Abstract
Vibrio vulnificus biotype 1 strains can be classified into two genotypes based on the PCR analysis of variations in the virulence-correlated gene (vcg). Genotype has been correlated with human infection for 90% of isolates from human cases having the vcgC sequence type and 87% of environmental strains having the vcgE variant. In this study we examined the dynamics of V. vulnificus populations and the distribution of the two genotypes recovered from oysters and surrounding estuarine wasters. Analysis of 880 isolates recovered from oysters showed a disparity in the ratio of the two genotypes, with those of the vcgE (E) genotype accounting for 84.4% of the population. In contrast, 292 isolates recovered from the waters surrounding the oyster sites revealed an almost equal distribution of the two genotypes. The levels of vcgC (C genotype) strains from both sources increased as a percentage of the population as water temperatures increased, while no culturable V. vulnificus cells were recovered from December through February. Our results suggest that there is a selective advantage for strains of the E genotype within oysters while survival of the C genotype strains may be favored by increased water column temperatures. These data suggest that the low incidence of infections may be due to the comparatively rare consumption of an oyster that contains a greater number of V. vulnificus vcgC genotype strains than of vcgE genotype strains. Levels of the two genotypes as well as seasonal dynamics within both oyster tissue and the surrounding waters may aid in identifying risk factors associated with human infection.
Filter-feeding shellfish concentrate microorganisms and often serve as a vector for transmission of disease. Vibrio vulnificus is indigenous to estuarine waters and shellfish worldwide and is commonly found in temperate waters with low to moderate salinities (1, 5, 9, 21, 23, 25, 31). Previous environmental studies have shown that oysters harboring this bacterium typically contain between 103 and 104 CFU per gram during the summer months, with only temperature showing a strong positive correlation to the levels of V. vulnificus recovered (25, 27, 31). When temperatures decline to below 13°C, the recovery of V. vulnificus diminishes as the cells enter a viable-but-nonculturable state (19).
This opportunistic human pathogen causes septicemia or gastroenteritis (following ingestion of contaminated shellfish) or wound infections (through exposure to contaminated seawater or seafood products) (6, 15). The incidence of disease in the United States parallels seasonal temperature fluctuations, with the highest rates of infection occurring when water temperatures are warmest between May and October (6, 15, 20, 25, 28). Host risk factors such as diabetes, chronic liver disease, and compromised immune status play a key role in the development of septicemia, while wound infections and gastroenteritis do not appear to be linked to underlying health conditions (15, 28). The Centers for Disease Control and Prevention estimates that between 18.5 and 26.5 million Americans may be at risk based on these factors (24), yet less than 100 cases are reported in the United States annually. The V. vulnificus populations found in oysters (Crassostrea virginica) have been shown to possess high levels of genetic diversity, yet isolates recovered from human cases have been typed to single strains (10, 13). Both the low incidence of infections and the determination that human cases arise from single strains imply that not all strains may be equally capable of causing human infection.
As reported by Rosche et al. (26), variations in the vcg gene provide a method for classifying biotype 1 strains of V. vulnificus into two distinct genotypes. Ninety percent of clinical isolates possess the vcgC sequence variant of the gene, while 87% of environmental isolates possess the vcgE sequence variation. The vcg gene has not been determined to code for any protein but appears to be genomically stable and serves as a basis for differentiating the two genotypes. Sequence variations in the 16S rRNA genes of biotype 1 strains similarly indicated that these strains could be classified into two groups (2). The 16S rRNA gene variations were identified (7, 18) as type B, which was also associated with clinical isolation (equivalent to our vcgC [C] genotype), and type A, which was associated with environmental isolation (our vcgE [E] genotype). Repetitive extragenic palindromic sequence-based PCR screening further showed that the variations in the 16S rRNA gene, along with those in the vcg gene sequences and variations in capsular polysaccharide gene sequences, strongly correlate with the two distinct genotype populations (3, 4). Studies conducted by Kim and Jeong (11) on water, oyster, and sediment samples collected in the Hadong area on the southern coast of South Korea in late August 1999 indicated that 35% of isolates possessed the type A 16S rRNA gene type while 65% possessed type B. Similar results were found for 208 pooled oyster and water samples taken from Galveston Bay in Texas between June 2000 and June 2001 (14).
Questions remain concerning the dynamics of and relationship between these two genotypes in oysters and estuarine waters, what may affect selection under environmentally diverse conditions, and how the occurrence of the two genotypes may relate to the incidence of disease. It is also unclear if levels of the two genotypes are unique to different geographical areas or certain environmental conditions. The primary goal of the studies reported here was to isolate V. vulnificus and identify the population structure within both oysters and estuarine waters in order to assess the levels of the two vcg genotypes and identify any seasonal population changes.
MATERIALS AND METHODS
Collection and treatment of samples.
Estuarine water and oyster samples were obtained at regular intervals between July 2005 and June 2006 from Alligator Bay on the eastern coast of North Carolina. A small number of samples were also collected from Cedar Key, FL, in August 2005 and August 2006. Oysters were collected from both subtidal and intertidal zones, with water samples taken from surrounding waters. Temperature and salinity measurements were taken at each site using a digital refractometer-thermometer (Atago; PAL-1). Samples were processed within one hour of collection. The external surfaces of the oysters were washed, oysters were opened with an alcohol-sterilized knife, and the entire oyster tissue was weighed and placed in a 50-ml tube. The tissue was homogenized at high speed for 60 seconds with sterile one-half strength (18-ppt) artificial seawater (ASW) in a Waring blender fitted with a 12- to 37-ml mini-sample container. A 101 dilution of each oyster homogenate was plated onto the newly developed revised cellobiose-polymixin B-colistin (CPC+) isolation agar (30) and incubated at 37°C for 24 h. To increase the sensitivity of detection of cells in estuarine waters, 10-ml water samples or 1-ml samples diluted with 9 ml of sterile one-half-strength ASW were filtered by vacuum suction onto 0.22-μm filters, which were placed directly onto CPC+ agar plates with incubation at 37°C for 24 h.
