Abstract
Maize produces a suite of allelopathic secondary metabolites, the benzoxazinoids. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one and 2,4-dihydroxy-2H-1,4-benzoxazin-3-one reside as glucosides in plant tissue and spontaneously degrade to 6-methoxy-2-benzoxazolinone (MBOA) and 2-benzoxazolinone (BOA) upon plant cell disruption. Several maize-associated fungi in the genus Fusarium can metabolize MBOA and BOA. BOA tolerance levels in 10 species of Fusarium and in the maize endophytes Nigrospora oryzae, Acremonium zeae, and Periconia macrospinosa were characterized. BOA tolerance ranged from 0.25 to 1.10 mg/ml among species. The influence of substrate alteration by one species on the subsequent growth of another species was assessed in the presence and absence of BOA. The colony area of the secondary colonizer in heterospecific interactions was compared to that in autospecific interactions (one isolate follows itself). In the presence of BOA, four of six secondary colonizers had greater growth (facilitation) when primary colonizers had higher BOA tolerance than the secondary colonizer. When the primary colonizer had lower tolerance than the secondary, three of six secondary colonizers were inhibited (competition) and three not significantly affected. In BOA-free medium, the number of isolates that were facilitated or inhibited was the same regardless of the tolerance level of the primary colonizer. Two of six secondary colonizers were facilitated, two inhibited, and two not significantly affected. This study provides some support for facilitation in stressful conditions under the Menge-Sutherland model. The results are not consistent with the corresponding prediction of competition in the absence of stress. The hypothesis drawn from these data is that in the presence of a toxin, fungal species that detoxify their substrate can enhance the colonization rate of less tolerant fungi.
Maize harbors a diverse community of nonmycorrhizal fungal endophytes (16). Several species in the fungal genus Fusarium, notably F. verticillioides, F. subglutinans, F. proliferatum, and F. graminearum, are commonly associated with maize as pathogens or asymptomatic endophytes. These species can produce secondary metabolites that are toxic to livestock, including moniliformin, fumonisin, and deoxynivanol (15, 29). F. verticillioides is frequently encountered as an asymptomatic maize endophyte, but as a pathogen it can cause seedling blight and stem, seed, root, and ear rot. This species is globally distributed and has a wide host range, including sorghum, millet, and sugar cane, but it is most frequently isolated from maize. In some localities, maize fields have rates of greater than 90% infection with F. verticillioides, which can be transmitted via seed, crop residue in soil, or airborne conidia (33). Other common preharvest endophytes include Acremonium zeae, Periconia macrospinosa, and Nigrospora oryzae (44; M. Saunders and L. M. Kohn, unpublished data).
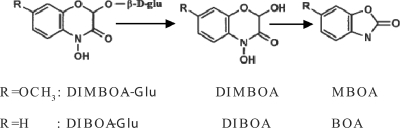
Maize has several mechanical and protein-based mechanisms of resistance to microbial invasion (5, 11, 13, 42). In addition, maize contains a suite of toxic secondary metabolites, the benzoxazinoids (also known as cyclic hydroxamic acids). The primary benzoxazinoids found in maize are 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA) and its desmethoxy derivative, 2,4-dihydroxy-2H-1,4-benzoxazin-3-one (DIBOA). These compounds originate in the cell vacuole as biologically inactive β-glucosides, 2-[2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one]-β-d-glucopyranoside and its desmethoxy derivative, and are enzymatically converted to the biologically active benzoxazinoids upon cell disruption (20). The benzoxazinoids are unstable in aqueous solution and spontaneously degrade to the corresponding benzoxazolinones, 6-methoxy-2-benzoxazolinone (MBOA) and 2-benzoxazolinone (BOA) (Fig. 1). The benzoxazolinones are biologically active and stable in aqueous solution. They can be allelopathic and protect against herbivory, fungal, and bacterial infection (4, 12, 26, 28, 45). Several species within the Poaceae, including wheat, rye, maize, teosinte, and some wild barley species, produce benzoxazinoids (34, 38).
FIG. 1.
Benzoxazinoids and their degradation products. The biologically inactive compounds DIMBOA-Glu and DIBOA-Glu reside in plants and are enzymatically converted by the plant to the fungitoxic aglucones DIMBOA and DIBOA. These compounds spontaneously degrade to the biologically active compounds MBOA and BOA. (Adapted from reference 32 with permission.)
DIMBOA and DIBOA are synthesized upon germination and accumulate within the tissues of the growing seedling, thereby providing a constitutive defense against invasion (10, 23). The highest levels of DIMBOA and DIBOA are found in the young seedling, and the concentrations of these compounds decrease linearly with age. The concentration of DIMBOA has been shown to peak at between 1 to 10 mmol per kg−1 (fresh weight) at 7 days after germination. At approximately 15 days, the concentration of DIMBOA decreases to about 0.3 to 3 mmol per kg−1 (fresh weight) (1). DIMBOA and DIBOA are formed systemically and are actively exuded from root tissue (35, 39).
F. verticillioides is known to tolerate MBOA and BOA. Tolerance is associated with metabolic conversion of MBOA and BOA to N-(2-hydroxy-4-methoxyphenyl)malonamic acid and N-(2-hydroxy-phenyl)malonamic acid, respectively (36, 46). Other important maize-associated fungi, including F. subglutinans, F. proliferatum and F. graminearum, are also known to detoxify MBOA and BOA (17). Fusarium species are highly variable in their tolerance to these compounds, with F. verticillioides demonstrating the highest tolerance. This characteristic has been suggested to increase the ecological fitness of F. verticillioides in maize fields (17).
A fungal species in host tissue may have a facilitative, competitive, or neutral effect on subsequent fungal infection. For example, in a study of 135 saprophytic fungi, Clonostachys rosea was found to suppress conidiation of several Fusarium species by up to 80% on maize stalks. Some species suppressed conidiation only slightly or not at all (27). Given that F. verticillioides is often in maize seed, and can begin to inhabit tissue upon seedling germination, this species has the potential to influence the colonization success of subsequent fungal invaders.
