaerobic spirochete. These findings suggest that spirochetes may play a significant role in maintaining the anaerobic environment at diseased sites in periodontitis.
Oral spirochetes have been strongly implicated as part of the periodontopathic microbiota. They are present as the predominant organisms associated with the progression of periodontal diseases. Treponema denticola, a small cultivable oral spirochete, is frequently isolated from periodontal pockets (27, 43). Using in vitro studies, this spirochete was shown to express a variety of potential virulence factors, i.e., adhesins, proteinases, cytotoxic factors, chemotaxis, and peptidases as well as other virulence factors (15, 20, 21, 23, 24).
In long-term studies of periodontal diseases, two types of gaseous properties were detected. First, low oxygen levels were observed in periodontal pockets. Since periodontal pockets are generally considered to be highly anaerobic, anaerobes compose the major flora, especially in deep periodontal pockets. T. denticola was classified as an obligate anaerobe which cannot grow in the presence of atmospheric oxygen because it initiated growth only when there was less than 0.5% oxygen in the cultures (16, 25). The predominance of anaerobic bacteria in subgingival plaque samples suggests that the pocket environment is anaerobic. Mettraux et al. (26, 30) reported that the oxygen tension (pO2) at the bottom of pockets ranged from 5 to 27 mm Hg, with a mean value of 13.3 mm Hg, and the oxygen sensitivities of resident organisms coincided with their proportional distribution in pockets at various pO2s and depths. Deep pockets have a lower pO2 than moderate pockets (7 to 10 mm/12.0 mm Hg versus 5 to 6 mm/15.7 mm Hg) and contain higher proportions of spirochetes.
In addition to oxygen differences, volatile thiol compounds were found in all periodontal pockets deeper than 3 mm and most often in the deepest regions of these pockets. Hydrogen sulfide (H2S) was predominant among these thiol compounds at elevated concentrations of up to 2 mM (22, 34, 37, 41) in the pockets associated with periodontitis. Several reports have also indicated that some oral bacteria, including spirochetes, can produce H2S from the metabolism of human serum proteins, l-cysteine, glutathione, and other sulfur-containing compounds (7, 8, 13, 38, 39), all of which are available in periodontal pockets (1, 52). Only low levels of l-cysteine (around 0.08 mM) are present in infected periodontal pockets. However, since polymorphonuclear leukocytes contain up to 4 mM of glutathione and significant amounts of glutathione may be released when polymorphonuclear leukocytes are damaged in the pockets, the tripeptide may be the major substrate for hydrogen sulfide formation in the pockets (3, 7, 32, 36). Despite these observations, the metabolic pathway leading to H2S production from glutathione in periodontal pockets has not been determined. T. denticola resides at the bottom of deep pockets which contain high levels of H2S and low pO2. Nevertheless, the relationship between the two gases in the pockets is unknown. H2S has been shown to be cytotoxic for a variety of host cells, such as gingival fibroblasts, epithelial cells, and HeLa cells (2, 9, 19, 29, 34, 35, 40, 49). Whether this microbial metabolite possesses other etiological and pathogenic properties in periodontal pockets still remains to be determined.
The strict anaerobe T. denticola was found to be capable of growing in aerobic gaseous environment (31, 47). Therefore, the spirochete has recently been described as a facultative anaerobic spirochete, which can grow with and without oxygen (4), in contrast to its earlier classification as an anaerobic bacterium (44, 45). Since T. denticola did not grow with the use of normal cultivation procedures aerobically, the more recent observations that the spirochete can grow under aerobic conditions may need to be reevaluated in relation to the contributions of this organism to pathogenicity in periodontal pockets. While evidence for T. denticola being able to grow under aerobic conditions was reported, neither the oxygen nor the H2S concentrations in the cultures were documented in those studies.
Recently, we have described a metabolic pathway for H2S production from glutathione as a substrate in T. denticola (8). We reasoned that investigations regarding the two gases, oxygen and H2S, in in vitro cultures and in in vivo periodontal pockets are needed to further define pathogenicity in periodontal diseases. In the present study, we have investigated the capacity of T. denticola to survive and grow under conditional aerobic conditions as well as a likely mechanism to explain this property.
MATERIALS AND METHODS
Materials.
Unless otherwise indicated, all chemicals and reagents were obtained from Sigma Chemical Company, St. Louis, MO.
Bacterial strains and culture conditions.
T. denticola ATCC 35405, ATCC 35404, and ATCC 33520 and Treponema vincentii ATCC 35580 were obtained from the American Type Culture Collection (Manassas, VA). T. denticola GM-1 was isolated from human periodontal pockets (33, 51). All strains were stored at −78 °C in 15% glycerol medium. For normal cultivation, the spirochetes were grown in GM-1 medium (51), which contains 2% heat-inactivated rabbit serum and 6 mM l-cysteine, in screw-cap tubes (120 by 9 to 10 mm). The bacteria were incubated either anaerobically in a Coy anaerobic chamber (5% CO2, 10% H2, and 85% N2) or aerobically in a 37°C warm room for the times appropriate for the experimental design. The culture purity was checked by dark-field microscopy at a magnification of ×400. T. denticola reference strain 35405 was used for the majority of the analyses.
Determination of bacterial growth.
