Abstract
Recent studies have suggested that the structural Fe(III) within phyllosilicate minerals, including smectite and illite, is an important electron acceptor for Fe(III)-reducing microorganisms in sedimentary environments at moderate temperatures. The reduction of structural Fe(III) by thermophiles, however, has not previously been described. A wide range of thermophilic and hyperthermophilic Archaea and Bacteria from marine and freshwater environments that are known to reduce poorly crystalline Fe(III) oxides were tested for their ability to reduce structural (octahedrally coordinated) Fe(III) in smectite (SWa-1) as the sole electron acceptor. Two out of the 10 organisms tested, Geoglobus ahangari and Geothermobacterium ferrireducens, were not able to conserve energy to support growth by reduction of Fe(III) in SWa-1 despite the fact that both organisms were originally isolated with solid-phase Fe(III) as the electron acceptor. The other organisms tested were able to grow on SWa-1 and reduced 6.3 to 15.1% of the Fe(III). This is 20 to 50% less than the reported amounts of Fe(III) reduced in the same smectite (SWa-1) by mesophilic Fe(III) reducers. Two organisms, Geothermobacter ehrlichii and archaeal strain 140, produced copious amounts of an exopolysaccharide material, which may have played an active role in the dissolution of the structural iron in SWa-1 smectite. The reduction of structural Fe(III) in SWa-1 by archaeal strain 140 was studied in detail. Microbial Fe(III) reduction was accompanied by an increase in interlayer and octahedral charges and some incorporation of potassium and magnesium into the smectite structure. However, these changes in the major element chemistry of SWa-1 smectite did not result in the formation of an illite-like structure, as reported for a mesophilic Fe(III) reducer. These results suggest that thermophilic Fe(III)-reducing organisms differ in their ability to reduce and solubilize structural Fe(III) in SWa-1 smectite and that SWa-1 is not easily transformed to illite by these organisms.
Dissimilatory Fe(III) reduction has an important influence on the geochemistry of both moderate-temperature (8 to 42°C) and hot (42 to 121°C) environments (27, 28). In freshwater and marine sediments and submerged soils, mesophilic Fe(III)-reducing organisms are responsible for the anaerobic oxidation of a substantial portion of organic carbon and often play an important role in the bioremediation of organic and metal contaminants at moderate temperatures (26). In hot anaerobic environments, such as the deep continental subsurface and in marine and terrestrial hydrothermal zones, hyperthermophilic microorganisms can oxidize hydrogen and metabolize short- and long-chain organic acids and aromatic compounds to carbon dioxide by using Fe(III) as the electron acceptor (12, 14, 18, 27, 53, 54). All hyperthermophiles available in pure culture that have been tested to date can reduce Fe(III), suggesting that the ability to reduce Fe(III) is a highly conserved characteristic of hyperthermophilic microorganisms (12-18, 27, 29, 53, 56).
Recent studies suggest that the two main sources of Fe(III) in sedimentary environments are ferrihydrite and Fe(III)-bearing phyllosilicates including the iron-rich smectite SWa-1 (hereinafter SWa-1) (28, 51). The addition of both ferrihydrite and SWa-1 (31, 33, 46) to methanogenic sediments promotes the activity of indigenous Fe(III)-reducing microorganisms and switches the terminal electron-accepting process in these sediments from methane production to Fe(III) reduction.
Fe(III) hydroxides have been the focus of most studies of microbial Fe(III) reduction (26), and the importance of Fe(III)-bearing phyllosilicates for Fe(III) respiration has only recently been recognized (28, 51). Thus far, structural Fe(III) reduction has been tested only in mesophilic microorganisms (4, 6, 7, 21, 23, 25, 45, 47, 50, 58).
Here, we report on the potential for the microbial reduction of structural Fe(III) in SWa-1 by various thermophilic and hyperthermophilic Archaea and Bacteria, originally isolated with Fe(III) oxide as the terminal electron acceptor, from a variety of hot environments. The results suggest that phyllosilicates can serve as an electron acceptor for the growth of Fe(III)-reducing microorganisms in sedimentary environments at elevated temperatures. However, the conversion of smectite to illite as previously reported for the mesophilic Shewanella oneidensis (19) was not observed.
MATERIALS AND METHODS
Sources of organisms and culture conditions.
The microbial strains used in this study are listed in Table 1. They were originally enriched and isolated with poorly crystalline Fe(III) oxide (30) as the sole electron acceptor, from a wide variety of hydrothermal environments. Strict anaerobic culturing techniques were used throughout (1, 37). Gases were passed through a column of hot copper filings to remove traces of oxygen. Strain 121 (16); strains 136, 139, 140, 296, 297, and 301 and Geothermobacter ehrlichii (13); and Geoglobus ahangari (18) were grown in modified marine enrichment medium (18), supplemented with the following (per liter): Difco yeast extract, 0.10 g; Na2SeO4, 0.02 g; vitamin mixture (32), 10 ml; trace mineral solution (41), 10 ml. Geothermobacterium ferrireducens was grown in freshwater enrichment medium (15) containing the following components (per liter): poorly crystalline Fe(III) oxide, 100 mmol/liter; MgCl2·6H2O, 0.33 g; CaCl2·2H2O, 0.33 g; KCl, 0.33 g; KH2PO4, 0.33 g; NH4Cl, 0.25 g; NaHCO3, 2.5 g; Na2SeO4, 100 μM; vitamin solution (32), 10 ml; trace mineral solution (32), 10 ml. The culture medium (10 ml) was dispensed into 26-ml anaerobic pressure tubes (Bellco Glass, Inc., Vineland, NJ) and sparged with oxygen-free H2-CO2 (80:20%, vol/vol) gas for 12 to 15 min to remove dissolved oxygen. The tubes were then sealed with thick butyl rubber stoppers and autoclaved prior to medium supplementation with 0.25 mM l-cysteine-HCl and 1.3 mM FeCl2·4H2O (from concentrated anaerobic stock solutions). The final pH of the medium was ca. 6.8 to 7.0 under either N2-CO2 (in the case of G. ehrlichii) or H2-CO2 (80:20%, vol/vol, 101 kPa) atmosphere (at room temperature).