The objective of this work was to test the influence of BOA biotransformation on the outcome of indirect interactions between common maize-associated fungi. This work is a complement to ongoing field studies aimed at determining the influence of benzoxazinoid production on fungal colonization in maize fields. The approach taken was threefold. First, BOA tolerance levels in 10 species of Fusarium and the common maize endophytes Nigrospora oryzae, Acremonium zeae, and Periconia macrospinosa were characterized. Second, sequential inoculations were performed in vitro to determine the influence of substrate alteration by one species on the growth of another in the presence or absence of BOA. The effect of F. verticillioides as a primary colonizer on subsequent fungal colonization and the influence of BOA on the outcome of species interactions were determined. Third, the isolates used in sequential inoculation experiments were evaluated for their ability to degrade BOA in the medium.
MATERIALS AND METHODS
Characterization of BOA tolerance in maize endophytes.
A panel of 10 Fusarium species (two isolates of each species), two isolates of A. zeae, two isolates of N. oryzae, and two isolates of P. macrospinosa were scored for growth or no growth on a range of concentrations of BOA (Table 1). Potato dextrose agar (PDA) (Difco, Detroit, MI) was amended with BOA (Sigma Chemical Co., St Louis, MO) in each of the following concentrations of BOA with respect to PDA: 0.00, 0.25, 0.50, 0.75, 1.00, 1.10, 1.20 mg/ml. Medium was aliquoted into quadrant petri dishes containing 5.00 ml medium per quadrant. The treatment concentrations in PDA were made from a stock solution of BOA in anhydrous ethanol (EtOH) (100 mg/ml). The 0.00% BOA control was a 1.20% concentration of anhydrous EtOH in PDA, to match the highest concentration of BOA solution in the treatments (1.20 mg/ml). The addition of EtOH to the medium did not inhibit the growth of any of the strains used in this study. Strains were incubated for 14 days in the dark at 22°C and scored for growth or no growth at a given concentration. These experiments were repeated twice with four replicates per isolate.
TABLE 1.
BOA tolerance levels of common maize endophytes
| Group | Highest concn (mg/ml) of BOA tolerated | Isolate |
|---|---|---|
| I | 1.10 | Fusarium verticillioides JFL A-00149, F. verticillioides JFL A-00999 |
| II | 1.00 | Fusarium subglutinans JFL E-00990, F. subglutinans JFL E-02192, Nigrospora oryzae MS 81 |
| III | 0.75 | Fusarium konzum JFL I-11615, F. konzum JFL I-11616, Gibberella zeae JFL 3639, G. zeae JFL 3634, Fusarium circinatum JFL H-10847, F. circinatum JFL H-10850, Fusarium nygamai JFL G-5111, F. nygamai JFL G-5112, Nigrospora oryzae MS 581 |
| IV | 0.50 | Fusarium thapsinum JFL F-4093, F. thapsinum JFL F-4094, Fusarium sacchari JFL B-03852, F. sacchari JFL B-03853, Fusarium fujikuroi JFL C-01993, F. fujikuroi JFL C-01995, Fusarium proliferatum JFL D-04853, F. proliferatum JFL D-04854, Acremonium zeae NRRL 6415, A. zeae NRRL 13540, |
| V | 0.25 | Periconia macrospinosa MS 31, P. macrospinosa MS 113 |
Strains assessed for BOA tolerance.
The following strains were scored for BOA tolerance: Fusarium verticillioides JFL A-00149 (= FGSC 7600) and JFL A-00999 (= FGSC 7603), Fusarium sacchari JFL B-03582 (= FGSC 7610) and JFL B-03853 (= FGSC 7611), Fusarium fujikuroi JFL C-01993 and C-01995, Fusarium proliferatum JFL D-04853 (= FGSC 7614) and JFL D-04854 (= FGSC 7615), Fusarium subglutinans JFL E-00990 (= FGSC 7616) and JFL E-02192 (= FGSC 7617), Fusarium thapsinum JFL F-4093 (= FGSC 7056) and JFL F-4094 (= FGSC 7057), Fusarium nygamai JFL G-5111 and JFL G-5112, Fusarium circinatum JFL H-10847 and JFL H-10850, Fusarium konzum JFL I-11615 and JFL I-11616, Gibberella zeae JFL 3639 and JFL 3634, Acremonium zeae NRRL 6415 and NRRL 13540) Nigrospora oryzae MS 81 (= DAOM 239206) and MS 581 (= DAOM 239207), and Periconia macrospinosa MS 31 (= DAOM 239204) and MS 113 (= DAOM 239205).
All isolates of Fusarium were obtained directly from J. F. Leslie (Kansas State University, Manhattan, KS), A. zeae was obtained from D. Wicklow (U.S. Department of Agriculture, Peoria, IL), and N. oryzae and P. macrospinosa were isolated from maize in a related field experiment in Ontario in 2005 (Saunders and Kohn, unpublished data).
Designations for sources of strains are as follows: DAOM, National Mycological Herbarium, Agriculture and Agri-Food Canada, Ottawa, Ontario; FGSC, Fungal Genetics Stock Center, Department of Microbiology, University of Kansas Medical Center, Kansas City, KS; JFL, John F. Leslie, Department of Plant Pathology, Kansas State University, Manhattan; KS; MS, Megan Saunders, Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario; and NRRL, Northern Regional Research Laboratory, USDA, ARS, Peoria, IL.
Isolation of fungal endophytes from maize.
N. oryzae and P. macrospinosa were isolated from the roots of maize seedlings grown in Ridgetown, Ontario. Root segments approximately 2.00 to 3.00 mm in diameter and 2.00 cm in length were surface sterilized in sequential washes of 70.00% EtOH (2 min) and 0.53% NaOCl (2 min) and finally were rinsed in sterile distilled water (2 min). Root tissue was plated onto PDA amended with 1.00 g/liter streptomycin sulfate and 0.25 g/liter neomycin sulfate. The efficiency of the surface sterilization procedure was tested using the tissue imprint method described by Schulz and Boyle (37), and the procedure was found to be effective.