To determine the effects of various factors on bacterial growth, 1-day cultures, whose optical density at a wavelength of 660 nm (OD660) was approximately 0.16 (about 3 × 108 cells/ml), were inoculated into complete or basic GM-1 medium (GM-1 broth without l-cysteine) at the indicated volumes (mostly 6 ml) and starting concentrations (mostly an OD660 of 0.032). Each of the supplements was added at a final concentration of 6 mM to the broth. The spirochetes were grown routinely in static culture at 37°C, and the growth rate was determined spectrophotometrically by OD660 with a Beckman DU-65 spectrophotometer at different times.
Determination of dissolved oxygen.
Oxygen levels in the cultures were assessed by using a SM600 dissolved oxygen meter (Milwaukee Instruments, Inc.). Based on the manufacturer's instructions, the electrode was calibrated to zero before every measurement and the concentration of oxygen in each sample was measured at room temperature.
Hydrogen sulfide detection.
Spent growth supernatant harvested from bacterial cultures by centrifugation was used for samples. Hydrogen sulfide production in the cultures was quantified as described by Siegel (42) with some modifications. Briefly, 3 μl of 0.02 M N,N-dimethyl-p-phenylenediamine sulfate in 7.2 N HCl and 3 μl of 0.3 M FeCl3 in 1.2 N HCl were added sequentially to 54 μl of spent growth supernatant in a 1.5-ml microcentrifuge tube. The reaction mixture was mixed thoroughly by vortexing and sealed with Parafilm. The absorbance at 620 nm was determined after color development for 30 min at room temperature. The sulfide concentration was determined from an Na2S standard curve. All analyses were carried out in triplicate unless otherwise indicated.
Standard curve for H2S.
The method of Carlsson et al. (14) was used to determine H2S production. A 200 mM concentration of disodium sulfide (Na2S) was stored at −20°C as a stock solution. H2S was produced by neutralizing disodium sulfide in phosphate-buffered saline (PBS) or GM-1 medium to pH 7.4 with the same molarity of HCl. Production of H2S in the reactions was determined by chemical methods as described above.
Determination of enzymatic activities.
The synthetic substrates for the measurement of gamma-glutamyltransferase (GGT), cysteinylglycinase (CGase), and cystalysin activities were Na-γ-glutamyl-4-nitroaniline, Cys-Gly, and l-cysteine, respectively. These substrates represent the means to determine the three peptidase activities involved in a stepwise enzymatic pathway previously characterized in our laboratory for T. denticola (6). Spirochete cells were harvested from 1-day cultures by centrifugation at 6,000 × g for 10 min, washed twice with 0.1 M PBS (pH 7.0), and resuspended in the same buffer to an OD660 of 6.0. In the controls the spirochete suspension was replaced by PBS. All related measurements were carried out in triplicate.
GGT was assayed by the method of Makinen and Makinen (28) with minor modifications. The reaction mixture contained 44 μl of 0.1 M PBS at pH 7.0 and 6 μl of 20 mM Na-γ-glutamyl-4-nitroaniline in a 1.5-ml microcentrifuge tube. The reaction was initiated by the addition of substrate, and the mixture was incubated at 37 °C for 1 h. Absorption at 405 nm was monitored in a Multiskan MCC (Fisher Scientific).
CGase activity was measured by a modification of the procedures of Cappiello et al. (6) based on the specific reaction between cysteine and ninhydrin (46). The reaction mixture (final volume, 80 μl) contained 0.4 mM MnCl2, 10 mM dithiothreitol, and 2 mM Cys-Gly in 50 mM Tris-HCI buffer (pH 7.3). The reaction was started by the addition of a 12-μl aliquot of washed spirochetes. After incubation for 1 hour at 37°C, the reaction was stopped by adding 5% trichloroacetic acid and the incubation mixture was centrifuged at 12,000 × g for 1 min. Supernatant (50 μl) was added to 50 μl of glacial acetic acid and 50 μl of a reagent prepared by dissolving 250 mg of ninhydrin in 10 ml of acetic acid-0.6 M phosphoric acid (3:2). The mixture was placed in a boiling bath for 10 min and then cooled on ice. After cooling, 150 μl of the mixture was diluted with 0.3 ml of 95% ethanol and used for spectrophotometric measurements at 540 nm.
Cystalysin (cysteine desulfhydrase) activity was determined by the colorimetric assay described by Chu et al. (10) with a reaction mixture consisting of 47.6 μl of 0.1 M PBS at pH 7.0, 10-μl aliquots of washed spirochetes, and 2.4 μl of 50 mM l-cysteine. The mixture was incubated at 37 °C for 1 h. The end product H2S was determined by the chemical assay described above.
Specific experimental design and methods. (i) Effect of inoculum concentrations on cell growth.
As starting cultures, T. denticola cultures at an OD660 of 0.16 were inoculated into GM-1 medium at the indicated concentrations in 6-ml volumes for 72 h of incubation at 37°C. The growth yield of the cells was determined for comparison between anaerobic and aerobic gaseous conditions.
(ii) Effect of culture depth on the proliferation of the spirochete.
The 1-day cultures of T. denticola 35405 were diluted to an OD 660 of 0.032 with GM-1 medium and then placed in tubes measuring 9 to 10 mm by 120 mm. Volumes of 1, 2, 3, 6, and 10 ml yielded liquid column heights of about 0.6, 1.5, 2.3, 5.3, and 7.1 cm, respectively. The growth yield was determined every 24 h or 72 h of incubation at 37°C in an anaerobic chamber or under aerobic conditions (warm room), and these cultures were passaged at 30% inoculation densities as indicated.
(iii) Correlation between growth yields, H2S production, and O2 reduction in T. denticola cultures.