TABLE 1.
Organisms used in this study
| Strain (other designations) | Name | Lineage | Freshwater (FW) or marine (M) | Source | Reference |
|---|---|---|---|---|---|
| FW1a (JCM 12379 = ATCC BAA-426) | Geothermobacterium ferrireducens | Bacteria, Thermodesulfobacteria, Geothermobacterium | FW | Sediment from Obsidian Pool in Yellowstone National Park, WY | 15 |
| SS015 (JCM 12418 = ATCC BAA-635 = DSM 15274) | Geothermobacter ehrlichii | Bacteria, Deltaproteobacteria, Geobacteraceae, Geothermobacter | M | Diffuse-flow vent fluid from the deep-sea hydrothermal chimney called Bag City in the Pacific Ocean (46°N, 130°W) | 13 |
| 234 (JCM 12378 = ATCC BAA-426) | Geoglobus ahangari | Archaea, Euryarchaeota, Archaeoglobaceae, Geoglobus | M | Hydrothermal chimney at Guaymas Basin (27°N, 111°W), in the Pacific Ocean at the depth of 2,000 m | 18 |
| 136 | Geogemma pacifica | Archaea, Crenarchaeota, Desulfurococcales, Pyrodictiaceae, Geogemma | M | Sulfide rock from an active hydrothermal vent sulfide chimney called Godzilla in the Pacific Ocean (47°N, 129°W) | Unpublished data |
| 139 | Ferroglobus pacificus | Archaea, Euryarchaeota, Archaeoglobaceae, Ferroglobus | M | Sulfide rock from an active hydrothermal vent sulfide chimney called Godzilla in the Pacific Ocean (47°N, 129°W) | Unpublished data |
| 140 | Archaea, Euryarchaeota, Archaeoglobaceae | M | Sulfide rock from an active hydrothermal vent sulfide chimney called Godzilla in the Pacific Ocean (47°N, 129°W) | Unpublished data | |
| 121 | Geogemma barossii | Archaea, Crenarchaeota, Desulfurococcales, Pyrodictiaceae, Geogemma | M | Vent fluid from an active hydrothermal sulfide chimney called Finn in the Pacific Ocean (47°N, 129°W) | 16 |
| 296 | Geogemma indica | Archaea, Crenarchaeota, Desulfurococcales, Pyrodictiaceae, Geogemma | M | Sulfide rock samples collected from the Kairei hydrothermal vent field, located along the Central Indian Ridge (25°S, 70°E) in the Indian Ocean | Unpublished data |
| 297 | Ferroglobus indicus | Archaea, Euryarchaeota, Archaeoglobaceae, Ferroglobus | M | Sulfide rock samples collected from the Kairei hydrothermal vent field, located along the Central Indian Ridge (25°S, 70°E) in the Indian Ocean | Unpublished data |
| 301 | Archaea | M | Sulfide rock samples collected from the Edmonds vent field located along the Central Indian Ridge (23°S, 69°E) in the Indian Ocean | Unpublished data |
In order to determine the ability of the strains to use structural Fe(III) of smectite as the terminal electron acceptor, poorly crystalline Fe(III) oxide was omitted from the medium. The well-characterized ferruginous smectite SWa-1, from Grant County, WA (Source Clays Repository of the Clay Minerals Society), which has a nearly ideal formula and lacks significant amounts of impurities, was used as a source of structural Fe(III). The composition of SWa-1 shows significant amount of Fe(III) in the octahedral sheet: [Na0.87(Si7.29Al0.62)(Fe3+2.67Fe2+0.01Al1.08Mg0.23)O20(OH)4] (34). SWa-1 was prepared as previously described (47) and contained 2,869 mmol Fe(III)/g and 0.016 mmol Fe(II)/g (dry weight). SWa-1 was added from concentrated anaerobic and sterile stock solution at the particle load of 2.84 g/liter, which corresponded to a final concentration of 9.2 mmol/liter Fe(III). The medium was then inoculated with 10% of the culture (freshly grown to late log phase) to be evaluated and was incubated in the dark and without agitation. With the exception of G. ehrlichii, which was grown with dl-malate (10 mM) as the electron donor, all other organisms were grown with hydrogen as the electron donor and structural Fe(III) in SWa-1 as the sole electron acceptor (Table 2). All cultures were incubated at their optimal temperature for growth. Strain 121 was incubated at both 105°C (optimal temperature) and 115°C, in order to be able to compare the results of the two different incubation temperatures. Growth studies were initiated with a 10% inoculum of a freshly grown culture that had previously been transferred twice in a medium with H2 as the electron donor and SWa-1 as the sole electron acceptor. The incubation period for each strain was determined when no further increase in cell number and no further increase in Fe(II) production were observed at its optimum growth temperature. Cell growth and production of Fe(II) were monitored as described below.
TABLE 2.