Sequential inoculation experiments.
From the initial screen for BOA tolerance, two groups of species (referred to as interaction set 1 and interaction set 2) were chosen for sequential inoculation. The members of each interaction set were chosen so that commonly co-occurring species that have different BOA tolerance profiles would comprise each set. Interaction set 1 included Fusarium verticillioides (JFL 00999), F. subglutinans (JFL 02192), and F. proliferatum (JFL 04854), and interaction set 2 included Nigrospora oryzae (MS 81), Acremonium zeae (NRRL 13540), and Periconia macrospinosa (MS 113).
Each experiment was repeated twice with a minimum of three replicates. To determine the effect of substrate alteration by one species on the growth of another, isolates were inoculated sequentially on the same medium, using a technique maintaining sterility for both inoculations. For interaction set 1, two types of media were prepared in standard petri dishes: PDA amended with BOA (0.50 mg/ml), and PDA with 0.5% EtOH. For interaction set 2, PDA amended with 0.25 mg/ml BOA and PDA amended with 0.25% EtOH were prepared in standard petri dishes. These concentrations were chosen because they were the highest tolerated by the most sensitive species within each interaction set. Cellophane membranes (VWR International, Mississauga, Ontario) were cut into 9- by 9-cm squares and sterilized by autoclaving for 20 min at 121°C in double-distilled water. The membranes were overlaid smoothly on the surface of each petri dish. The center of the membrane was inoculated with a mycelial plug 5 mm in diameter (taken with a no. 1 cork borer). Plates were incubated at 22°C in the dark, and radial growth measurements were taken at 72 h by tracing the colony margin on the plate. Directly after the measurement was taken, the membrane supporting the fungal colony was removed and replaced with a new sterile membrane. The medium that had been biotransformed by the preceding colonizing isolate was then inoculated with the second colonizer. Radial growth measurements of the second colonizers were taken at 72 h. At the completion of the experiment, the colony area measurements were traced onto paper, scanned, and saved as electronic files. Areas of the colonies were measured using the ImageJ digital analysis program (version 1.32; W. S. Rasband, U.S. National Institutes of Health, Bethesda, MD [http://rsb.info.nih.gov/ij/]).
Prior to beginning these experiments, it was determined that all isolates grew on the surface of the membrane without penetration. F. graminearum could not be included because it rapidly degraded the cellophane membrane.
Assessment of presence of BOA in the medium.
The degradation of BOA was evaluated using thin-layer chromatography (TLC) as described by Glenn et al. (17). Media and inocula of the focal strains were prepared as described in the previous section. After 72 h of growth, the cellophane membranes were removed, and a plug of medium was taken from the center of the area previously covered by the colony. Glass-backed silica gel TLC plates (10 by 20 cm) precoated with F254, a fluorescent indicator, were used (VWR International, Mississauga, ON). TLC plates were placed in a 50°C oven for 10 min prior to use to remove ambient moisture. Plugs of medium were placed on oven-dried plates, with the upper surface down, for 30 s. The compounds were then mobilized in toluene-ethyl acetate-formic acid (50:40:10), allowed to air dry, and subsequently viewed and photographed using a 254-nm UV lamp (Mineralight UVSII; Ultra-Violet Products Inc., San Gabriel, CA). TLC plates were run with a BOA standard spot (10 ng) on each plate. In addition, uninoculated, BOA-amended PDA was screened. One plug from the center of each colony was sampled. The intensity of a spot is a qualitative, relative measurement of the amount of BOA in the medium. A darker spot indicates a greater quantity of BOA. High-pressure liquid chromatography (HPLC) was used to verify that the compound viewed using TLC was truly BOA. A second set of TLC plates was created to purify the compound for HPLC, using a protocol similar to that described by Glenn et al. (18). After the first colonizer had grown for 72 h, the colony-containing membrane was removed. A total of 19 plugs were taken from the center of each petri plate using a no. 5 cork borer and processed as described above. TLC plates were left to dry under a laminar flow hood overnight, after which the silica gel containing the putative BOA spots was scraped off of the plate, eluted in methanol, and filtered through a 0.45-nm nylon filter. HPLC was performed on an HP series 1100 variable-wavelength detector, using a Perkin-Elmer Brownlee C18 column (column dimensions, 250 by 4.6 mm; bead size, 5 μm). The mobile phase was 40% aqueous methanol for the entire 10-min run. The flow rate was 1.00 ml per minute. The elutant (15 μl) was injected onto the column and monitored at 280 nm at room temperature.
Statistical analyses.
In this study the objective of the sequential inoculations was to determine if a species would grow faster when it succeeded unlike species (heterospecific interaction) than when it followed itself (autospecific interaction). If the colony area of a species is greater in a heterospecific than in an autospecific interaction, the heterospecific sequence is interpreted as facilitative. If the area of a species is less in a heterospecific than in an autospecific interaction, the heterospecific sequence is competitive. Tukey-Kramer honestly significant difference (HSD) tests were conducted to compare colony area values for different treatments. The threshold for statistical significance was set at a P value of ≤0.05. The analyses were conducted using JMPin (version 5.1; SAS Institute Inc., Cary, NC). Interaction set 1 and interaction set 2 were analyzed separately.
RESULTS
Qualitative characterization of BOA tolerance.
Five categories of BOA tolerance were recognized (Table 1), and the highest concentrations tolerated were as follows: group I, 1.10% BOA (F. verticillioides); group II, 1.00% (F. subglutinans and N. oryzae); group III, 0.75% (F. konzum, F. graminearum, F. circinatum, F. nygamai, and N. oryzae); group IV, 0.50% (F. thapsinum, F. sacchari, F. fujikuroi, F. proliferatum, and A. zeae); and group V, 0.25% (P. macrospinosa). No species were able to grow in a 1.20% concentration. The two isolates of N. oryzae differed in tolerance. There was no variation in tolerance levels between the two isolates tested in any of the other species.