As starting cultures, T. denticola cultures at an OD660 of 0.16 were inoculated into GM-1 medium at the indicated final concentrations in 6-ml volumes for 24 h of incubation at 37 °C.
(iv) Kinetics of H2S formation and oxygen reduction in T. denticola cultures.
This experiment was to test the kinetic changes of H2S production and oxygen reduction after the addition of T. denticola cells into the medium. Before the experiment, the GM-1 medium was stored under normal room conditions, i.e., air-saturated medium with an initial O2 concentration of 4.6 mg/liter (5.8% O2). One-day 20% (culture volume/medium volume) cultures of T. denticola were added to the medium to an OD660 of 0.04 (final concentration) and cultivated under aerobic conditions. Samples were taken at the indicated incubation times, and the concentrations of H2S and O2 were determined.
(v) Hydrogen sulfide addition and oxygen removal in the medium.
In this experiment, sodium sulfide (Na2S) was used to generate H2S in the culture medium, since Na2S will be converted to H2S at neutral pH. The same molarity of Na2S and HCl at the indicated concentrations (0, 0.0625, 0.125, 0.25, 0.5, 1, 2, and 4 mM) was added to 6-ml volumes of GM-1 medium. After 30 min of incubation, O2 levels were determined versus H2S concentrations; kinetic analysis of O2 reduction with the addition of 2 mM Na2S in the media at various times was performed by determining the concentrations of oxygen in the medium.
(vi) Effects of S-containing compounds and substrates of cystalysin on the growth of T. denticola and H2S production under aerobic conditions.
Basic GM-1 medium without l-cysteine (including all other medium compounds and supplements) was used in the experiment. Each of the selected S- or thiol-containing compounds (cystathionine, sodium sulfate, glutathione, l-cysteine, and d-cysteine) and other compounds as substrates of cystalysin, including alanine, was used to replace l-cysteine as a supplement in the basic GM-1 medium. T. denticola was inoculated into the basic GM-1 medium separately containing each of the compounds at a concentration of 6 mM under aerobic and anaerobic conditions. After 2 days of aerobic incubation, the cell growth and H2S formation were determined for each sample.
Statistical analysis.
One-way analysis of variance and the Student-Newman-Keuls test were used to examine differences between groups. The level of statistical significance was a P value of <0.05.
RESULTS
Effect of inoculum concentration on cell growth.
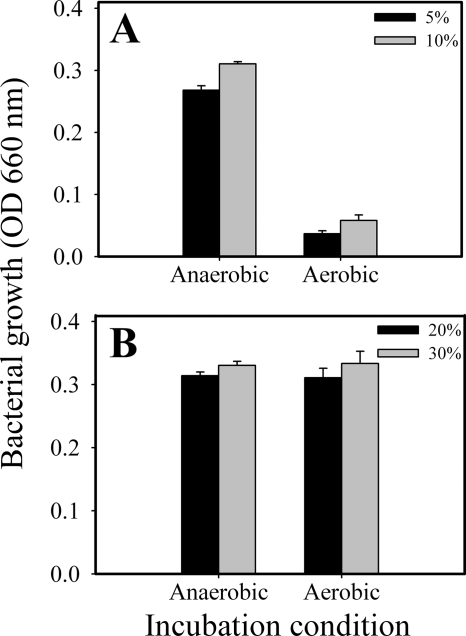
The growth yields of the cells determined with various inoculum concentrations under both anaerobic and aerobic gaseous conditions are shown in Fig. 1. As predicted, while the cultivation results demonstrated that the organisms were able to grow at all tested inoculation rates in the anaerobic chamber with an OD660 of at least 0.25 (Fig. 1A and B), the spirochete failed to grow (OD660 of <0.08) with the regular (routine) inocula (≤10% inoculation [culture volume/broth volume]) under aerobic conditions (Fig. 1A). These results showed that T. denticola was not like other facultatively anaerobic organisms and could not grow under aerobic conditions. However, the organism was able to grow well under aerobic conditions when the inoculum density was increased to ≥20% (Fig. 1B). These results indicated that T. denticola was able to conditionally grow in an aerobic environment and that the inoculum densities were important for this property. If the inoculum density was sufficient to support its initial survival and proliferation, the gaseous environments had less effect on the growth yields. The ODs of the cultures grown under aerobic conditions were similar to those of cultures grown under anaerobic conditions. The microscopic morphologies and motilities of the organisms were comparable (data not shown).
FIG. 1.
Effect of inoculum concentration on cell growth under both anaerobic and aerobic gasses with regular (routine) (A) and conditional (B) inoculation. T. denticola cultures with an initial OD660 of 0.16 were inoculated into GM-1 medium under the indicated conditions in 6-ml volumes for 72 h of incubation. The regular (routine) inoculum (A) represents ≤10% inoculation (culture volume/broth volume), and the conditional inoculum (B) represents ≥20% inoculation (culture volume/broth volume). Error bars indicate standard deviations.