Results of structural Fe(III) reduction by thermophilic and hyperthermophilic Fe(III)-reducing organisms in the third successive 10% transfera
| Strain | Growth temp (°C) | Structural Fe(III) reduced
|
Iron released into solution (mmol/liter)
|
|||
|---|---|---|---|---|---|---|
| Concn (mmol/liter) | % of initial structural Fe(III) | Fe(II) | [Fe(II) + Fe(III)] | Fe(III) | ||
| FW-1a | 85 | 0.00 ± 0.03 | 0 | 0.00 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.01 |
| SS015 | 55 | 0.83 ± 0.07 | 9.0 | 0.69 ± 0.01 | 0.74 ± 0.07 | 0.04 ± 0.08 |
| 234 | 85 | 0.00 ± 0.01 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 136 | 95 | 1.01 ± 0.02 | 11.0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 139 | 95 | 0.58 ± 0.06 | 6.3 | 0.00 ± 0.02 | 0.05 ± 0.05 | 0.05 ± 0.07 |
| 140 | 90 | 1.00 ± 0.03 | 10.9 | 0.61 ± 0.03 | 0.69 ± 0.01 | 0.08 ± 0.04 |
| 121 | 105 | 0.78 ± 0.01 | 8.5 | 0.11 ± 0.01 | 0.25 ± 0.00 | 0.14 ± 0.01 |
| 121 | 115 | 0.62 ± 0.01 | 6.7 | 0.16 ± 0.04 | 0.27 ± 0.00 | 0.11 ± 0.04 |
| 296 | 100 | 0.85 ± 0.00 | 9.2 | 0.00 ± 0.01 | 0.05 ± 0.00 | 0.27 ± 0.01 |
| 297 | 100 | 0.92 ± 0.00 | 10.0 | 0.00 ± 0.02 | 0.09 ± 0.04 | 0.09 ± 0.06 |
| 301 | 95 | 1.39 ± 0.03 | 15.1 | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.01 |
All organisms were incubated at their optimum growth temperatures. Strains 136, 139, 140, 121, 296, 297, and 301 were each incubated for 3 days. G. ehrlichii (strain SS015) was incubated for 7 days. Neither cell growth nor Fe(II) production was detected in the tubes containing G. ahangari (strain 234) and G. ferrireducens (strain FW-1a) with SWa-1 as the sole electron acceptor and H2 as the sole electron donor after 14 days of incubation. The data are means ± standard deviations of triplicate replicates.
Measurement of Fe(III) reduction.
Previous studies have shown that both 0.5 N HCl and dilute HF can completely extract Fe(II) from smectite, but only hydrofluoric acid is effective at extracting Fe(III) (23). For this reason, total structural Fe(II) and Fe(III) in the clay were determined with hydrofluoric acid extraction followed by the 1,10-phenanthroline assay (49) as modified by Komadel and Stucki (20). Reduction of structural Fe(III) bound in the SWa-1 was monitored by measuring the accumulation of Fe(II) over time. Fe(II) was extracted from the clay sample with 0.5 N HCl for 1 h and then separated from clay particles with microcentrifugation. The Fe(II) content was determined with ferrozine as described previously (32). To assess the concentration of dissolved iron, the SWa-1 slurry samples were filtered through a 0.2-μm filter in the anaerobic chamber. Fe(II) and total Fe were measured in the filtrate by the ferrozine assay (33).
Microscopy.
Cells were routinely examined with a Zeiss Axioskop 20 phase-contrast microscope equipped with a UV lamp, an excitation filter (LP 420), and a red-attenuation filter (BG 38). Cell numbers were determined by counting DAPI (4′,6′-diamino-2-phenylindole)-stained cells under oil immersion (×1,000 magnification) with an ocular grid and UV light (10). Cells were stained by adding a 0.5- to 1-ml aliquot of the culture to about 0.5 to 1 ml of 1× DAPI solution for 5 min; were filtered through a black Isopore membrane filter (0.2-μm pore diameter; Millipore); and then rinsed with water, air dried, and examined under UV light.
Transmission electron microscopy of thin sections of SWa-1 H2-grown cells was carried out as previously described (15). Briefly, cells were fixed for 1 h with glutaraldehyde (5%, vol/vol) in 0.1 M cacodylate buffer (pH 7.2), washed with cacodylate buffer, and postfixed with osmium tetroxide (1%, vol/vol) for 2 h. Samples were dehydrated through increasing concentrations of an ethanol series and embedded in epoxy resin, which polymerized at 70°C for 8 h. Thin sections (70 nm) were stained with 5% uranyl acetate and 0.4% lead citrate and examined with a JEOL 100S microscope. Cells of G. ehrlichii and strain 140 growing on smectite also were stained with ruthenium red to demonstrate the presence of an exopolysaccharide (EPS) matrix with a modification of the method of Vladut-Talor et al. (57). Briefly, samples of cells were prefixed with 0.2% glutaraldehyde and 0.15% ruthenium red (Sigma-Aldrich Chemical Co., St. Louis, MO) in 0.1 M cacodylate buffer (pH 7.2) for 30 min at room temperature, followed by fixation in 1% glutaraldehyde and 0.05% ruthenium red in 0.1 M cacodylate buffer (pH 7.2) for 2 h. Fixed cells were then washed five times with 0.1 M cacodylate buffer (pH 7.2) containing 0.05% ruthenium red and stained with 2% (vol/vol) OsO4 and 0.05% ruthenium red in 0.1 M cacodylate buffer (pH 7.2) for 2 h. The stained cells were then washed in 0.1 M cacodylate buffer and dehydrated in 70% (vol/vol) ethanol containing 0.05% ruthenium red, followed by 90% (vol/vol) ethanol without ruthenium red and two final washes in 100% ethanol. Dehydrated samples were embedded in Spurr resin. The thin sections (70 nm) of the embedded samples were prepared and examined with a JEOL 100S microscope.
X-ray diffraction.