Sequential inoculation of species pairs.
Mean colony areas for the three treatments in BOA-amended and BOA-free media are shown in Fig. 2 and 3. The three treatments are as follows: (i) solo control (growth of a strain on previously uncolonized medium), (ii) autospecific interaction (growth of a strain following itself), and (iii) heterospecific interaction (growth of a strain following an unlike species). The statistical significance of the difference between the mean colony area values of a single strain for each treatment is indicated. One-way ANOVAs were conducted to test for a significant difference between experimental replicates, and no significant differences were detected (results not shown). Figures 4 and 5 compare the outcomes of autospecific and heterospecific interactions for the secondary colonizer. Values listed are the percent differences between mean colony area values in the autospecific interaction compared to a heterospecific interaction. A positive value indicates a greater mean colony area in the heterospecific interaction than in the autospecific interaction (facilitation). A negative value indicates that the colony area of the heterospecific interaction was less than that in the autospecific interaction (competition).
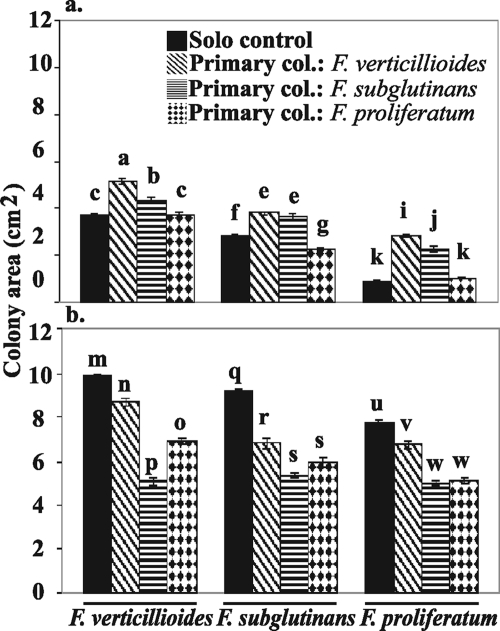
FIG. 2.
Interaction set 1 (Fusarium verticillioides, F. subglutinans, and F. proliferatum) mean colony area values of solo control or secondary colonizers on the BOA-amended medium (a) and on the BOA-free medium (b). Solo control, growth of a species on previously uncolonized medium; primary col., growth of secondary colonizer following F. verticillioides, F. subglutinans, or F. proliferatum as indicated. Error bars indicate standard errors. Tukey-Kramer HSD tests were used to compare mean colony area values of the same species in different treatments. The same letter above two bars indicates that there is no significant difference between means.
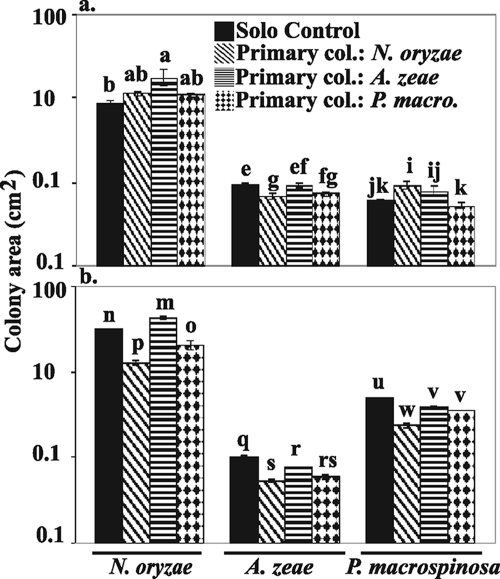
FIG. 3.
Interaction set 2 (N. oryzae, A. zeae, and P. macrospinosa) mean colony area values of solo control or secondary colonizers on the BOA-amended medium (a) and on the BOA-free medium (b). Note that data are plotted on a log scale. Solo control, growth of a species on previously uncolonized medium; primary col., growth of secondary colonizer following N. oryzae, A. zeae, or P. macrospinosa as indicated. Error bars indicate standard errors. Tukey-Kramer HSD tests were used to compare mean colony area values of the same species in different treatments. The same letter above two bars indicates that there is no significant difference between means.
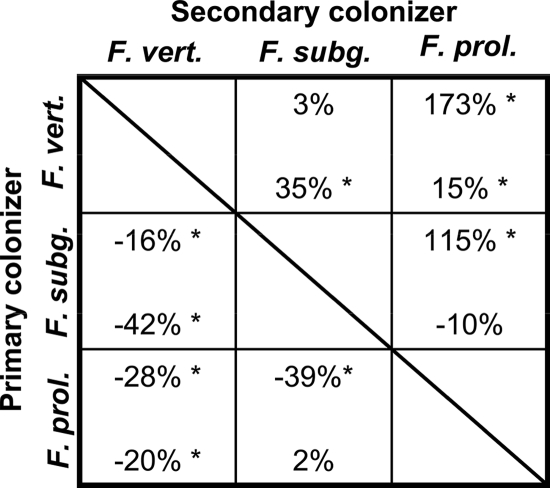
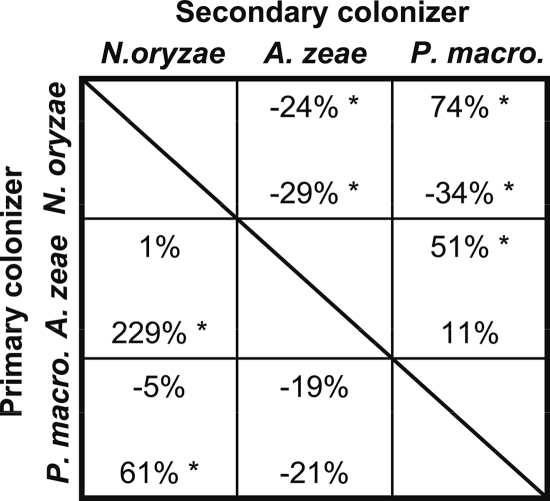
FIG. 4.
