Abstract
In vivo force microscopy measurements of Acidithiobacillus ferrooxidans revealed a repulsive force that was due to the presence of extracellular polymers on the bacterium's surface. Measured force-distance profiles were fit to steric force theory to estimate the density and thickness values of these exopolymers. The polymer densities were 3.4 × 1016 to 7.1 × 1016 molecules m−2, and the equilibrium thickness was 29 nm.
Extracellular polymeric substances (EPS) in large part control the interactions between Acidithiobacillus ferrooxidans and sulfide minerals (10-11, 17, 19-20). EPS play an integral part in the attachment of bacteria to sulfides and help facilitate the dissolution of metal sulfides (10-11, 17, 19-20) because of their abilities to complex Fe(III) ions, which attack the surfaces of sulfide minerals (10-11, 19). The variability in molecular weight and composition of EPS makes it difficult to determine their size and density (10). Here, we used atomic force microscopy (AFM) to observe intermolecular forces as a probe approached the surfaces of individual cells of A. ferrooxidans. By comparing the observed force-distance profiles to the steric force model, we were able to determine the length and density of EPS on the surfaces of living A. ferrooxidans bacteria in an aqueous solution. To the best of our knowledge, this represents the first time that the structure and architecture of EPS have been determined under environmentally relevant conditions (e.g., acidic pH) with A. ferrooxidans in vivo.
A. ferrooxidans (ATCC 23270) was cultivated on soluble Fe(II) at pH ∼2 in ATCC medium 2039 without Wolfe's trace mineral solution. After at least 4 days of growth time, cells were briefly centrifuged (10,500 × g) and deposited onto hydrophobic glass slides made according to Lower et al. (12). Fluorescence microscopy with fluorescein isothiocyanate-labeled concanavalin A (9, 11) revealed that EPS was present on the prepared cells. A NanoScope IV BioScope AFM (Veeco-Digital Instruments) was used to collect force curves on single cells at a frequency of 0.5 to 1.0 Hz and a relative trigger of 100 nm.
AFM measurements were performed in 0.1 M NaCl adjusted to pH ∼2 with sulfuric acid. Prior to use, V-shaped Si3N4 force probes were cleaned in a 3:1 (vol/vol) mixture of sulfuric acid and hydrogen peroxide, rinsed with water, and dried under nitrogen gas. AFM cantilevers had a spring constant of 0.08 N m−1, as determined by the method of Cleveland et al. (5). Raw data showing the photodiode detector signal (in V) as a function of the movement of the z-piezoscanner (in nm) were converted to force (nN) versus separation (nm) values (4, 8).
It was relatively simple to ensure that the AFM tip was on a bacterium, as control measurements revealed a very strong attractive force as the tip came into contact with a hydrophobic slide (see the supplemental material). Conversely, approach curves on the cells revealed only repulsive forces, as described below. A total of 450 force profiles were collected on 10 different bacterial cells prepared from three separate growth cultures. Average force profiles (per bacterium probed) were calculated using Scanning Probe Image Processor software (version 3.2).
Steric force theory (2-4) can be used to determine the force (F) as a function of the distance (D) at which the AFM probe approaches the surface of an A. ferrooxidans bacterium:
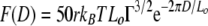 |
(1) |
where Lo is the equilibrium thickness of a polymer (in m) on the cell surface (i.e., exopolymer thickness), Γ is the polymer surface density (in m−2), r is the radius of the probe tip (2.0 × 10−8 m), kB is the Boltzmann constant (1.381 × 10−23 J K−1), and T is temperature (298 K).
Averaged force-separation profiles (Fig. 1) were mathematically fit (MATLAB, version 7.4) to the simplified steric equation 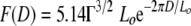 , where Lo and D are in nm and Γ is in nm−2 (Table 1). In other words, the steric model was used to reveal the probe's response to EPS on A. ferrooxidans. In addition to strain ATCC 23270, some force measurements were also conducted on A. ferrooxidans ATCC 19859. The approach force data for both strains were very similar (see the supplemental material).
