Abstract
Efficient lactic acid production from cane sugar molasses by Lactobacillus delbrueckii mutant Uc-3 in batch fermentation process is demonstrated. Lactic acid fermentation using molasses was not significantly affected by yeast extract concentrations. The final lactic acid concentration increased with increases of molasses sugar concentrations up to 190 g/liter. The maximum lactic acid concentration of 166 g/liter was obtained at a molasses sugar concentration of 190 g/liter with a productivity of 4.15 g/liter/h. Such a high concentration of lactic acid with high productivity from molasses has not been reported previously, and hence mutant Uc-3 could be a potential candidate for economical production of lactic acid from molasses at a commercial scale.
Lactic acid can be used as a preservative, acidulant, and flavor in food, textile, and pharmaceutical industries. It could become a commodity chemical for the production of lactate esters, propylene glycol, propylene oxide, acrylic acid, 2,3-pentanedione, propanoic acidacetaldehyde, and dilactide (3, 15). It has also been increasing in importance as a feedstock for manufacture of polylactic acid (PLA), which could be a good substitute for synthetic plastic derived from petroleum feedstock. Approximately 90% of the total lactic acid produced worldwide is by bacterial fermentation (20). The chemical synthesis of lactic acid always leads to racemic mixture, which is major disadvantage. Fermentative production of lactic acid offers great advantage in producing optically pure l- or d-lactic and also dl-lactic acid, depending on the strain selected for fermentation. The optical purity of lactic acid is crucial factor in the physical properties of PLA, and it is l(+)-lactic acid that can be polymerized to a high-crystal PLA suited to commercial uses such as fibers and films (14).
Most studies within production of lactic acid have focused on the use of pure substrates such as glucose (9, 12) or lactose (5) for the production of lactic acid. The use of natural substrates like starch (4, 10, 11, 18) and cellulose (2, 6, 16) is economically unfavorable because they are very expensive and also require pretreatment in order to release fermentable sugars. The manufacturing cost of lactic acid can be significantly reduced if waste products such as whey or molasses containing fermentable sugars could be used for the production of lactic acid. India is one of the largest countries producing more than 20 million tons of cane sugar from sugar cane. During this process, a large amount of molasses is generated as the by-product, which contains 40 to 60% sucrose, which can be converted to lactic acid by the use of microorganisms.
In this paper, we describe the efficient conversion of molasses sugar by a mutant strain, Lactobacillus delbrueckii Uc-3, for lactic acid production. The mutant was isolated by UV mutagenesis followed by selection on the basis of a bigger zone of acid formation on sucrose-based medium (7). The mutant is reported to utilize glucose preferentially from high concentrations of hydrolyzed cane sugar resulting in coproduction of lactic acid and fructose (13). Recently, we have reported the complete utilization of bagasse-derived cellulose to lactic acid with an 80% yield using this strain (2), which was attributed to the presence of cellobiose and cellotriose enzymes present in the mutant strain (1).
For the evaluation of lactic acid production from cane molasses, experiments were performed in 250-ml, screw-cap flasks at 42°C with shaking at 150 rpm. The flask contained 100 ml production medium consisting of hydrolyzed 10 g cane molasses sugar, 4.0 g CaCO3, and 0.5 g yeast extract. The cane molasses was obtained from Godavari Sugar Mills Limited, Sameerwadi, India. The molasses contained sucrose (31%), glucose (9.5%), fructose (10%), and nitrogen (0.95%). The cane molasses sugar was hydrolyzed by adding 1 ml of 20% H2SO4 in 100 ml of molasses solution. The acidified molasses solution was heated in a boiling water bath for 20 min. The cane molasses contained 46 to 48% reducing sugar. The pH of the medium was adjusted to 6.5 with 4.0 M KOH prior to sterilization. The flasks were inoculated (5% inoculum) with culture grown in hydrolyzed, sucrose-based medium (7). The culture samples harvested after suitable time intervals were centrifuged at 2,000 × g for 20 min to separate the cells. The supernatant was analyzed for sugar and lactic acid and for determination of the pH of fermented broth. Lactic acid was analyzed by high-pressure liquid chromatography with UV or refractive index detectors using an Aminex HPX-87H column, and sugar was analyzed by the dinitrosalicylic acid method as reported earlier (7). The dry cell weight was determined by a calibration curve related with optical density at 660 nm to dry weight (g/liter). One unit of optical density corresponded to 0.76 g/liter of dry cell weight.