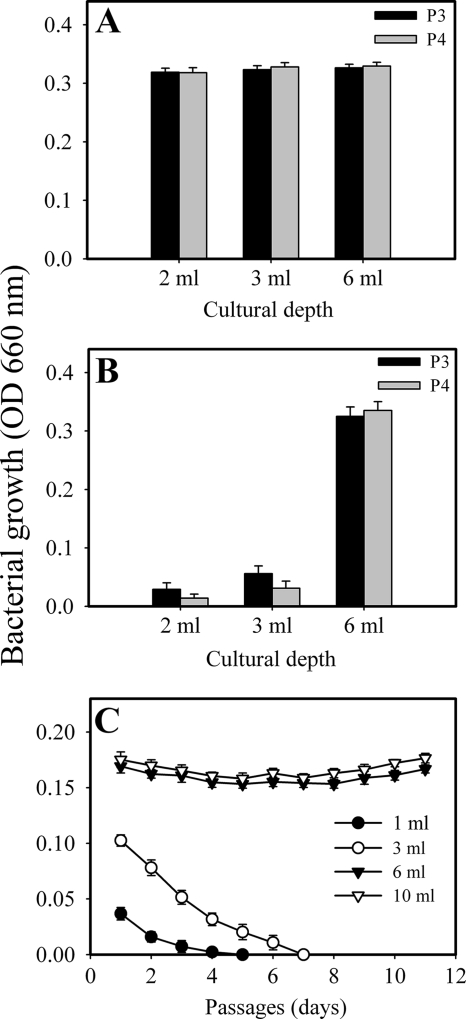
Effect of culture depth on proliferation of the spirochete.
The results showed that the organism grew and could be passaged at all culture depths in the anaerobic chamber (Fig. 2A). Under aerobic conditions, the organism can be continuously transferred over 11 passages at ≥6 ml of broth depth. However, the spirochete survived and grew for only a few passages at ≤3 ml of broth depth and dramatically failed to grow in 1 ml of broth depth (Fig. 2B and C). These results clearly show that the depth of the cultures significantly influenced the ability of the spirochete to grow under aerobic conditions and indicated that T. denticola only conditionally grew aerobically at appropriate broth depths.
FIG. 2.
Effect of broth depth on cell proliferation under both anaerobic (A) and aerobic (B and C) conditions. T. denticola cultures at a final OD660 of 0.032 were placed in tubes at the indicated volumes. The growth yield was determined every 72 h (A and B) or every 24 h (C), and the bacteria were passaged as indicated (A and B) or 30% (C). Panel C represents the growth following continuous inoculation. Error bars indicate standard deviations.
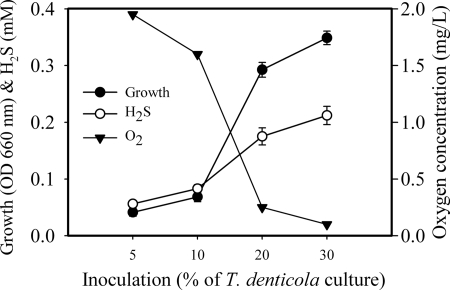
Correlation between growth yield, H2S production, and O2 reduction in T. denticola cultures under aerobic conditions.
To address the question of why T. denticola was able to grow only at higher inoculum densities and in deeper cultures, potential correlations between growth yield, H2S production, and O2 reduction in T. denticola cultures under aerobic conditions were examined. As seen in Fig. 3, hydrogen sulfide generation was significantly increased with significant growth of T. denticola. No significant levels of H2S were determined in poorly growing cultures with regular inocula equal to or less than 10% (OD660 = 0.016) under aerobic conditions. However, the dissolved H2S concentrations were determined to be over 0.2 mM when the spirochete grew well (with an OD660 of more than 0.3). Interestingly, in contrast to the changes in H2S, the dissolved oxygen levels were significantly decreased with increased cellular growth. When the organism grew very poorly, oxygen concentrations of more than 1.6 mg/liter (2% O2) were detected, which is about 85% of that in control media without bacteria (data not shown) and simulates microaerophilic conditions (31). In actively growing cultures, the oxygen concentrations dramatically dropped to less than 0.25 mg/liter (0.3% O2), which is less than 10% of that of air-saturated water (data not shown) or the oxygen levels in an anaerobic jar (25). These results indicated that cell growth yields were positively correlated with H2S production and negatively correlated with O2 concentration during aerobic cultivation. It is further suggested that microanaerobic environments can be generated in T. denticola cultures under aerobic conditions.
FIG. 3.
Correlation between growth rate, H2S production, and O2 reduction in cultures of T. denticola under aerobic condition. T. denticola cultures at a starting OD660 of 0.16 were added to GM-1 medium at the indicated concentrations in 6-ml volumes for 72 h of incubation. Data are shown for bacterial growth and H2S and O2 levels in the cultures. Error bars indicate standard deviations.
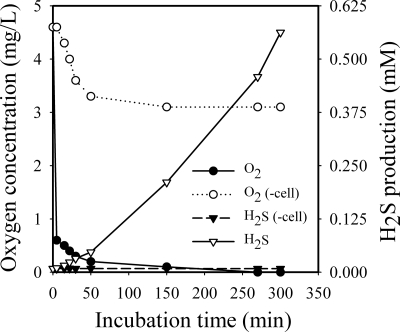
Kinetics of H2S formation and oxygen reduction in T. denticola cultures.
To understand whether H2S production from T. denticola cells will result in a reduction of oxygen in the growth cultures, H2S and oxygen levels in the culture medium were determined after the addition of T. denticola compared to broth alone. The results presented in Fig. 4 show the changes of O2 and H2S levels in cell-free medium versus medium containing bacteria. As indicated (Fig. 4), the O2 levels fell sharply in the initial a few minutes and were lowest over 150 min. The O2 levels decreased to less than 0.2 mg/liter (0.25% O2) versus about 3.1 mg/liter (3.9% O2) for medium without cells. On the other hand, a significant rise in H2S levels in the fresh cultures was observed at 15 min after the addition of T. denticola cells, and a maximum concentration of about 0.5 mmol/liter was determined at 5.5 h. No measurable H2S was observed either in the medium without T. denticola or in the medium without l-cysteine and with T. denticola (data not shown). These results strongly suggest that the spirochete itself or H2S produced by the organism from its substrate l-cysteine, or both, had the ability to remove almost all of the O2 from the cultures.