, where Lo and D are in nm and Γ is in nm−2 (Table 1). In other words, the steric model was used to reveal the probe's response to EPS on A. ferrooxidans. In addition to strain ATCC 23270, some force measurements were also conducted on A. ferrooxidans ATCC 19859. The approach force data for both strains were very similar (see the supplemental material).
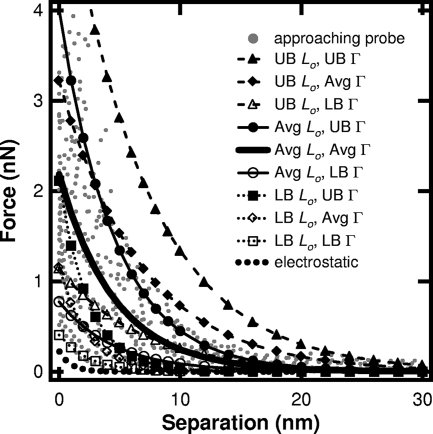
FIG. 1.
Observed force profiles for a probe on A. ferrooxidans in pH 2, 0.1 M saline solution. Steric force curves were calculated using the average (Avg), lower bound (LB), and upper bound (UB) values obtained by fitting measured force data (gray dots) to equation 1, where the tip radius is 20 nm. Lo, equilibrium thickness; Γ, polymer density. UB values are averages plus SD; LB values are averages minus SD. Also shown is the maximum electrostatic force that is expected between A. ferrooxidans (−5 mV) and the AFM tip (−5 mV) in pH 2, 0.1 M NaCl solution.
TABLE 1.
Calculated values of exopolymer thickness (Lo) and surface density (Γ) for a tip radius of 20 nm
| Cell | Lo (95% CI) (nm) | Γ (95% CI) (10−2)/nm−2 | R2 | RMSEb |
|---|---|---|---|---|
| 1a | 12.7 (9.6, 15.9) | 8.0 (6.5, 9.5) | 0.76 | 0.15 |
| 2 | 31.4 (29.5, 33.2) | 4.9 (4.6, 5.1) | 0.96 | 0.06 |
| 3 | 8.1 (7.7, 8.6) | 13.8 (13.2, 14.4) | 0.98 | 0.05 |
| 4 | 8.2 (7.1, 9.4) | 10.4 (9.5, 11.4) | 0.90 | 0.10 |
| 5 | 35.6 (34.3, 36.8) | 7.8 (7.5, 8.2) | 0.98 | 0.05 |
| 6 | 31.6 (28.1, 35.0) | 3.7 (3.4, 3.9) | 0.86 | 0.11 |
| 7a | 368.4 (362.8, 374.1) | 4.6 (4.5, 4.6) | 0.99 | 0.42 |
| 8 | 36.9 (35.0, 38.8) | 5.9 (5.5, 6.2) | 0.96 | 0.07 |
| 9 | 46.1 (43.0, 49.1) | 5.0 (4.6, 5.3) | 0.93 | 0.10 |
| 10 | 31.5 (29.1, 33.8) | 5.6 (5.2, 6.0) | 0.94 | 0.09 |
These two cells were considered outliers, as force profiles for cell 1 displayed an attractive force, rather than a repulsive one, and the Lo value for cell 7 was grossly different from that of the other cells. Therefore, force measurements on these two bacteria were not included in the computation of average values for Lo and Γ.
RMSE, root mean squared error.
We assume that the electrostatic force was also present. However, this force type is unlikely to predominate at the length scale of observed forces. This is because the Debye length is only 1.0 nm for a 0.1 M NaCl solution, and the surface potentials of the AFM probe and bacteria are very small at pH 2: ∼0 mV for the AFM tip and −5 to 0 mV for A. ferrooxidans (1, 6-7, 16, 21). Figure 1 shows the maximum expected contribution of the electrostatic force, calculated according to reference 13, assuming that both the bacterium and the tip have surface potentials of −5 mV. Clearly, the steric force predominates under these experimental conditions.