Approximately 1 to 2 ml of the microbially reduced SWa-1 was dropped onto clean petrographic slides to produce an oriented mount of SWa-1 to diffract 00l reflections (38). The air-dried oriented mount was scanned using Cu radiation at 35 kV and 15 mA filtered with a graphite monochromator from 2° 2θ to 32° 2θ at approximately 1° 2θ per minute. The air-dried mount was then solvated in ethylene glycol vapor for 24 h at room temperature and scanned using the conditions mentioned above. The d001 of smectite group minerals characteristically expands to 17Å after solvation with ethylene glycol. The X-ray diffraction patterns were interpreted by comparison to known mineral diffraction patterns of phyllosilicate minerals provided in the work of Moore and Reynolds (38).
Chemical analysis of initial and bioreduced clay.
Major oxides in clay samples were determined in Activation Laboratories by an inductively coupled plasma-optical emission spectroscopy method. Prior to major oxide determination, clay samples were Na saturated with 1 M NaCl solution and dialyzed in deionized water overnight. The structural formulae of the control and the bioreduced smectites were calculated following the procedures described in the work of Moore and Reynolds (38).
RESULTS
Structural Fe(III) reduction by thermophilic and hyperthermophilic Fe(III)-reducing organisms.
Out of 10 organisms tested, eight (G. ehrlichii and strains 121, 136, 139, 140, 296, 297, and 301) grew with SWa-1 as the sole electron acceptor and reduced 6.3 to 15.1% of the structural Fe(III). Some of the cultures (strains 136 and 301) reduced structural Fe(III) but did not dissolve it, and some others (strain 140 and G. ehrlichii) dissolved a substantial portion of iron (Table 2). In general, the extent of Fe(III) reduction did not correlate with the extent of iron dissolution (Table 2). Although G. ahangari (strain 234) and G. ferrireducens (strain FW-1a) were isolated on poorly crystalline Fe(III) oxide as the sole electron acceptor and H2 as the sole electron donor, they were not capable of coupling the reduction of structural Fe(III) associated with SWa-1 to the oxidation of H2 (Table 2).
Growth of strain 140 on SWa-1.
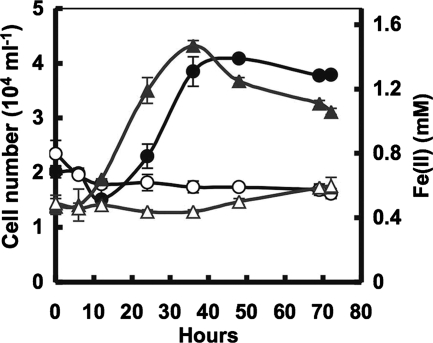
Growth was determined by an increase in cell number parallel to the production of Fe(II), resulting from reduction of structural Fe(III) bound within the lattice of phyllosilicates, over the incubation period at the optimal temperature. A 10% inoculum of a culture that had previously been transferred twice in medium with H2 as the electron donor and SWa-1 as the electron acceptor reduced 1 mmol/liter (ca. 11%) of the 9-mmol/liter structural Fe(III) after 36 h of incubation at the optimal growth temperature of 90°C (Fig. 1). Because Fe(III) is located in the octahedral sheets of this smectite, only a modest part of Fe(III) can be reduced by strain 140, and Fe(III) reduction can support only a small microbial population (Fig. 1). Due to the “masking” effect of clay minerals, cell number, which was determined by direct cell count using epifluorescence microscopy, might have been underestimated. Cell growth was not detected in any of the controls. Controls included inoculated tubes with SWa-1 as the sole electron acceptor, without H2 (H2:CO2, 80:20%, vol/vol, 101 kPa) as the electron donor (Fig. 1); inoculated tubes without the electron acceptor, with H2 (H2:CO2, 80:20%, vol/vol, 101 kPa) as the electron donor (data not shown); and uninoculated tubes with SWa-1 and H2 (H2:CO2, 80:20%, vol/vol, 101 kPa), incubated at 90°C. There was no significant reduction of Fe(III) in control tubes without H2 (Fig. 1) or in uninoculated controls (data not shown). Furthermore, Fe(III) reduction was associated with a gradual change in color of the SWa-1 (Fig. 2), which was absent in the controls, suggesting that Fe(III) reduction was not abiotic but a direct consequence of metabolic activity of strain 140 during growth on SWa-1. Similar results were also observed for strains 121, 136, 139, 140, 296, 297, and 301 and G. ehrlichii (data not shown).
FIG. 1.
Growth of strain 140 at 90°C with H2 as the electron donor and structural Fe(III) of SWa-1 as the electron acceptor. Solid circles, cells with donor; solid triangles, Fe(II) with donor; open circles, cells without donor; open triangles, Fe(II) without donor. The results are the means of duplicate cultures.
FIG. 2.
Tubes showing a gradual color change from jade green (oxidized smectite form, 0 h) to dark greenish blue (reduced form, 36 h), which is proportional to the amount of Fe(II) accumulation in the tubes and very characteristic of smectite reduction as a result of the growth of strain 140 with H2 as the electron donor and structural Fe(III) of SWa-1 as the electron acceptor.
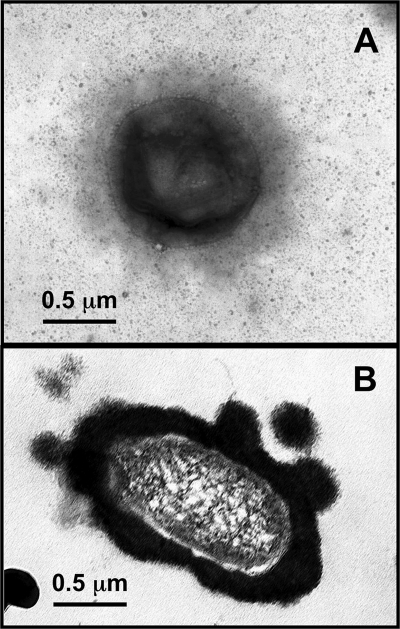
Major oxide analysis indicated that Fe(III) reduction was accompanied by a decrease in iron content and by incorporation of some magnesium, sodium, and potassium (Table 3). The reduction of structural Fe(III) in SWa-1 led to an increase in layer charge within the octahedral sheet, resulting in an increase in interlayer cations (Table 4). Electron microscopic examination of ruthenium red-stained cells of strain 140 and G. ehrlichii growing with SWa-1 smectite as the electron acceptor revealed a thick layer of electron-dense material around the cells, consistent with an EPS matrix (Fig. 3A and B, respectively). The thick electron-dense material was not observed in association with cells of other strains under investigation.
