Abstract
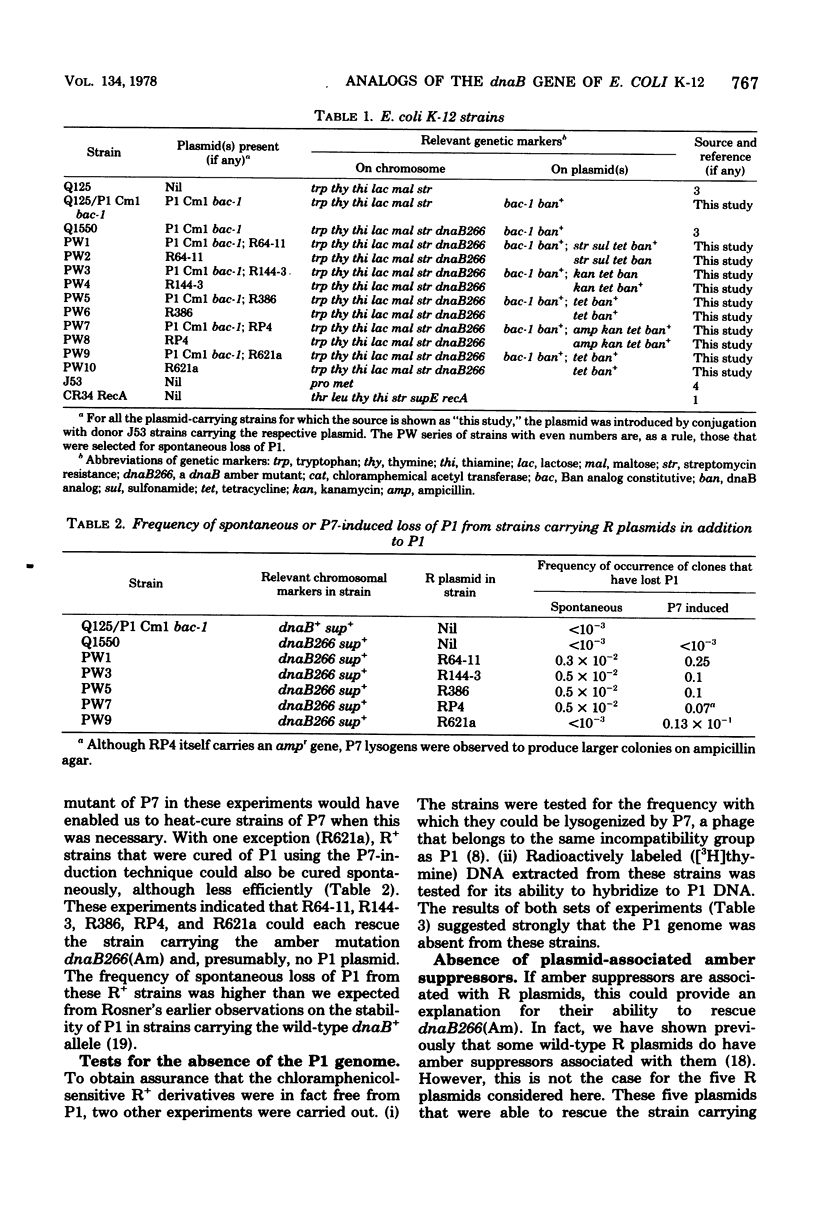
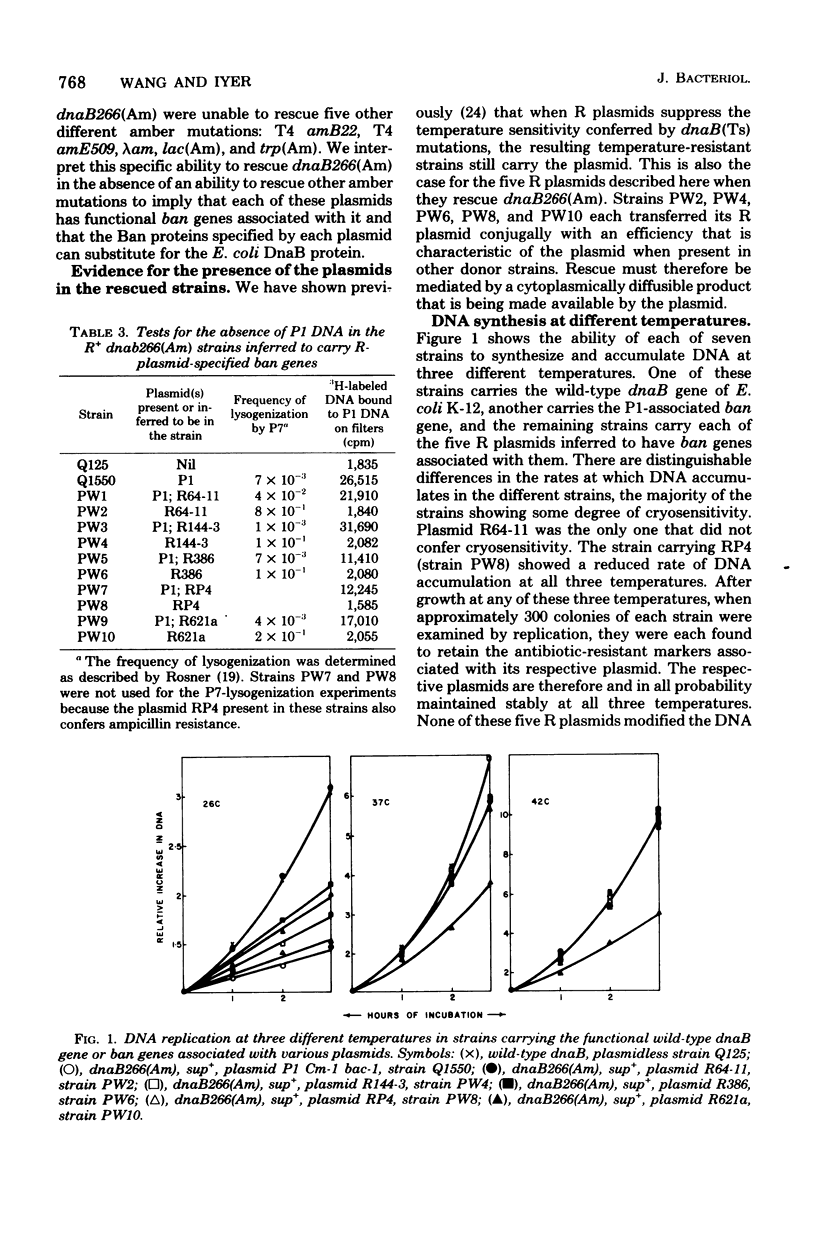
The dnaB266(Am) mutation in Escherichia coli K-12 is an amber mutation such that strains carrying this mutation are not viable in a sup+ strain. With five different R plasmids, it has been possible to construct viable R+ derivatives of this amber mutant and show that the plasmids themselves do not carry amber suppressors. This is interpreted as evidence for the presence of dnaB analog genes associated with these plasmids. Plasmid-positive strains carrying these genes often showed some degree of cryosensitivity of DNA synthesis and colony-forming ability. These observations indicate that the presence of dnaB analog genes in association with R plasmids must be relevant to the plasmid state or to some aspect of conjugative ability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanson G. S., Iyer V. N. dnaB gene of Escherichia coli K-12 affects superinfection inhibition between F' plasmids. J Bacteriol. 1975 Jul;123(1):137–146. doi: 10.1128/jb.123.1.137-146.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Jaffé-Brachet A., Touati-Schwartz D., Yarmolinsky M. B. A dnaB analog specified by bacteriophage P1. J Mol Biol. 1975 May 25;94(3):341–366. doi: 10.1016/0022-2836(75)90207-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E., Barth P. T., Grinter N. J. Compatibility properties of P1 and PhiAMP prophages. Mol Gen Genet. 1975 Dec 1;141(3):263–267. doi: 10.1007/BF00341804. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Kogoma T. Two types of temperature sensitivity in DNA replication of an Escherichia coli dnaB mutant. J Mol Biol. 1976 May 5;103(1):191–197. doi: 10.1016/0022-2836(76)90059-0. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Leidner J., Tremblay G. Y. DNA-DNA hybridization on filters at low temperature in the presence of formamide or urea. Biochimie. 1971;53(10):1111–1114. doi: 10.1016/s0300-9084(71)80201-8. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- LEDERBERG J. Cell genetics and hereditary symbiosis. Physiol Rev. 1952 Oct;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Wechsler J. A. DNA replication in dnaB mutants of Escherichia coli: gene product interaction and synthesis of 4 S pieces. J Mol Biol. 1975 Feb 15;92(1):145–163. doi: 10.1016/0022-2836(75)90095-9. [DOI] [PubMed] [Google Scholar]
- Louarn J., Funderburgh M., Bird R. E. More precise mapping of the replication origin in Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):1–5. doi: 10.1128/jb.120.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J Mol Biol. 1975 May 25;94(3):327–340. doi: 10.1016/0022-2836(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Compatibility between two F' factors in an Escherichia coli strain bearing a chromosomal mutation affecting DNA synthesis. J Mol Biol. 1971 Apr 28;57(2):319–333. doi: 10.1016/0022-2836(71)90349-4. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Schlicht M., Lanka E., Mikolajczyk M., Edelbluth C. DNA synthesis in an Escherichia coli dna B dnaC mutant. Mol Gen Genet. 1977 Feb 28;151(1):11–16. doi: 10.1007/BF00446907. [DOI] [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Wang P. Y., Iyer V. N. Suppression and enhancement of temperature sensitivity of dnaB mutations of Escherichia coli K12 by conjugative plasmids. Plasmid. 1977 Nov;1(1):19–33. doi: 10.1016/0147-619x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Wright M., Hurwitz J. Association of DNA-dependent and -independent ribonucleoside triphosphatase activities with dnaB gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):783–787. doi: 10.1073/pnas.71.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun T., Vapnek D. Electron microscopic analysis of bacteriophages P1, P1Cm, and P7. Determination of genome sizes, sequence homology, and location of antibiotic-resistance determinants. Virology. 1977 Mar;77(1):376–385. doi: 10.1016/0042-6822(77)90434-2. [DOI] [PubMed] [Google Scholar]