FIG. 4.
Kinetic analysis of H2S production and O2 reduction in T. denticola aerobic cultivations. T. denticola cultures (OD660 = 0.048, final concentration) were cultivated under aerobic conditions. After 0, 5, 15, 22,30, 50, 150, 270, and 300 min of incubation, the changes in O2 levels were determined for cell-free medium (○) and medium plus bacteria (•). Changes in H2S levels are shown for medium without cells (▾) and for medium with cells (▿).
Hydrogen sulfide addition and oxygen reduction in the medium.
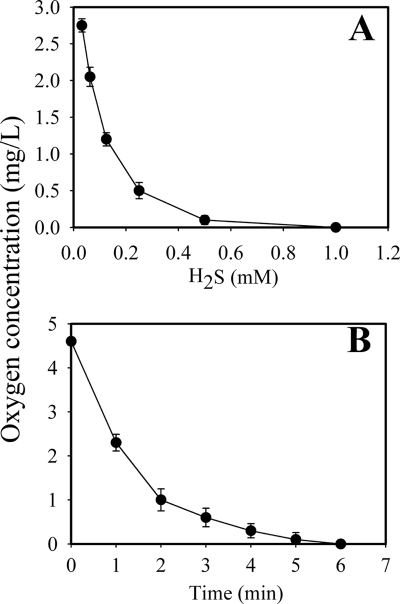
In order to directly determine whether or not H2S was capable of removing O2 from aerobic cultures, oxygen reduction was determined following the addition of equal molar concentrations of Na2S and HCl. The addition of 0.5 mM Na2S/H2S was able to remove 27.0 ng oxygen from GM-1 medium; i.e., this caused complete removal of oxygen (98%) (Fig. 5A). When 2 mM Na2S plus HCl was added to the culture medium, H2S dramatically reduced oxygen levels to undetectable within 6 min (Fig. 5B). These results strongly indicated that the concentrations of H2S alone were sufficient to rapidly remove O2 almost completely from the culture medium and resulted in the formation of an anaerobic environment in the medium.
FIG. 5.
Demonstration of oxygen removal by the addition of H2S in GM-1 medium (A) and kinetic analysis of O2 reduction with the addition of H2S (B). Various concentrations of H2S (Na2S and HCl at the same molarity) were separately added to 6-ml volumes of GM-1 medium in tubes. After 30 min of incubation, O2 levels were determined versus H2S concentrations (A). Kinetics of O2 reduction were assayed following the addition of 2 mM H2S (B). Error bars indicate standard deviations.
Effects of S-containing compounds and substrates of cystalysin on the growth of T. denticola and H2S production under aerobic conditions.
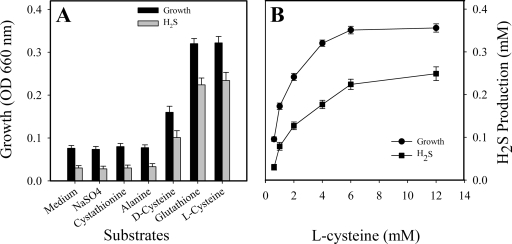
We have described an enzyme pathway for H2S production from glutathione, Cys-Gly, and l-cysteine as substrates (8, 12). To further investigate the possible mechanism for the ability of T. denticola to grow under aerobic conditions, some sulfur-containing compounds and substrates of cystalysin were selected for the replacement of l-cysteine as a supplement in GM-1 medium for supporting T. denticola growth under aerobic conditions. The organism grew well in the presence of l-cysteine or glutathione, and such growth was accompanied by significant levels of H2S (P < 0.01) (Fig. 6A). In contrast, when l-cysteine was replaced by alanine or cystathionine, the substrates for cystalysin and other thiol-containing compounds in the basic GM-1 medium, essentially no H2S was detected in the cultures and the spirochete failed to grow (Fig. 6A). A dose-dependent positive correlation with l-cysteine, H2S production, and treponeme growth was observed (Fig. 6B). Similar results were obtained in the presence of glutathione (data not shown). H2S as one of the enzyme products is not required for T. denticola growth under anaerobic conditions (data not shown). These results support the hypothesis that only those thiol-containing compounds effective as substrates for cystalysin or gamma-glutamyltransferase producing H2S can support T. denticola growth under aerobic conditions. Identical results were obtained with T. denticola 35404 and 33520 and the clinical isolate GM-1 (data not shown), each of which contained the intact three-enzyme pathway. By contrast, T. vincentii failed to grow under the conditions tested in this study, since it was not able to produce H2S from glutathione, Cys-Gly, or l-cysteine (data not shown).
FIG. 6.
Effects of thiol compounds on growth of T. denticola and H2S production under aerobic conditions. (A) Effects of substrates for cystalysin and GGT as well as other compounds on T. denticola growth. (B) Positive correlation of H2S production and growth with dose dependence in the presence of l-cysteine in the culture. Error bars indicate standard deviations.