TABLE 3.
Results of chemical analysis of control SWa-1 (incubated without microorganisms) and SWa-1 bioreduced by strain 140a
| SWa-1 | Major element oxide for SWa-1 (wt %)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | |
| Control | 46.56 | 8.76 | 25.34 | 0.012 | 1.26 | 0.14 | 2.36 | 0.18 | 0.614 | 0.34 |
| Bioreduced | 43.58 | 8.51 | 22.52 | 0.014 | 2.42 | 0.10 | 2.64 | 0.25 | 0.595 | 2.32 |
The incubation period was 3 days (at 90°C).
TABLE 4.
Calculated formulae for control SWa-1 (incubated without microorganisms) and SWa-1 microbially reduced by strain 140
| SWa-1 smectite | Formulae | Charge
|
||
|---|---|---|---|---|
| Interlayer | Octahedral | Tetrahedral | ||
| Control | Mg0.07, Ca0.01, K0.02, Na0.35; Fe(III)1.45, Ti0.04, Al0.4Fe(II)0.03, Mg0.08; (Si3.60Al0.4)O10(OH)2 | 0.53 | 0.07 | 0.40 |
| Bioreduced | Mg0.13, Ca0.01, K0.01, Na0.42; Fe(III)1.26, Al0.39, Ti0.04, Fe(II)0.14, Mg0.17; (Si3.57, Al0.43)O10(OH)2 | 0.71 | 0.27 | 0.43 |
FIG. 3.
Electron micrograph of thin section of strain 140 (A) and G. ehrlichii (B) (growing with SWa-1 as the electron acceptor and H2 as the electron donor) stained with ruthenium red and showing the production of an EPS material around the cells.
X-ray diffraction analysis of bioreduced SWa-1.
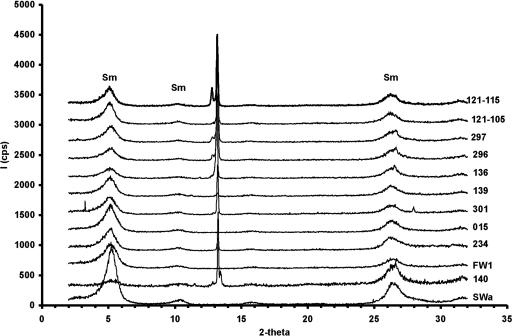
It has previously been reported that Fe(III)-reducing microorganisms play a role in the transformation of smectite to illite (19). As shown in Fig. 4, smectite is the predominant phase in these bioreduced clays based on the presence of a strong diffraction peak at 5° 2θ after solvation with ethylene glycol vapor. This peak corresponds to a d-spacing of 17Å and is interpreted to be the d001 spacing for smectite. The strong diffraction peaks observed at 26.6° 2θ are lower-order 00l reflections for smectite group minerals. There were no peaks observed at 8.8° 2θ which correspond to the strong diffraction peak (d001) for illite. The low peak/saddle ratio (ratio of the peak at 5° 2θ and saddle at 4° 2θ) is also indicative of the presence of smectite and the absence of a mixed layered illite-smectite phase. The peaks observed in the area of 13° 2θ are the diffraction of crystalline components of the growth medium, which dried with the microbially reduced clays in producing the oriented mounts. The presence of illite as either a mixed layer of illite-smectite or illite itself was not recognized from the diffraction patterns by comparison to published patterns of illite-smectite and illite (reference 38 and references therein).
FIG. 4.
X-ray diffraction patterns of initial and bioreduced SWa-1, after solvation with ethylene glycol. All organisms tested are listed on the far right and at the end of each corresponding X-ray diffraction pattern. Starting from the bottom, the strains are SWa-1 (control), followed by strain 140, G. ferrireducens (strain FW-1a), G. ahangari (strain 234), and G. ehrlichii (strain SS015) as well as strains 301, 139, 136, 296, 297, and 121 at 105°C and strain 121 at 115°C.
DISCUSSION
The results demonstrate that some thermophilic and hyperthermophilic Fe(III)-reducing microorganisms are capable of reducing the structural Fe(III) in smectite, suggesting that microbial reduction of structural Fe(III) in phyllosilicate minerals is possible in hot environments.
Extent of Fe(III) reduction and iron dissolution.
Our study showed that 6.3 to 15.1% of initial Fe(III) was reduced in a model SWa-1 smectite by thermophilic and hyperthermophilic organisms (Table 2). This is 20 to 50% less than that reported for Fe(III) reduction of the same SWa-1 by mesophilic Fe(III) reducers. The extent of Fe(III) reduction in SWa-1 was 20 to 62% by mesophilic enrichment cultures (6, 24), 20% by Geobacter spp. (24), 20 to 46% by Shewanella spp. (21, 24), and ca. 50% by Desulfitobacterium hafniense (formerly Desulfitobacterium frappieri) (47). The experiments with both mesophilic and thermophilic organisms were performed under anaerobic conditions at circumneutral pH. The main difference is higher temperature, but the extent of Fe(III) reduction in our study does not seem to correlate with the temperature (Table 2). Therefore, physical-chemical factors cannot explain the lesser extent of structural Fe(III) reduction by thermophiles. Most probably the overall decrease in the extent of Fe(III) reduction is related to differences in the electron transfer pathways.