Fitting the measured forces to steric force theory produced average polymer density and equilibrium thickness values (± standard deviations [SD]) of 7.1 × 1016 (± 3.4 × 1016) m−2 and 28.7 (±13.5) nm, respectively (see average curve in Fig. 1). As shown in equation 1, the tip's radius can have a significant impact on the values estimated for polymer thickness and surface density. Therefore, we also determined these values for a tip whose radius is 60 nm, which is the maximum tip radius quoted by the manufacturer. Scanning electron micrographs collected in our laboratory (images not shown) confirmed that the tips used in our experiments had radii of less than 60 nm. With a tip radius of 60 nm, the estimated polymer density decreases to 3.4 × 1016 (± 1.6 × 1016) m−2 while the equilibrium thickness of EPS remains the same.
Our estimate of polymer thickness is smaller than that (85 ± 28 nm) determined from electron micrographs of A. ferrooxidans by Rojas et al. (18). This could be due to the fact that our analyses were conducted in solution rather than vacuum. Another difference is that we cultured our cells on soluble Fe(II), whereas Rojas's cells were grown on pyrite discs, which, according to Gehrke et al. (10), would cause up to a 12-fold increase in EPS production over cells grown with iron(II) sulfate as the energy source. It would be useful to compare the EPS thickness and density values determined from force data collected on A. ferrooxidans, which are cultured on ferrous sulfate, pyrite, and elemental sulfur. However, solids like elemental sulfur and iron oxide precipitates from pyrite oxidation adhere to the outer surface of a bacterium (1, 22). Therefore, it would be difficult to determine whether a particular force curve was due to EPS or, more likely, the mineral particles on the outside of the cell.
Our estimate of polymer density seems reasonable compared to the density of lipopolysaccharide (LPS), the dominate polymer on the outer surface of gram-negative bacteria. An average EPS density of 1016 molecules per m2 for A. ferrooxidans is smaller than the estimate of 2 × 1017 to 5 × 1017 m−2 for LPS on Escherichia coli (13-15). Our density estimate means that an A. ferrooxidans bacterium has 51,000 to 105,000 exopolymers on its outer surface, assuming a cell surface area of 1.5 μm2. This is much smaller than the 1 million to 3 million molecules of LPS on E. coli (13-15).
To the best of our knowledge, this is the first study to determine the physical architecture of EPS molecules in their native state on A. ferrooxidans in vivo. We have shown that living cells of A. ferrooxidans within an acidic solution have exopolymers that span outwards from the cell surface 29 nm and are spaced with a density of 1016 polymers m−2, which corresponds to approximately 50,000 to 100,000 EPS molecules per bacterium. These physical dimensions help to constrain the length scale and distribution of EPS molecules that A. ferrooxidans may use to bind to sulfide minerals. These dimensions also serve as a quantitative measure of the reaction space around A. ferrooxidans. Specifically, this work provides an in vivo estimate of the amount of EPS that is available to complex Fe(III), which in turn catalyzes the oxidation of sulfide minerals.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation awards 0411935 and 0525297.
We thank R. Yongsunthon for her involvement in preliminary AFM experiments, J. Bigham and O. Tuovinen for their insight into this work, and Z. Oestreicher and five anonymous reviewers for their constructive comments. S.K.L. acknowledges the support of J. Tak.