DISCUSSION
In the present study, we investigated the effect of H2S production, O2 availability, and changes in the levels of l-cysteine on the in vitro growth conditions for T. denticola. The results suggested a model for T. denticola growth under aerobic conditions, based upon several observations. First, it was demonstrated that T. denticola did not survive and grew under routine aerobic conditions (<20% inoculation) (Fig. 1A). However, the spirochete was conditionally capable of growth under these atmospheric conditions (Fig. 1B, 2, and 3). This observation is not in agreement with previous reports (16, 25, 44, 45) which suggested that this spirochete is an obligate anaerobe. Nevertheless, others have previously proposed that T. denticola was a facultative anaerobic spirochete (31, 47). In contrast, our findings revealed that the organism is only conditionally capable of surviving and growing under strict aerobic conditions (Fig. 1B and 3). The second relevant observation is the demonstration that the significant levels of dissolved O2 in the cultures of T. denticola grown under normal atmospheric conditions were reduced to <0.5%, facilitating the growth of the spirochete under microanaerobic conditions (Fig. 4). Furthermore, our data suggest that the addition of T. denticola cells resulted in the coupling of O2 removal from the culture medium to the simultaneous production of H2S (Fig. 4). Third, it was observed that H2S production from T. denticola cultures was always accompanied by the removal of dissolved O2 in the culture medium under aerobic conditions (Fig. 4). Furthermore, the coupling of H2S production with O2 removal was found to be dose dependent (Fig. 5). Therefore, H2S production might be the real trigger for removal of dissolved O2 in the growth cultures and the creation of anaerobic conditions for promoting the growth of T. denticola. Finally, following a process of screening several S-containing compounds, peptides, and amino acids, l-cysteine and glutathione appeared to be the main sources for H2S production in T. denticola culture medium under aerobic conditions and the major supplements for supporting the growth of T. denticola (Fig. 6). Based upon previous publications, all growth media for T. denticola, including TYGVS, MTYGS, GM-1, and PTY broth (8, 18, 31, 47), contained l-cysteine as one of the major supplements. T. denticola can utilize this substrate to produce H2S (10), which reduces O2 and creates a microanaerobic environment to facilitate the growth of the spirochete under aerobic conditions. We have previously described a stepwise enzyme pathway for H2S production. Three enzymes, GGT, CGase, and cystalysin, were found to be involved in converting any of the three substrates glutathione, Cys-Gly, and l-cysteine into H2S (8, 10, 11). Therefore, we propose that the three-enzyme pathway is likely to be an important player in the coupling of H2S production to O2 removal in mediating the survival of T. denticola under aerobic conditions.
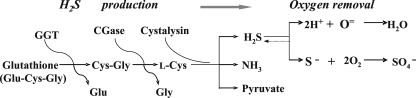
Taking all of the present findings together, we propose a model for H2S production involving a stepwise enzyme pathway in T. denticola which produces a microanaerobic environment for promoting the growth of the organism (Fig. 7). To this end, culture media containing one of the three substrates, i.e., glutathione, Cys-Gly, or l-cysteine, can be converted by T. denticola cells in a stepwise manner to the end product H2S. When acted on under certain conditions, H2S releases 2H+ and sulfide (S2−). The strong reducing reagent H2S will subsequently react with dissolved O2 and produce H2O and sulfate (50). Therefore, such changes in the medium will provide protective conditions supporting the survival and growth of T. denticola under aerobic conditions. The culture depth and the size of the inocula are two additional factors which favor the creation of such a microanaerobic environment.
FIG. 7.
Proposed model for H2S production in T. denticola and creation of an anaerobic microenvironment in aerobic cultures. Our model entails the presence of an H2S-producing enzyme pathway which is critical in the development of a local microanaerobic environment for T. denticola and the promotion of the subsequent conditional growth of the organism under aerobic conditions.
To better explain the capacity of T. denticola to grow under aerobic conditions, oxygen metabolism and the oxidative enzymes, including NAD (NADH) oxidase (5), oxidoreductase, peptidohydrolase (47), and peptidases as well as other enzymes (31), have been previously studied. Thus far, no specific oxidative enzymes had been found to be capable of removing dissolved O2 from the culture medium. In this study, our results have demonstrated that in T. denticola, GGT and cystalysin activities were increased under aerobic conditions compared to anaerobic conditions (data not shown). The mechanism for the up-regulation of these enzymatic systems is not clear and remains to be studied.
Some current views suggest that anaerobes may have some ability to metabolize oxygen, but these organisms usually lack sufficient defenses against the toxic metabolites of oxygen such as H2O2, O2·−, or OH· (5, 29). T. denticola 35405 was shown to possess a moderate oxygen metabolic activity based mainly on NADH oxidases, and it also produces NADH peroxidase and superoxide dismutase, which are known to be protective against oxidative damage (5, 26). These protective enzymes, even if they may contribute to survival in aerobic environments, do not appear to be primarily involved in T. denticola growth under aerobic conditions for the following reasons. First, our experiments showed that only those S-containing compounds capable of producing H2S can support T. denticola growth under aerobic conditions. This indirectly demonstrated that the ability to metabolize oxygen is not a likely possibility for the organism to sustain aerobic growth. Second, the gaseous environment had no remarkable effect on the catalase, peroxidase, and superoxide dismutase activities (47). Third, it has been shown previously that fresh clinical isolates of anaerobic bacteria can tolerate oxygen exposure for about 8 h at room temperature (48), which suggests that oxygen tolerance based on the function of superoxide dismutase is not sufficient to support growth under aerobic conditions. Our findings have also shown that deeper broth cultures with higher H2S versus lower O2 levels favor cell proliferation and are in agreement with earlier clinical observations (15, 16, 21, 25, 26, 30). It was reported that H2S is a major metabolic end product detected in deep periodontal pockets which is produced by resident periodontopathic microbiota (8, 41).