Eight out of 10 of the thermophilic and hyperthermophilic Fe(III)-reducing organisms tested solubilized structural iron in SWa-1. The concentration of iron in solution underestimates the amount of iron liberated from the SWa-1 structure, since a substantial portion of liberated iron can be sorbed by cells and clay minerals (2, 9, 11, 55). In addition, the extent of measured iron dissolution did not correlate with the extent of microbial Fe(III) reduction (Table 2). A small amount (0.04 to 0.14 mmol/liter) of soluble Fe(III) was detected in all cultures for which iron dissolution was documented (Table 2). A similar phenomenon was observed by O'Reilly and coworkers (39), who documented partial alteration of NAu-1 smectite by both aerobic and Fe(III)-respiring cultures of Shewanella oneidensis, resulting in formation of poorly crystalline smectite, amorphous aluminosilicates, and small quantities of soluble Fe(III). The highest amounts of iron were solubilized by a bacterium in the family Geobacteraceae, G. ehrlichii, and by archaeal strain 140. Interestingly, both strains also produced copious EPS material during growth on SWa-1, as demonstrated by its ability to bind to ruthenium red (Fig. 3). Ruthenium red is a polycationic dye that specifically binds to anionic EPSs. Previous studies (3, 35, 52) have shown that acidic EPSs may bind cations such as Fe3+ and other trace metals. This and the fact that as much as 99% of dissolved Fe in the ocean is thought to be bound to organic ligands (43) strongly suggest that anionic microbial EPS materials play a key role in Fe dissolution. Furthermore, in the above-mentioned cultures of G. ehrlichii and strain 140 grown with SWa-1 and H2 the dissolved iron was mainly in the form of Fe(II), which is consistent with earlier studies (22) showing that organic ligands are capable of removing structural Fe(II) from SWa-1.
Smectite-to-illite transformation.
The kinetics of the smectite-to-illite conversion via an intermediate illite-smectite (I-S) mixed-layer phase in sediment diagenesis has been extensively studied (36). The type of stacking order, the percentage of illite layers in I-S, and the K-Ar ages of I-S are very useful in predicting the generation of crude oil and natural gas from source rocks such as shales and in gaining insights into how these hydrocarbons may have been generated in relation to the timing of the formation of hydrocarbon traps (40). As the burial temperature increases to 100 to 150°C in deep subsurface sediment, smectite is converted to illite via a solid-state transformation producing a mixed-layer phase, I-S, or by a dissolution-precipitation process. In the former solid-state mechanism, layer charge is created in smectite by substitution of Al3+ for Si4+ primarily as well as by the substitution of Mg2+ for Al3+ in the octahedral layer. After creation of layer charge, cations (primarily K+) are attracted into the interlayer. Approximately 1 atom of K is added per formula unit during illite formation. In a dissolution-precipitation mechanism, illite is formed from the dissolution of smectite without forming an intermediate I-S mixed-layer phase. The mesophilic Fe(III)-reducing bacterium Shewanella oneidensis catalyzed the smectite-to-illite transformation via a dissolution-precipitation mechanism in a laboratory study (19).
While our results did not show the formation of illite or I-S, our chemical analyses of control and bioreduced SWa-1 indicated that structural Fe(III) reduction by hyperthermophilic strain 140 was associated with an increase in layer charge in the octahedral sheet followed by the incorporation of magnesium, sodium, and potassium (mostly magnesium) into the structure. The amount of layer charge and the interlayer cations formed in the time period of this study (3 days) were not quantitatively sufficient to form an illite-like structure (Table 4). However, this does not preclude formation of illite from smectite via a solid-state transformation as the result of microbially mediated redox cycling. In an earlier study of the effect of chemical iron redox cycling in SWa-1 on potassium fixation, the amount of fixed K was documented to increase with each redox cycle, resulting in conversion of smectite to a more illitic form (48). Therefore, our study supports the idea of possible microbial involvement in the conversion of smectite to illite in the subsurface (5).
Implications for Fe(III) cycling in hot environments.
The results indicate that Fe(III)-bearing phyllosilicates such as smectite could potentially contribute to the microbial cycling of iron in hydrothermal environments (8, 42, 44). It remains to be seen whether thermophilic or hyperthermophilic microorganisms can reduce the Fe(III) in other Fe(III)-bearing phyllosilicates formed by hydrothermal metamorphism of mafic rocks (serpentine, talc, and chlorite). The inability of G. ferrireducens and G. ahangari to reduce the structural Fe(III) in smectite, despite their ability to reduce Fe(III) hydroxide, suggests that the mechanisms for electron transfer to these sources of solid-phase Fe(III) are different. Further studies are required to clarify the pathways leading to Fe(III) reduction in phyllosilicates versus Fe(III) hydroxides.
Acknowledgments
We thank Anna-Louise Reysenbach (supported by National Science Foundation grant OCE-0242038) and John A. Baross for the vent samples.
This work was supported by a start-up package provided to K.K. by the Department of Microbiology and Molecular Genetics and the Michigan Agricultural Experiment Station at Michigan State University and by a grant (MCB-0348085) to D.R.L. and K.K. from the “Microbial Observatories Program” of the National Science Foundation.
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge, T. J. 1989. Role of cellular design in bacterial metal accumulation and mineralization. Annu. Rev. Microbiol. 43:147-171. [DOI] [PubMed] [Google Scholar]
- 3.Decho, A. W. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes, p. 73-153. In M. Barnes (ed.), Oceanography marine biology annual review. Aberdeen University Press, Aberdeen, United Kingdom.