The parent strain Lactobacillus delbrueckii NCIM 2365 and mutant Uc-3 were evaluated initially for lactic acid production in molasses-based fermentation medium using 102 g/liter of cane molasses sugar and various concentrations of yeast extract. The mutant Uc-3 produced 4.5 times more lactic acid than the parent strain within 24 h using 102 g/liter of molasses sugar (Table 1). Lactic acid bacteria are generally fastidious organisms requiring complex nutrients such as amino acids and vitamins for cell growth. Yeast extract is the most commonly used nitrogen source which provides vitamin B complex content in addition to organic nitrogen to lactic acid bacteria (19). It was found that 2.5 g/liter yeast extract was sufficient to obtain maximum lactic acid with high productivity. The requirement of a smaller amount of yeast extract as a nitrogen source could be attributed to the presence of enough nitrogen in molasses. Further experiments were performed using 5 g/liter of yeast extract because we used higher molasses sugar concentrations. In order to investigate the influence of molasses concentration on lactic acid production, Lactobacillus delbrueckii mutant Uc-3 was grown using 110 to 500 g/liter of molasses (equivalent to 51 to 240 g/liter of total sugar). As shown in Table 2, the final lactic acid concentration increased with increases in the initial molasses sugar concentration up to 190 g/liter. A sharp decrease in lactic acid production was observed at 240 g/liter of molasses sugar concentration. This could be probably due to substrate inhibition, a phenomenon observed in traditional batch fermentation. Maximum lactic acid production (166 g/liter) was obtained within 40 h of fermentation with an initial molasses sugar concentration of 190 g/liter. At all molasses sugar concentrations, the lactic acid yields were 0.88 to 0.96 g/g based on the total sugar consumed and the highest yield (0.96 g/g) and productivity (4.3 g/liter/h) were obtained at 148 g/liter of molasses sugar concentration.
TABLE 1.
Lactic acid production by parent and mutant strains in cane molasses sugara
| Yeast extract concn (g/liter) | Result for:
|
|||||
|---|---|---|---|---|---|---|
| Parent strain
|
Mutant Uc-3
|
|||||
| Dry cell wt (g/liter) | Lactic acid concn (g/liter) | Productivity (g/liter/h) | Dry cell wt (g/liter) | Lactic acid concn (g/liter) | Productivity (g/liter/h) | |
| 0 | 4.5 ± 0.3 | 8.0 ± 0.7 | 0.33 ± 0.05 | 5.8 ± 0.6 | 43.6 ± 3.5 | 1.80 ± 0.1 |
| 2.5 | 5.0 ± 0.4 | 14.0 ± 1.2 | 0.58 ± 0.06 | 9.2 ± 0.8 | 74.0 ± 5.0 | 3.08 ± 0.2 |
| 5 | 5.7 ± 0.3 | 16.4 ± 1.1 | 0.68 ± 0.04 | 8.7 ± 0.6 | 73.0 ± 3.7 | 3.04 ± 0.1 |
| 10 | 5.8 ± 0.3 | 16.8 ± 1.1 | 0.70 ± 0.04 | 9.8 ± 0.7 | 73.2 ± 4.0 | 3.05 ± 0.2 |
The cultures were grown anaerobically at 42°C with shaking (150 rpm). The concentration of molasses sugar was 102 g/liter. The samples were removed after 24 h of fermentation and analyzed for growth and lactic acid. The values are averages of three independent experiments.
TABLE 2.
Effect of initial hydrolyzed molasses sugar concentration on fermentation time required, lactic acid production, dry cell weight, and lactic acid productivity of mutant Uc-3a
| Molasses sugar concn (g/liter) | Lactic acid concn in g/liter (time of analysis [h])b | Dry cell wt (g/liter) | Lactic acid yield (g/g) | Productivity (g/liter/h) |
|---|---|---|---|---|
| 51 | 40.0 ± 2.4 (16) | 7.42 ± 0.4 | 0.88 | 2.50 ± 0.2 |
| 102 | 84.6 ± 3.8 (30) | 8.75 ± 0.3 | 0.94 | 2.82 ± 0.1 |
| 123 | 105.0 ± 6.5 (30) | 14.0 ± 0.9 | 0.95 | 3.50 ± 0.2 |
| 148 | 129.0 ± 7.0 (30) | 14.0 ± 0.8 | 0.96 | 4.30 ± 0.2 |
| 190 | 166.0 ± 7.5 (40) | 15.1 ± 0.8 | 0.95 | 4.15 ± 0.2 |
| 240 | 88.2 ± 5.0 (48) | 2.70 ± 0.2 | 0.94 | 1.83 ± 0.1 |
The values are averages of three independent experiments.
The values in parentheses indicate the time points (in hours) when the samples were analyzed.