Percent differences between mean colony area values in the autospecific and heterospecific interactions for interaction set 1 (Fusarium verticillioides, F. subglutinans, and F. proliferatum). Mean colony area was measured at 72 h. Percent differences in colony area for the BOA-amended environment are on the top line of each cell, and those for the BOA-free environment are on the bottom. A positive value indicates a facilitative interaction and a negative value a competitive one. Asterisks indicate values where the heterospecific and autospecific mean colony area values are significantly different.
FIG. 5.
Percent differences between mean colony areas in the autospecific and heterospecific interactions for interaction set 2 (Nigrospora oryzae, Acremonium zeae, and P. macrospinosa). Mean colony area was measured at 72 h. Percent differences for the BOA-amended environment are on the top line of each cell, and those for the BOA-free environment are on the bottom. A positive value indicates a facilitative interaction and a negative value a competitive one. Asterisks indicate values where the heterospecific and autospecific mean colony area values are significantly different.
In the presence of BOA, four out of six secondary colonizers were facilitated when the primary colonizers had higher BOA tolerance than the secondary colonizer (Fig. 4 and 5, above the diagonal). For example, in interaction set 1, F. verticillioides (Table 1, group I) had the greatest impact on the colony area of the least BOA-tolerant strain, F. proliferatum (group IV), increasing its colony area by 173% (Fig. 4). In interaction set 1, the isolates with the highest BOA tolerance, F. verticillioides and F. subglutinans (group II), achieved a larger colony area in the autospecific interaction than in the solo control (Fig. 2a). When the primary colonizer had lower tolerance than the secondary, three out of six secondary colonizers were inhibited (competition), and the remaining three had interactions that were not significant (neutral) (Fig. 4 and 5, below the diagonal).
In BOA-free medium, two out of six secondary colonizers were facilitated, two were inhibited, and two were not significantly affected (Fig. 4 and 5). The solo control of all strains in the BOA-free medium had a larger colony area than in the autospecific interaction (Fig. 2b and 3b).
In some cases the direction of heterospecific interactions was different in the BOA-amended and BOA-free media. In four out of six examples where the primary colonizer was more tolerant than the secondary colonizer, the direction of interaction changed depending on the medium. For example, when F. proliferatum followed F. subglutinans, the mean colony area was greater in the presence of BOA but was not affected in the BOA-free medium. When P. macrospinosa (group V) followed N. oryzae (group II), the mean colony area of P. macrospinosa was greater in the BOA-amended medium and decreased in the BOA-free medium.
Some interactions were the same in direction, if not in magnitude, in both BOA-amended and BOA-free media. The colony area of F. verticillioides was always greater in autospecific interactions than in heterospecific interactions (Fig. 2). When F. verticillioides was a primary colonizer, the colony area of heterospecific secondary colonizers was greater, with the exception of F. subglutinans in BOA-amended medium.
Assessment of biodegradation of BOA in the medium.
The degradation of BOA in the medium was evaluated using TLC and HPLC. In interaction set 1, the plugs taken from medium in which F. verticillioides and F. subglutinans were incubated produced a spot of BOA lighter than that produced by F. proliferatum and the uninoculated control plate (Fig. 6). In interaction set 2, plugs from both N. oryzae and A. zeae produced a spot of BOA lighter than that of P. macrospinosa and the control plate. The results show that BOA tolerance is in part associated with chemical alteration of BOA. We observed no variation in intensity of BOA spotting on the TLC plates between biological replicates. Results of the HPLC analysis confirm that the compound identified using TLC was BOA. The retention times of the samples, including the BOA standard, were nearly identical, all peaks were symmetrical, and only one peak per chromatograph was observed (results not shown). Results could not be quantified because the sequential inoculation experiments were tractable only on solid medium.
FIG. 6.
Qualitative assessment of presence of BOA in the medium. Plugs were taken from the center of the colony after 72 h of incubation, after removal of the cellophane membrane. BOA spots were identified using a BOA standard (10 ng).
DISCUSSION
The patterns of BOA tolerance observed in this study generally conform to expectations under the assumption that tolerant fungi will be associated with benzoxazinoid-producing hosts. The species in groups I and II, i.e., F. verticillioides, F. subglutinans, and N. oryzae, are most frequently isolated from maize. The isolates in groups III, IV, and V are associated with a variety of hosts, not all of which are known to produce benzoxazinoids (14, 19, 22, 24, 44, 47).
Nine out of the 13 species compared here were previously tested for BOA tolerance. Glenn et al. (17) assessed the ability of 29 Fusarium species to grow in a 1.00-mg/ml concentration of BOA. Eleven of these species were tolerant. Tolerance of this concentration in F. verticillioides, F. subglutinans, F. nygamai, F. thapsinum, F. sacchari, and F. fujikuroi was also observed in the present study. Tolerance of this concentration in F. graminearum, F. circinatum, and F. proliferatum was not repeated in the present study, in which the highest concentration tolerated by F. graminearum and F. circinatum was 0.75 mg/ml and the highest concentration tolerated by F. proliferatum was 0.50 mg/ml. With five exceptions, different strains were used in the two studies. The five strains that were used in the previous and present studies had the same tolerance test results. Divergent tolerance results might be due to fixed differences between strains or to differences between environments in the two studies, influencing growth kinetics. For example, the previous study was conducted with standard petri dishes at 23°C, while the present study used subdivided petri dishes (5 ml medium per compartment) at 22°C; in both studies the focal tests were performed with PDA amended with BOA.