- 4.Dong, H. L., J. E. Kostka, and J. Kim. 2003. Microscopic evidence for microbial dissolution of smectite. Clays Clay Miner. 51:502-512. [Google Scholar]
- 5.Eslinger, E., P. Highsmith, D. Albers, and B. deMayo. 1979. Role of iron reduction in the conversion of smectite to illite in bentonites in the Disturbed Belt, Montana. Clays Clay Miner. 27:327-338. [Google Scholar]
- 6.Gates, W. P., A. M. Jaunet, D. Tessier, M. A. Cole, H. T. Wilkinson, and J. W. Stucki. 1998. Swelling and texture of iron-bearing smectites reduced by bacteria. Clays Clay Miner. 46:487-497. [Google Scholar]
- 7.Gates, W. P., H. T. Wilkinson, and J. W. Stucki. 1993. Swelling properties of microbially reduced ferruginous smectite. Clays Clay Miner. 41:360-364. [Google Scholar]
- 8.Geptner, A., H. Kristmannsdottir, J. Kristjansson, and V. Marteinsson. 2002. Biogenic sapontite from an active submarine hot spring, Iceland. Clays Clay Miner. 50:174-185. [Google Scholar]
- 9.Glasauer, S., S. Langley, and T. J. Beveridge. 2001. Sorption of Fe (hydr)oxides to the surface of Shewanella putrefaciens: cell-bound fine-grained minerals are not always formed de novo. Appl. Environ. Microbiol. 67:5544-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaisi, D. P., H. L. Dong, and C. Liu. 2007. Influence of biogenic Fe(II) on the extent of microbial reduction of Fe(III) in clay minerals nontronite, illite, and chlorite. Geochim. Cosmochim. Acta 71:1145-1158. [Google Scholar]
- 12.Kashefi, K. 2005. Living hell: life at high temperatures. Biochemist 27:6-10. [Google Scholar]
- 13.Kashefi, K., D. E. Holmes, J. A. Baross, and D. R. Lovley. 2003. Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal vent. Appl. Environ. Microbiol. 69:2985-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashefi, K., D. E. Holmes, D. R. Lovley, and J. M. Tor. 2004. Potential importance of dissimilatory Fe(III)-reducing microorganisms in hot sedimentary environments, p. 199-211. In W. S. D. Wilcock, E. F. DeLong, D. S. Kelley, J. A. Baross, and S. C. Cary (ed.), The subseafloor biosphere at mid-ocean ridges, vol. 144. American Geophysical Union, Washington, DC. [Google Scholar]
- 15.Kashefi, K., D. E. Holmes, A.-L. Reysenbach, and D. R. Lovley. 2002. Use of Fe(III) as an electron acceptor to recover previously uncultured hyperthermophiles: isolation and characterization of Geothermobacterium ferrireducens gen., nov., sp. nov. Appl. Environ. Microbiol. 68:1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashefi, K., and D. R. Lovley. 2003. Extending the upper temperature limit for life. Science 301:934. [DOI] [PubMed] [Google Scholar]
- 17.Kashefi, K., and D. R. Lovley. 2000. Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl. Environ. Microbiol. 66:1050-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashefi, K., J. M. Tor, D. E. Holmes, C. Gaw Van Praagh, A.-L. Reysenbach, and D. R. Lovley. 2002. Geoglobus ahangari, gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int. J. Syst. Evol. Microbiol. 52:719-728. [DOI] [PubMed] [Google Scholar]
- 19.Kim, J., H. L. Dong, J. Seabaugh, S. W. Newell, and D. D. Eberl. 2004. Role of microbes in the smectite-to-illite reaction. Science 303:830-832. [DOI] [PubMed] [Google Scholar]
- 20.Komadel, P., and J. W. Stucki. 1988. Quantitative assay of minerals for Fe2+ and Fe3+ using 1,10-phenanthroline. III. A rapid photochemical method. Clays Clay Miner. 36:379-381. [Google Scholar]
- 21.Kostka, J. E., D. D. Dalton, H. Skelton, S. Dollhopf, and J. W. Stucki. 2002. Growth of iron(III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth on a variety of oxidized iron forms. Appl. Environ. Microbiol. 68:6256-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostka, J. E., E. Haefele, R. Viehweger, and J. W. Stucki. 1999. Respiration and dissolution of iron(III) containing clay minerals by bacteria. Environ. Sci. Technol. 33:3127-3133. [Google Scholar]
- 23.Kostka, J. E., J. W. Stucki, K. H. Nealson, and J. Wu. 1996. Reduction of structural Fe(III) in smectite by a pure culture of Shewanella putrefaciens strain MR-1. Clays Clay Miner. 44:522-529. [Google Scholar]
- 24.Kostka, J. E., J. Wu, K. H. Nealson, and J. W. Stucki. 1999. The impact of structural Fe(III) reduction by bacteria on the surface chemistry of smectite clay minerals. Geochim. Cosmochim. Acta 63:3705-3713. [Google Scholar]
- 25.Li, Y.-L., H. Vali, S. K. Sears, J. Yang, B. L. Deng, and C. L. Zhang. 2004. Iron reduction and alteration of nontronite NAu-2 by a sulfate-reducing bacterium. Geochim. Cosmochim. Acta 68:3251-3260. [Google Scholar]
- 26.Lovley, D. R. 2003. Cleaning up with genomics: applying molecular biology to bioremediation. Nat. Rev. Microbiol. 1:35-44. [DOI] [PubMed] [Google Scholar]
- 27.Lovley, D. R. 2004. Potential role of dissimilatory iron reduction in the early evolution of microbial respiration, p. 301-313. In J. Seckbach (ed.), Origins, evolution and biodiversity of microbial life. Kluwer, Dordrecht, The Netherlands.