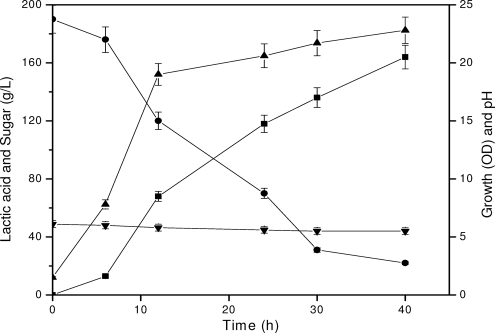
The profile of growth (optical density), pH, lactic acid production, and molasses sugar utilization is shown in Fig. 1. The maximum amount of lactic acid (166 g/liter) was produced from 190 g/liter of molasses sugar within 40 h of fermentation, with an increase in optical density from 1.5 to 21.6 and a decrease in pH from 6.0 to 5.3. The decrease in pH of the fermentation is not significant, probably due to buffering action of molasses. We observed a drastic pH drop from 6.8 to 4.5 of the fermented broth when sucrose-based production medium was used for lactic acid production (7). Hence, molasses-based production medium could be an advantage for maintaining the pH of the fermentation medium above 5.3, at which fermentation rates are faster. This could be one of the reasons for the high lactic acid productivity observed in molasses-based medium.
FIG. 1.
Profile of lactic acid production, growth, pH, and sugar utilization during fermentation by Lactobacillus delbrueckii mutant Uc-3 using a molasses sugar concentration of 190 g/liter. OD, optical density. •, molasses sugar; ▪, lactic acid; ▴, growth; ▾, pH.
Table 3 summarizes the results obtained from this work and from other literature previously reported on lactic acid concentration, lactic acid yield, and productivity in batch fermentation of molasses. Recently, Wee et al. (17) reported that a maximum lactic acid concentration of 134.9 g/liter was obtained at a molasses concentration of 333 g/liter (equivalent to 170 g/liter of total sugar) with productivity of 1.5 g/liter/h using Enterococcus faecalis. The high productivity of 4.3 was obtained at lower molasses concentrations. However, their requirement for yeast extract was very high (15 g/liter) to obtain the highest productivity of lactic acid. The Lactobacillus delbrueckii mutant Uc-3 used in this study could produce 166 g/liter of lactic acid from 400 g/liter of molasses (equivalent to 190 g/liter of total sugar). It is noteworthy that we required a very small amount of yeast extract (5 g/liter) to obtain high lactic acid productivity even at a high molasses concentration. Therefore, the mutant Uc-3 proved to be an efficient strain with very high productivity for production of lactic acid from a high concentration of molasses.
TABLE 3.
Comparison of different lactic acid-overproducing strains grown on molasses in batch fermentation
| Strain used (yeast extract concn [g/liter]) | Lactic acid concn (g/liter) | Molasses sugar concn (g/liter) | Lactic acid yield (g/g) | Fermentation time (h) | Productivity (g/liter/h) | Reference |
|---|---|---|---|---|---|---|
| Lactobacillus delbrueckii NCIMB 8130 (50) | 90.0 | 100 | 0.98 | 24 | 3.8 | 8 |
| Enterococcus faecalis RKY1 (15) | 95.7 | 102 | 0.95 | 24 | 4.0 | 17 |
| 127.0 | 136 | 0.95 | 60 | 2.1 | 17 | |
| 134.9 | 170 | 0.93 | 90 | 1.5 | 17 | |
| Lactobacillus delbrueckii Uc-3 (5) | 129.0 | 148 | 0.96 | 30 | 4.3 | This work |
| 166.0 | 190 | 0.95 | 40 | 4.15 | This work |
In conclusion, the Lactobacillus delbrueckii mutant Uc-3 proved to be a promising strain for the production of lactic acid from molasses. The requirement for yeast extract is brought down to minimum because molasses contains enough of the nitrogen source necessary to grow such fastidious organisms. Also the buffering capacity of the molasses could be an advantage in maintaining the pH of the medium above pH 5.3 during fermentation. Molasses was also proven to be an economically feasible raw material for industrial production of lactic acid since it is fortified with enough nutrients necessary for growth of lactic acid bacteria.
Acknowledgments
We acknowledge financial support from the NMITLI Division of the Council of Scientific and Industrial Research, New Delhi, India.