Tolerance to BOA in F. konzum, N. oryzae, A. zeae, and P. macrospinosa is reported in the present study for the first time. F. konzum is a recently described species that appears to be endemic to the Konza Prairie in Kansas, where it is associated with wild grasses (47). Benzoxazinoid levels in hosts of F. konzum have not been ascertained. Several agriculturally important members of the Poaceae produce benzoxazinoids, but few wild grasses have been investigated. P. macrospinosa was isolated frequently from maize in a related field experiment (Saunders and Kohn, unpublished data), and A. zeae is a common maize endophyte (44). A. zeae decreases BOA in the medium and therefore has a mechanism of tolerance to BOA, although it cannot grow in concentrations above 0.50 mg/ml (Fig. 6). Species with relatively low BOA tolerance that are frequently associated with benzoxazinoid-producing hosts may survive in planta by any of several mechanisms. Benzoxazinoids and their derivatives are highest in concentration in the vascular tissue of the seedling and could be avoided spatially or temporally. Another strategy for colonization without tolerance could be concurrent colonization with a species that is able to detoxify benzoxaxinoid degradation products.
In the field of community ecology, researchers have often focused on the role of interspecific competition in community organization and function. Recent studies indicate the prevalence of interspecific facilitation in these processes as well (for a review, see reference 6). A general trend emerging from this work is that primary colonizers often dramatically affect the establishment of communities by ameliorating stress in the environment, consequently facilitating colonization by additional species (6, 8, 40).
The modified Menge-Sutherland model (MSM) is a conceptual model proposing a trade-off between interspecific competitive ability and stress tolerance (6, 30, 31). The direction of species interactions should therefore change predictably along a gradient of environmental stress, such that in less stressful conditions, species interactions are competitive, leading to the ecological dominance of one or a few species (40). In relatively high levels of environmental stress, well-adapted species are predicted to facilitate the population growth of community members. For example, environmentally dependent facilitation can occur when abiotic stress is ameliorated. Callaway (7) evaluated the outcome of interactions between salt marsh plants and found that winter annual plants can facilitate the growth of perennial shrubs by creating shade, which acts to increase water availability and reduce salinity. Other studies have shown that when the stress of salinity is removed, species interactions become competitive rather than facilitative (see reference 3). The outcome of this dynamic is predicted to increase with the size or density of the facilitative individuals (9). The goal of these studies is not only to quantify the outcome of species interactions under specific conditions but to determine the various factors that contribute to the outcome of these interactions. Data describing the primary variables that shape species interactions will enable us to better predict community change over time.
While our study is not an explicit test of the MSM, this conceptual model provided expectations against which observed data could be compared, offering a useful ecological framework for interpreting our results. In this study we measured the outcome of species interactions in the presence or absence of an environmental stress while keeping the starting population size constant. The extent of support for the MSM can be seen in Fig. 4 and 5, above the diagonal. In the presence of BOA, in four cases out of six, a primary colonizer with higher BOA tolerance facilitated a secondary colonizer with lower tolerance. In the absence of BOA, only two of these six interactions evidenced the competition effect expected under the MSM. Evidence is stronger for facilitation under stress than for corresponding competition in the absence of stress. The MSM is not sufficient to describe this system. A hypothesis that merits general testing is that under environmental stress, fungal species that detoxify their substrate can enhance the colonization rate of less tolerant fungi.
It is possible that several factors other than BOA tolerance and metabolism may have contributed to the direction and strength of interactions between species. For example, drawing essential nutrients from the medium could be a mechanism of competition for the primary colonizer. The degree to which a species was detrimental to the growth of itself (autospecific interaction) in the absence of BOA could be an approximate, relative measure of essential nutrient use by that species. In both interaction sets, the species most inhibited in the autospecific interaction in BOA-free medium was neutral or competitive with other species in the same medium (F. subglutinans and N. oryzae in interaction sets 1 and 2, respectively). This pattern would be expected if F. subglutinans and N. oryzae depleted a greater essential nutrient load than the other species. In contrast, in both interaction sets, the species least detrimental to its own growth in BOA-free medium was neutral or facilitative to heterospecifics (F. verticilloides and A. zeae).
Another factor that may have contributed to the outcome of these species interactions is the production of antifungal compounds. The Fusarium species tested here each produce a suite of toxigenic compounds (15). One of the most toxic and abundant of these compounds is fumonisin B-1 (FB-1), a known fungitoxin produced by all of the Fusarium species used in this study (21). F. verticillioides and F. subglutinans are not sensitive to FB-1, and F. proliferatum is sensitive only to concentrations of 40 mM and higher. The isolate of F. subglutinans used in the sequential competition experiment does not produce FB-1 (25). Because the other focal strains are not sensitive to low concentrations of FB-1 and Fusarium species do not produce mycotoxins in high quantities on PDA, it is unlikely that production of this compound influenced our results.
Several Fusarium mycotoxins, such as fusaric acid, are known to have antibacterial properties that may influence interactions between Fusarium spp. and bacteria in the maize environment (2). Little is known about the effects of Fusarium mycotoxins on filamentous fungi. It is possible that mycotoxins other than FB-1 also have antifungal properties. This would be an important factor to consider when implementing biocontrol strategies. A consideration of both facilitative and competitive interactions between microbes could be a meaningful way to evaluate potential biocontrol agents.
Species used in interaction set 2 are also known to produce antifungal compounds. An organic extract from N. oryzae inhibited the colony growth and spore germination of several Fusarium species (41), and A. zeae produces pyrrocidines, compounds that are antagonistic to F. verticillioides and Aspergillus flavus (43). Consequently, we cannot distinguish competition for nutrients from a potential antifungal interaction.
Many experimental studies have shown that positive and negative interactions co-occur in stressful environments, and the beneficial component of the interaction often outweighs the negative component (see references 3 and 9). In this study, the outcome of some heterospecific interactions changed with addition of BOA to the medium. This may be because the effect of BOA detoxification is stronger than the effect of unknown mechanisms of competition in the presence of BOA, while in the absence of BOA, mechanisms such as nutrient competition or production of antifungal compounds alter the direction of the interaction. Such a dynamic would be explained by the MSM. Further work is needed to address these factors directly.