At least five genera of anaerobic oral microorganisms commonly found in the deepest portion of these pockets have the capacity to form H2S, and the most potent producers of H2S were T. denticola and the black-pigmented species Porphyromonas gingivalis, Bacteroides intermedius, Bacteroides loescheii, and Porphyromonas endodontalis (39, 41). Deep pockets also were found to have a lower pO2 than moderate pockets and to contain higher proportions of strictly anaerobic spirochetes such as T. denticola (26). We speculate that the mechanisms by which these prokaryotes create this environmental niche in periodontal pockets may be similar to the coupling of H2S production with removal of dissolved O2 as demonstrated in this study. Therefore, these findings suggest that spirochetes may play a significant role in maintaining the anaerobic environment at diseased sites in periodontitis and also suggest that local oxygen therapy may serve as a novel treatment for necrotizing periodontal disease (17).
Acknowledgments
We thank David Kolodrubetz and Howard Kuramitsu (University of Buffalo) for their excellent discussions and criticism of this work.
This work was supported by NIH grant DE-13819.
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Afonsky, D. 1961. Saliva and its relation to oral health. University of Alabama Press, Tuscaloosa, AL.
- 2.Beauchamp, R. O., Jr., J. S. Bus, J. A. Popp, C. J. Boreiko, and D. A. Andjelkovich. 1984. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 13:25-97. [DOI] [PubMed] [Google Scholar]
- 3.Bilzer, M., and B. H. Lauterburg. 1991. Glutathione metabolism in activated human neutrophils: stimulation of glutathione synthesis and consumption of glutathione by reactive oxygen species. Eur. J. Clin. Investig. 21:316-322. [DOI] [PubMed] [Google Scholar]
- 4.Brock, T. D. 1974. Biology of microorganisms, p. 313-318. Prentice-Hall, Inc., Englewood Cliffs, NJ.
- 5.Caldwell, C. E., and R. E. Marquis. 1999. Oxygen metabolism by Treponema denticola. Oral Microbiol. Immunol. 14:66-72. [DOI] [PubMed] [Google Scholar]
- 6.Cappiello, M., A. Lazzarotti, F. Buono, A. Scaloni, C. D. Ambrosio, P. Amodeo, B. L. M. Endez, P. Pelosi, A. D. Corso, and U. Mura. 2004. New role for leucyl aminopeptidase in glutathione turnover. Biochem. J. 378:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, J., J. T. Larsen, and M. B. Edlund. 1993. Peptostreptococcus micros has a uniquely high capacity to form hydrogen sulfide from glutathione. Oral Microbiol. Immunol. 8:42-45. [DOI] [PubMed] [Google Scholar]
- 8.Chu, L., Z. Dong, X. Xu, D. Cappelli, and J. Ebersole. 2002. Role of glutathione metabolism of Treponema denticola in bacterial growth and virulence expression. Infect. Immun. 70:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, L., J. L. Ebersole, and S. C. Holt. 1999. Hemoxidation and binding of the 46-kDa cystalysin of Treponema denticola leads to a cysteine-dependent hemolysis of human erythrocytes. Oral Microbiol. Immunol. 14:293-303. [DOI] [PubMed] [Google Scholar]
- 10.Chu, L., J. L. Ebersole, G. P. Kurzban, and S. C. Holt. 1997. Cystalysin, a 46-kilodalton cysteine desulfhydrase from Treponema denticola, with hemolytic and hemoxidative activities. Infect. Immun. 65:3231-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, L., A. Burgum, D. Kolodrubetz, and S. C. Holt. 1995. The 46-kilodalton-hemolysin gene from Treponema denticola encodes a novel hemolysin homologous to aminotransferases. Infect. Immun. 63:4448-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu, L., X. Xu, Z. Dong, D. Cappelli, and J. D. Ebersole. 2003. Role for recombinant gamma-glutamyltransferase from Treponema denticola in glutathione metabolism. Infect. Immun. 71:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claesson, R., M. B. Edlund, S. Persson, and J. Carlsson. 1990. The production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol. Immunol. 5:137-142. [DOI] [PubMed] [Google Scholar]
- 14.Claesson, R., M. Granlund-Edstedt, S. Persson, and J. Carlsson. 1989. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect. Immun. 57:2776-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenno J. C., P. M. Hannam, W. K. Leung, M. Tamura, V. J. Uitto, and B. C. McBride. 1998. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 66:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredette, V., C. Plante, and A. Roy. 1967. Numerical data concerning the sensitivity of anaerobic bacteria to oxygen. J. Bacteriol. 93:2012-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaggl, A. J., H. Rainer, F. Grund, and F. M. Chiari. 2006. Local oxygen therapy for treating acute necrotizing periodontal disease in smokers. J. Periodontol. 77:31-38. [DOI] [PubMed] [Google Scholar]
- 18.Gaitonde, M. K. 1967. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman, L. S., and A. Gilman. 1955. The pharmacologic basis of therapeutics, 2nd ed. MacMillan Co., New York, NY.