Footnotes
Published ahead of print on 2 November 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Blake, R. C., II, E. A. Shute, and G. T. Howard. 1994. Solubilization of minerals by bacteria: electrophoretic mobility of Thiobacillus ferrooxidans in the presence of iron, pyrite, and sulfur. Appl. Environ. Microbiol. 60:3349-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt, H.-J., B. Cappella, and M. Kappl. 2005. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep. 59:1-152. [Google Scholar]
- 3.Butt, H.-J., M. Kappl, H. Mueller, R. Raiteri, W. Meyer, and J. Rühe. 1999. Steric forces measured with the atomic force microscope at various temperatures. Langmuir 15:2559-2565. [Google Scholar]
- 4.Camesano, T. A., and B. E. Logan. 2000. Probing bacterial electrosteric interactions using atomic force microscopy. Environ. Sci. Technol. 34:3354-3362. [Google Scholar]
- 5.Cleveland, J. P., S. Manne, D. Bocek, and P. K. Hansma. 1993. A nondestructive method for determining the spring constant of cantilevers for atomic force microscopy. Rev. Sci. Instrum. 64:403-405. [Google Scholar]
- 6.Considine, R. F., D. R. Dixon, and C. J. Drummond. 2000. Laterally-resolved force microscopy of biological microspheres-oocysts of Cryptosporidium parvum. Langmuir 16:1323-1330. [Google Scholar]
- 7.Devasia, P., K. A. Natarajan, D. N. Sathyanarayana, and G. Ramananda Rao. 1993. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces. Appl. Environ. Microbiol. 59:4051-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson, R. J., IV, and T. A. Camesano. 2004. Nanoscale investigation of pathogenic microbial adhesion to a biomaterial. Appl. Environ. Microbiol. 70:6012-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fife, D. J., D. F. Bruhn, K. S. Miller, and D. L. Stoner. 2000. Evaluation of a fluorescent lectin-based staining technique for some acidophilic mining bacteria. Appl. Environ. Microbiol. 66:2208-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehrke, T., J. Telegdi, D. Thierry, and W. Sand. 1998. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 64:2743-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harneit, K., A. Göksel, D. Kock, J.-H. Klock, T. Gehrke, and W. Sand. 2006. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 83:245-254. [Google Scholar]
- 12.Lower, B. H., R. Yongsunthon, F. P. Vellano III, and S. K. Lower. 2005. Simultaneous force and fluorescence measurements of a protein that forms a bond between a living bacterium and a solid surface. J. Bacteriol. 187:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lower, S. K. 2005. Directed natural forces of affinity between a bacterium and mineral. Am. J. Sci. 305:752-765. [Google Scholar]
- 14.Neidhardt, F. C., and H. E. Umbarger. 1996. Chemical composition of Escherichia coli, p. 13-14. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 15.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 16.Ohmura, N., K. Kitamura, and H. Saiki. 1993. Selective adhesion of Thiobacillus ferrooxidans to pyrite. Appl. Environ. Microbiol. 59:4044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogliani, C., and E. Donati. 1999. The role of exopolymers in the bioleaching of a non-ferrous metal sulphide. J. Ind. Microbiol. Biotechnol. 22:88-92. [Google Scholar]
- 18.Rojas, J., M. Giersig, and H. Tributsch. 1995. Sulfur colloids as temporary energy reservoirs for Thiobacillus ferrooxidans during pyrite oxidation. Arch. Microbiol. 163:352-356. [Google Scholar]
- 19.Sand, W., and T. Gehrke. 2006. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(II) ions and acidophilic bacteria. Res. Microbiol. 157:49-56. [DOI] [PubMed] [Google Scholar]
- 20.Savage, D. C., and M. Fletcher. 1985. Bacterial adhesion, p. 349-351. Plenum Press, New York, NY.
- 21.Senden, T. J., and C. J. Drummond. 1995. Surface chemistry and tip-sample interactions in atomic force microscopy. Colloids Surf. A 94:29-51. [Google Scholar]
- 22.Southam, G., and T. J. Beveridge. 1993. Examination of lipopolysaccharide (O-antigen) populations of Thiobacillus ferrooxidans from two mine tailings. Appl. Environ. Microbiol. 59:1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.