This study was designed to evaluate indirect species interactions, simulating a successional series where heterospecific mycelia do not intermingle. In some cases these species may also reside concurrently, with heterospecific hyphae in close proximity to one another. In such circumstances, factors such as BOA metabolism or mycotoxin production may change and shift the outcome of species interactions. The patterns observed here set an experimental baseline that is being compared with dynamics in planta in a related field study assessing the influence of F. verticillioides and benzoxazinoid production on endophyte community structure and dynamics (Saunders and Kohn, unpublished data). The investigation of mechanisms contributing to the outcome of species interactions, under controlled conditions, will supplement field data and increase our understanding of endophyte community assembly.
Acknowledgments
We thank J. F. Leslie and D. T. Wicklow for providing isolates, J. B. Anderson for helpful advice, and P. Mitrakos for assistance with HPLC.
This research was supported by a Discovery Grant to L.M.K. from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Argandona, V. H., and L. J. Corcuera. 1985. Distribution of hydroxamic acids in Zea mays tissues. Phytochemistry 24:177-178. [Google Scholar]
- 2.Bacon, C. W., D. M. Hinton, and A. Hinton. 2006. Growth-inhibiting effects of concentrations of fusaric acid on the growth of Bacillus mojavensis and other biocontrol Bacillus species. J. Appl. Microbiol. 100:185-194. [DOI] [PubMed] [Google Scholar]
- 3.Bertness, M. D., and S. M. Yeh. 1994. Cooperative and competitive interactions in the recruitment of marsh elders. Ecology 75:2416-2429. [Google Scholar]
- 4.Bravo, H. R., S. V. Copaja, and W. Lazo. 1997. Antimicrobial activity of natural 2-benzoxazolinones and related derivatives. J. Agric. Food Chem. 45:3255-3257. [Google Scholar]
- 5.Bravo, J. M., S. Campo, I. Murillo, M. Coca, and B. S. Segundo. 2003. Fungus- and wound-induced accumulation of mRNA containing a class II chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 52:745-759. [DOI] [PubMed] [Google Scholar]
- 6.Bruno, J. F., J. J. Stachowicz, and M. D. Bertness. 2003. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18:119-125. [Google Scholar]
- 7.Callaway, R. M. 1994. Facilitative and interfering effects of Arthrocnemum subterminale on winter annuals. Ecology 75:681-686. [Google Scholar]
- 8.Callaway, R. M., R. W. Brooker, P. Choler, Z. Kikvidze, C. J. Lortie, R. Michalet, L. Paolini, F. I. Pugnaire, B. Newingham, E. T. Aschehoug, C. Armas, D. Kikodze, and B. J. Cook. 2002. Positive interactions among alpine plants increase with stress. Nature 417:844-848. [DOI] [PubMed] [Google Scholar]
- 9.Callaway, R. M., and L. R. Walker. 1997. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958-1965. [Google Scholar]
- 10.Cambier, V., T. Hance, and E. de Hoffmann. 2000. Variation of DIMBOA and related compounds content in relation to the age and plant organ in maize. Phytochemistry 53:223-229. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z. Y., R. L. Brown, K. Rajasekaran, K. E. Damann, and T. E. Cleveland. 2006. Identification of a maize kernel pathogenesis-related protein and evidence for its involvement in resistance to Aspergillus flavus infection and aflatoxin production. Phytopathology 96:87-95. [DOI] [PubMed] [Google Scholar]
- 12.Corcuera, L. J., M. D. Woodward, J. P. Helgeson, A. Kelman, and C. D. Upper. 1978. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4h)-one, an inhibitor from Zea mays with differential activity against soft rotting Erwinia species. Plant Physiol. 61:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordero, M. J., D. Raventos, and B. Sansegundo. 1994. Differential expression and induction of chitinases and beta-1,3-glucanases in response to fungal infection during germination of maize seeds. Mol. Plant-Microbe Interact. 7:23-31. [Google Scholar]
- 14.Desjardins, A. E. 2003. Gibberella from A (venaceae) to Z (eae). Annu. Rev. Phytopathol. 41:177-198. [DOI] [PubMed] [Google Scholar]
- 15.Desjardins, A. E., and R. H. Proctor. 2001. Biochemistry and genetics of Fusarium toxins, p. 50-69. In B. A. Summerell, J. F. Leslie, D. Backhouse, W. L. Bryden, and L. W. Burgess (ed.), Fusarium: Paul E. Nelson memorial symposium. American Phytopathology Society Press, St. Paul, MN.