- 20.Holt, S. C., and T. E. Bramanti. 1991. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit. Rev. Oral Biol. Med. 2:177-281, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Holt, S. C., and J. L. Ebersole. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 38:72-122. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz, A., and L. E. Folke. 1973. Hydrogen sulfide production in the periodontal environment. J. Periodontol. 44:390-395. [DOI] [PubMed] [Google Scholar]
- 23.Jobin, M. C., I. Virdee, C. A. McCulloch, and R. P. Ellen. 2007. Activation of MAPK in fibroblasts by Treponema denticola major outer sheath protein. Biochem. Biophys. Res. Commun. 356:213-218. [DOI] [PubMed] [Google Scholar]
- 24.Li, H., S. Arakawa, Q. D. Deng, and H. Kuramitsu. 1999. Characterization of a novel methyl-accepting chemotaxis gene, dmcB, from the oral spirochete Treponema denticola. Infect. Immun. 67:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loesche, W. J. 1969. Oxygen sensitivity of various anaerobic bacteria. Appl. Microbiol. 18:723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loesche, W. J., F. Gusberti, G. Mettraux, T. Higgins, and S. Syed. 1983. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect. Immun. 42:659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loesche, W. J. 1988. The role of spirochetes in periodontal disease. Adv. Dent. Res. 2:275-283.10. [DOI] [PubMed] [Google Scholar]
- 28.Makinen, P. L., and K. K. Makinen. 1997. γ-Glutamyltransferase from the outer cell envelope of Treponema denticola ATCC 35405. Infect. Immun. 65:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquis, R. E. 1995. Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J. Ind. Microbiol. 15:198-207. [DOI] [PubMed] [Google Scholar]
- 30.Mettraux, G. R., F. A. Gusberti, and H. Graf. 1984. Oxygen tension (pO2) in untreated human periodontal pockets. J. Periodontol. 55:516-521. [DOI] [PubMed] [Google Scholar]
- 31.Mikx, F. H. M. 1997. Environmental effects on the growth and proteolysis of Treponema denticola ATCC 33520. Oral Microbiol. Immunol. 12:249-253. [DOI] [PubMed] [Google Scholar]
- 32.Mills, B. J., J. P. Richie, and C. A. Lang. 1990. Sample processing alters glutathione and cysteine values in blood. Anal. Biochem. 184:263-267. [DOI] [PubMed] [Google Scholar]
- 33.Moore, W. E. C. 1987. Microbiology of periodontal disease. J. Periodontal Res. 22:235-241. [DOI] [PubMed] [Google Scholar]
- 34.Morhart, R. E., L. J. Mata, A. J. Sinskey, and R. S. Harris. 1970. A microbiological and biochemical study of gingival crevice debris obtained from Guatemalan Mayan Indians. J. Periodontol. 41:644-649. [DOI] [PubMed] [Google Scholar]
- 35.Ng, W., and J. Tonzetich. 1984. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J. Dent. Res. 63:994-997. [DOI] [PubMed] [Google Scholar]
- 36.Page, R. C., and H. E. Schroeder. 1982. Periodontitis in man and other animals: a comparative review, p. 330. Karger, Basel, Switzerland.
- 37.Persson, S. 1992. Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiol. Immunol. 7:378-379. [DOI] [PubMed] [Google Scholar]
- 38.Persson, S., R. Claesson, and J. Carlsson. 1989. The capacity of subgingival microbiotas to produce volatile sulfur compounds in human serum. Oral Microbiol. Immunol. 4:169-172. [DOI] [PubMed] [Google Scholar]
- 39.Persson, S., M. B. Edlund, and R. Claesson. 1990. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 5:195-201. [DOI] [PubMed] [Google Scholar]
- 40.Reiffenstein, R. J., W. C. Hulbert, and S. H. Roth. 1992. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 32:109-134. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo, A. A. 1967. The possible role of hydrogen sulfide in human periodontal disease. I. Hydrogen sulfide production in periodontal pockets. Periodontics 5:233-236. [PubMed] [Google Scholar]
- 42.Siegel, L. M. 1965. A direct microdetermination for sulfide. Anal. Biochem. 11:126-132. [DOI] [PubMed] [Google Scholar]
- 43.Simonson, L. G., C. H. Goodman, J. J. Bial, and H. E. Morton. 1988. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect. Immun. 56:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smibert, R. M. 1984. Genus III Treponema Schaudinn 1905, 1728AL, p. 49-57. In N. R. Krieg and R. J. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 45.Smibert, R. M. 1985. Anaerobic spirochetes, p. 489-494. In E. H. Lennette, A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, DC.
- 46.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-782. [Google Scholar]
- 47.Syed, S. A., K. K. Makinen, P. L. Makinen, C. Y. Chen, and Z. Muhammad. 1993. Proteolytic and oxidoreductase activity of Treponema denticola ATCC 35405 grown in an aerobic and anaerobic gaseous environment. Res. Microbiol. 144:317-326. [DOI] [PubMed] [Google Scholar]
- 48.Tally, F. P., P. R. Stewart, V. L. Sutter, and J. E. Rosenblatt. 1975. Oxygen tolerance of fresh clinical anaerobic bacteria. J. Clin. Microbiol. 1:161-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. National Research Council. 1979. Hydrogen sulfide. University Park Press, Baltimore, Md.
- 50.Wang, C. L., A. M. Lum, S. C. Ozuna, D. S. Clark, and J. D. Keasling. 2001. Aerobic sulfide production and cadmium precipitation by Escherichia coli expressing the Treponema denticola cysteine desulfhydrase gene. Appl. Microbiol. Biotechnol. 56:425-430. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg, A., and S. C. Holt. 1990. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect. Immun. 58:1720-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, A., P. Handler, E. L. Smith, and D. Stetten. 1959. Principles of biochemistry, 2nd ed., p. 631. McGraw-Hill, New York, NY.