- 28.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 29.Lovley, D. R., K. Kashefi, M. Vargas, J. M. Tor, and E. L. Blunt-Harris. 2000. Reduction of humic substances and Fe(III) by hyperthermophilic microorganisms. Chem. Geol. 169:289-298. [Google Scholar]
- 30.Lovley, D. R., and E. J. P. Phillips. 1986. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl. Environ. Microbiol. 52:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovley, D. R., and E. J. P. Phillips. 1987. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53:2636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manceau, A., B. Lanson, D. Chateigner, J. Wu, D. Huo, W. P. Gates, and J. W. Stucki. 2000. Oxidation-reduction mechanism of iron in dioctahedral smectites. I. Crystal chemistry of oxidized reference nontronites. Am. Mineralogist 85:133-152. [Google Scholar]
- 35.Mancuso Nichols, C., J. P. Bowman, and J. Guezennec. 2005. Olleya marilimosa gen. nov., sp. nov., an exopolysaccharide-producing marine bacterium from the family Flavobacteriaceae, isolated from the Southern Ocean. Int. J. Syst. Evol. Microbiol. 55:1557-1561. [DOI] [PubMed] [Google Scholar]
- 36.Meunier, A., and B. D. Velde. 2004. Illite: origins, evolution, and metamorphism. Springer, Berlin, Germany.
- 37.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore, D. M., and R. C. Reynolds. 1997. X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, New York, NY.
- 39.O'Reilly, S. E., J. Watkins, and Y. Furukawa. 2005. Secondary mineral formation associated with respiration of nontronite, NAu-1 by iron-reducing bacteria. Geochem. Trans. 6:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pevear, D. R. 1999. Illite and hydrocarbon exploration. Proc. Natl. Acad. Sci. USA 96:3440-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pledger, R. J., and J. A. Baross. 1989. Characterization of an extremely thermophilic archaebacterium isolated from a black smoker polychaete (Paralvinella sp.) at the Juan de Fuca Ridge. Syst. Appl. Microbiol. 12:249-256. [Google Scholar]
- 42.Reysenbach, A. L., and E. Shock. 2002. Merging genomes with geochemistry in hydrothermal ecosystems. Science 296:1077-1082. [DOI] [PubMed] [Google Scholar]
- 43.Rue, E. L., and K. W. Bruland. 1995. Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibrium/adsorptive cathode stripping voltametric method. Mar. Chem. 50:117-138. [Google Scholar]
- 44.Russel, M. J., A. J. Hall, A. J. Boyce, and A. E. Fallick. 2005. 100th anniversary special paper: on hydrothermal convection systems and the emergence of life. Econ. Geol. 100:419-438. [Google Scholar]
- 45.Seabaugh, J. L., H. Dong, R. K. Kukkadapu, D. E. Eberl, J. P. Morton, and J. Kim. 2006. Microbial reduction of Fe(III) in the Fithian and Miloorina illites: contrasting extents and rates of bioreduction. Clays Clay Miner. 54:69-81. [Google Scholar]
- 46.Shelobolina, E. S., R. T. Anderson, Y. N. Vodyanitskii, A. V. Sivtsov, R. Yuretich, and D. R. Lovley. 2004. Importance of clay size minerals for Fe(III) respiration in a petroleum-contaminated aquifer. Geobiology 2:67-76. [Google Scholar]
- 47.Shelobolina, E. S., C. Gaw Van Praagh, and D. R. Lovley. 2003. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 20:143-156. [Google Scholar]
- 48.Shen, S., and J. W. Stucki. 1994. Effects of iron oxidation state on the fate and behaviour of potassium in soils, p. 173-185. In J. L. Havlin, J. Jacobson, P. Fixen, and G. Hergert (ed.), Soil testing: prospects for improving nutrient recommendations. SSSA special publication 40. Soil Science Society of America, Madison, WI.
- 49.Stucki, J. W. 1981. The quantitative assay of minerals for Fe2+ and Fe3+ using 1,10-phenanthroline. II. A photochemical method. Soil Sci. Soc. Am. J. 45:638-641. [Google Scholar]
- 50.Stucki, J. W., P. Komadel, and H. T. Wilkinson. 1987. Microbial reduction of structural iron(III) in smectites. Soil Sci. Soc. Am. J. 51:1663-1665. [Google Scholar]
- 51.Stucki, J. W., and J. E. Kostka. 2006. Microbial reduction of iron in smectite. C. R. Geosci. 338:468-475. [Google Scholar]
- 52.Sutherland, I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 53.Tor, J. M., K. Kashefi, and D. R. Lovley. 2001. Acetate oxidation coupled to Fe(III) reduction in hyperthermophilic microorganisms. Appl. Environ. Microbiol. 67:1363-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tor, J. M., and D. R. Lovley. 2001. Anaerobic degradation of aromatic compounds coupled to Fe(III) reduction by Ferroglobus placidus. Environ. Microbiol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 55.Urrutia, M. M., E. E. Roden, and J. M. Zachara. 1999. Influence of aqueous and solid-phase Fe(II) complexants on microbial reduction of crystalline iron(III) oxides. Environ. Sci. Technol. 33:4022-4028. [Google Scholar]
- 56.Vargas, M., K. Kashefi, E. L. Blunt-Harris, and D. R. Lovley. 1998. Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65-67. [DOI] [PubMed] [Google Scholar]
- 57.Vladut-Talor, M., T. Kauri, and D. J. Kushner. 1986. Effects of cellulose on growth, enzyme production, and ultrastructure of a Cellulomonas species. Arch. Microbiol. 144:191-195. [Google Scholar]
- 58.Wu, J., C. B. Roth, and P. F. Low. 1988. Biological reduction of structural iron in sodium-nontronite. Soil Sci. Soc. Am. J. 52:295-296. [Google Scholar]