- 16.Fisher, P. J., O. Petrini, and H. M. L. Scott. 1992. The distribution of some fungal and bacterial endophytes in maize (Zea mays L). New Phytol. 122:299-305. [DOI] [PubMed] [Google Scholar]
- 17.Glenn, A. E., D. M. Hinton, I. E. Yates, and C. W. Bacon. 2001. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 67:2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn, A. E., F. I. Meredith, W. H. Morrison, and C. W. Bacon. 2003. Identification of intermediate and branch metabolites resulting from biotransformation of 2-benzoxazolinone by Fusarium verticillioides. Appl. Environ. Microbiol. 69:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon, T. R. 2006. Pitch canker disease of pines. Phytopathology 96:657-659. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto, Y., and K. Shudo. 1996. Chemistry of biologically active benzoxazinoids. Phytochemistry 43:551-559. [DOI] [PubMed] [Google Scholar]
- 21.Keyser, Z., H. F. Vismer, J. A. Klaasen, P. W. Snijman, and W. F. O. Marasas. 1999. The antifungal effect of fumonisin B-1 on Fusarium and other fungal species. S. Afr. J. Sci. 95:455-458. [Google Scholar]
- 22.Klaasen, J. A., and P. E. Nelson. 1998. Identity of Fusarium nygamai isolates with long and short microconidial chains from millet, sorghum and soil in Africa. Mycopathologia 140:171-176. [DOI] [PubMed] [Google Scholar]
- 23.Klun, J. A., and J. F. Robinson. 1969. Concentration of 2 1,4-benzoxazinones in dent corn at various stages of development of plant and its relation to resistance of host plant to European corn borer. J. Econ. Entomol. 62:214-220. [Google Scholar]
- 24.Leslie, J. F. 1995. Gibberella fujikuroi: available populations and variable traits. Can. J. Bot. 73:S282-S291. [Google Scholar]
- 25.Leslie, J. F., R. D. Plattner, A. E. Desjardins, and C. J. R. Klittich. 1992. Fumonisin B1 production by strains from different mating populations of Gibberella fujikuroi (Fusarium section Liseola). Phytopathology 82:341-345. [Google Scholar]
- 26.Long, B. J., G. M. Dunn, J. S. Bowman, and D. G. Routley. 1977. Relationship of hydroxamic acid content in corn and resistance to corn leaf aphid. Crop Sci. 17:55-58. [Google Scholar]
- 27.Luongo, L., M. Galli, L. Corazza, E. Meekes, L. De Haas, C. L. Van der Plas, and J. Kohl. 2005. Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci. Technol. 15:229-242. [Google Scholar]
- 28.Macias, F. A., D. Marin, A. Oliveros-Bastidas, D. Castellano, A. M. Simonet, and J. M. G. Molinillo. 2006. Structure-activity relationship (SAR) studies of benzoxazinones, their degradation products, and analogues. Phytotoxicity on problematic weeds Avena fatua L. and Lolium rigidum Gaud. J. Agric. Food Chem. 54:1040-1048. [DOI] [PubMed] [Google Scholar]
- 29.Marasas, W. F. O., P. E. Nelson, and T. A. Toussoun. 1984. Toxigenic Fusarium species: identity and mycotoxicology. Pennsylvania State University Press, University Park.
- 30.Menge, B. A., and J. P. Sutherland. 1987. Community regulation: variation in disturbance, competition, and predation in relation to environmental-stress and recruitment. Am. Nat. 130:730-757. [Google Scholar]
- 31.Menge, B. A., and J. P. Sutherland. 1976. Species-diversity gradients: synthesis of roles of predation, competition, and temporal heterogeneity. Am. Nat. 110:351-369. [Google Scholar]
- 32.Morrissey, J. P., and A. E. Osbourn. 1999. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 63:708-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munkvold, G. P., D. C. McGee, and W. M. Carlton. 1997. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 87:209-217. [DOI] [PubMed] [Google Scholar]
- 34.Niemeyer, H. M. 1988. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry 27:3349-3358. [Google Scholar]
- 35.Park, W. J., F. Hochholdinger, and M. Gierl. 2004. Release of the benzoxazinoids defense molecules during lateral- and crown root emergence in Zea mays. J. Plant Physiol. 161:981-985. [DOI] [PubMed] [Google Scholar]
- 36.Richardson, M. D., and C. W. Bacon. 1995. Catabolism of 6-methoxy-benzoxazolinone and 2-belazoxazolinone by Fusarium moniliforme. Mycologia 87:510-517. [Google Scholar]
- 37.Schulz, B., and C. Boyle. 2005. The endophytic continuum. Mycol. Res. 109:661-686. [DOI] [PubMed] [Google Scholar]
- 38.Sicker, D., M. Frey, M. Schulz, and A. Gierl. 2000. Role of natural benzoxazinones in the survival strategy of plants. Int. Rev. Cytol. 198:319-346. [DOI] [PubMed] [Google Scholar]
- 39.Sicker, D., B. Schneider, L. Hennig, M. Knop, and M. Schulz. 2001. Glycoside carbamates from benzoxazolin-2(3H)-one detoxification in extracts and exudates of corn roots. Phytochemistry 58:819-825. [DOI] [PubMed] [Google Scholar]
- 40.Stachowicz, J. J. 2001. Mutualism, facilitation, and the structure of ecological communities. Bioscience 51:235-246. [Google Scholar]
- 41.Szewczuk, V., W. Kita, B. Jarosz, W. Truszkowska, and A. Siewinski. 1991. Metabolites of Nigrospora: growth-inhibition of some phytopathogenic fungi by organic extracts from Nigrospora oryzae (Berkeley and Broome) Petch. J. Basic Microbiol. 31:69-73. [Google Scholar]
- 42.Warfield, C. Y., and R. M. Davis. 1996. Importance of the husk covering on the susceptibility of corn hybrids to Fusarium ear rot. Plant Dis. 80:208-210. [Google Scholar]
- 43.Wicklow, D. T., S. Roth, S. T. Deyrup, and J. B. Gloer. 2005. A protective endophyte of maize: Acremonium zeae antibiotics inhibitory to Aspergillus flavus and Fusarium verticillioides. Mycol. Res. 109:610-618. [PubMed] [Google Scholar]
- 44.Wicklow, D. T., D. K. Weaver, and J. E. Throne. 1998. Fungal colonists of maize grain conditioned at constant temperatures and humidities. J. Stored Prod. Res. 34:355-361. [Google Scholar]
- 45.Yan, F., X. Liang, and X. Zhu. 1999. The role of DIMBOA on the feeding of Asian corn borer, Ostrinia furnacalis (Guenee) (Lep., Pyralidae). J. Appl. Entomol. 123:49-53. [Google Scholar]
- 46.Yue, Q., C. W. Bacon, and M. D. Richardson. 1998. Biotransformation of 2-benzoxazolinone and 6-methoxy-benoxazolinone by Fusarium moniliforme. Phytochemistry 48:451-454. [Google Scholar]
- 47.Zeller, K. A., B. A. Summerell, S. Bullock, and J. F. Leslie. 2003. Gibberella konza (Fusarium konzum) sp nov from prairie grasses, a new species in the Gibberella fujikuroi species complex. Mycologia 95:943-954. [PubMed] [Google Scholar]