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Adsul, M., J. Khire, K. Bastawde, and D. Gokhale. 2007. Lactic acid production from cellobiose and cellotriose by Lactobacillus delbrueckii mutant Uc-3. Appl. Environ. Microbiol. 73:5055-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adsul, M. G., A. J. Varma, and D. V. Gokhale. 2007. Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem. 9:58-62. [Google Scholar]
- 3.Akerberg, C., and G. Zacchi. 2000. An economic evaluation of the fermentative production of lactic acid from wheat floor. Bioresour. Technol. 75:119-126. [Google Scholar]
- 4.Altaf, M., B. J. Naveena, M. Venkateshwar, E. V. Kumar, and G. Reddy. 2006. Single step fermentation of starch to L(+) lactic acid by Lactobacillus amylophilus GV6 in SSF using inexpensive nitrogen sources to replace peptone and yeast extract—optimization by RSM. Proc. Biochem. 41:465-472. [Google Scholar]
- 5.Amrane, A., and Y. A. Prigent. 1996. A novel concept of bioreactor: specialized function two-stage continuous reactor, and its application to lactose conversion in to lactic acid. J. Biotechnol. 45:195-203. [Google Scholar]
- 6.Chen, R., and Y. Y. Lee. 1997. Membrane-mediated extractive fermentation for lactic acid production from cellulosic biomass. Appl. Biochem. Biotechnol. 63:435-448. [DOI] [PubMed] [Google Scholar]
- 7.Kadam, S. R., S. S. Patil, K. B. Bastawde, J. M. Khire, and D. V. Gokhale. 2006. Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Proc. Biochem. 41:120-126. [DOI] [PubMed] [Google Scholar]
- 8.Kotzamanidis, C., T. Roukas, and G. Skaracis. 2002. Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World J. Microbiol. Biotechnol. 18:441-448. [Google Scholar]
- 9.Kwon, S., I. K. Yoo, W. G. Lee, H. N. Chang, and Y. K. Chang. 2001. High rate continuous production of lactic acid by Lactobacillus bulgaricus in two-stage membrane cell-cycle bioreactor. Biotechnol. Bioeng. 73:25-34. [DOI] [PubMed] [Google Scholar]
- 10.Linko, Y. Y., and P. Javanainen. 1996. Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb. Technol. 19:118-123. [Google Scholar]
- 11.Ohkouchi, Y., and Y. Inoue. 2006. Direct production of L(+)-lactic acid from starch and food wastes using Lactobacillus manivotivorans LMG18011. Bioresour. Technol. 97:1554-1562. [DOI] [PubMed] [Google Scholar]
- 12.Olmos-Dichara, A., F. Ampe, J. L. Uribelarrea, A. Pareileux, and G. Goma. 1997. Growth and lactic acid production by Lactobacillus casei spp. rhamnosus in batch and membrane bioreactor: influence of yeast extract and tryptone enrichment. Biotechnol. Lett. 19:709-714. [Google Scholar]
- 13.Patil, S. S., S. R. Kadam, S. S. Patil, K. B. Bastawde, J. M Khire, and D. V. Gokhale. 2006. Production of lactic acid and fructose from media with cane sugar using mutant of L. delbrueckii NCIM 2365. Lett. Appl. Microbiol. 43:53-57. [DOI] [PubMed] [Google Scholar]
- 14.Sodegard, A., and M. Stolt. 2002. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 27:1123-1163. [Google Scholar]
- 15.Vardarajan, S., and D. J. Miller. 1999. Catalytic upgrading of fermentation derived organic acids. Biotechnol. Prog. 15:845-854. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh, K. V. 1997. Simultaneous saccharification and fermentation of cellulose to lactic acid. Bioresour. Technol. 62:91-98. [Google Scholar]
- 17.Wee, Y. J., J. N. Kim, J. S. Yun, and H. W. Ryu. 2004. Utilization of sugar molasses for economical L(+)-lactic acid production by batch fermentation of Enterococcus faecalis. Enzyme Microb. Technol. 35:568-573. [Google Scholar]
- 18.Xiodong, W., G. Xuan, and S. K. Rakshit. 1997. Direct fermentative production of lactic acid on cassava and other starch substrates. Biotechnol. Lett. 19:841-843. [Google Scholar]
- 19.Yoo, I. K., N. N. Cheng, E. G. Lee, Y. K. Cheng, and S. H. Moon. 1997. Effect of B vitamin supplementation on lactic acid production by Lactobacillus casei. J. Ferment. Bioeng. 84:172-175. [Google Scholar]
- 20.Zhou, S., K. T. Shanmugam, L. P. Yomano, T. B. Grabar, and L. O. Ingram. 2006. Fermentation of 12% (w/v) glucose to 1.2 M lactate by Escherichia coli strain SZ194 using mineral salts medium. Biotechnol. Lett. 28:663-670. [DOI] [PubMed] [Google Scholar]