Identification of V. vulnificus isolates.
Presumptive V. vulnificus colonies on CPC+ plates were counted and at least 10%, and sometimes all, of the presumptive V. vulnificus colonies were randomly selected, with single colonies picked onto heart infusion agar plates for PCR confirmation and typing. Final V. vulnificus totals and C and E genotype ratios were based on the percentage of isolates confirmed and typed by molecular testing compared to the presumptive counts recorded. Genotype was determined by PCR gene amplification using a triprimer set for the vcgC or vcgE gene by methods previously described (26). Occasional strains that amplified both variants of the vcg gene were further analyzed for their 16S rRNA gene types (GenBank accession numbers X76333 and X76334) by PCR analysis. Primer pairs were as follows: 16S type A F1, 5′ CAT GAT AGC TTC GGC TCA A 3′ and R1, 5′ CAC TAC CAC CTT CCT CAC GAC 3′; 16S type B F1, 5′ GCC TAC GGC CCA AAG AGG 3′, and R1, 5′ CCT GCG TCT CCG CTG GCT 3′. Genomic DNA was extracted using the QIAGEN DNeasy tissue kit in accordance with the manufacturer's instructions, with PCR amplification using the Promega GoTaq system in a Px2E thermal cycler. The cycling profile was 1 cycle at 94°C for 3 min, followed by 30 cycles of 94°C for 40 s, 58°C for 30 s, and 72°C for 60 s, with 1 final extension cycle of 72°C for 2 min.
RESULTS
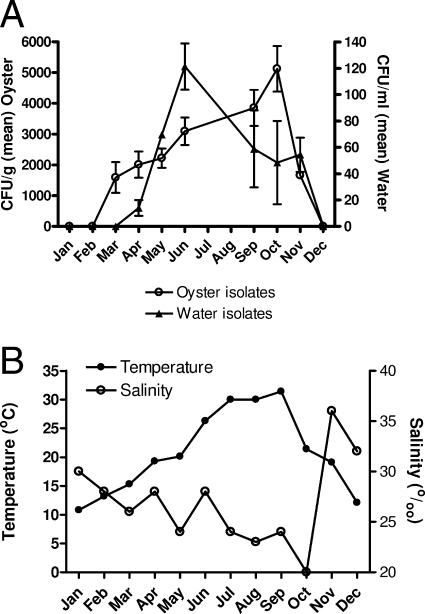
The seasonal variations in water temperature and salinity, as well as the total isolates of V. vulnificus recovered from both oyster tissue and water over the course of these studies, are shown in Fig. 1. Large seasonal fluctuations in the populations of this pathogen were evident during the 12 months of the study, with cells recovered from water reaching their highest levels in June, while the highest recovery of cells in oysters occurred several months later.
FIG. 1.
Seasonal distribution of V. vulnificus cells in oysters and surrounding waters of Alligator Bay, NC. (A) Mean of the total V. vulnificus isolates recovered from oysters (CFU/g) and water (CFU/ml). (B) Temperature (°C) and salinity (0/00) of the waters sampled.
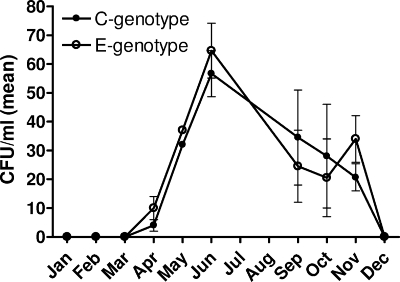
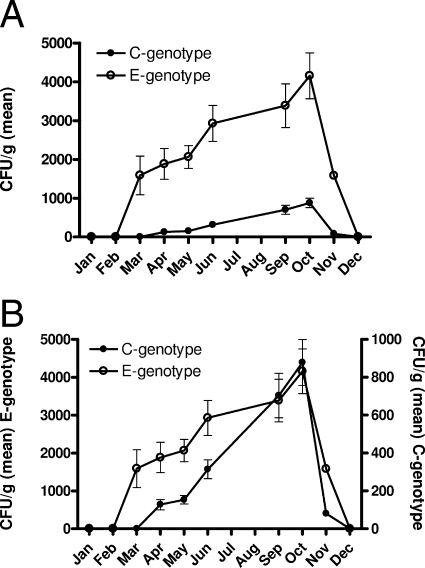
A total of 100 oysters were tested, of which 15% contained no detectable V. vulnificus. PCR analysis of the vcgE and vcgC gene variants in 880 isolates recovered from the remaining 85 oysters and 292 isolates recovered from estuarine water samples identified a marked disparity in the ratios of the C and E genotypes between the two sources. Of the 292 total isolates recovered from water, 137 (46.9%) were of the E genotype and 155 (53.1%) were of the C genotype. Overall, there were an almost equal number of both genotypes found in water samples (Fig. 2), with a peak in total recovery from the water column highest in June for both genotypes. In contrast, oyster isolates were predominately of the E genotype (Fig. 3A). Indeed, of the 743 V. vulnificus isolates recovered from oysters, 84.4% were of the E genotype while only 15.6% were of the C genotype. The seasonal trends for both the C and E genotypes, however, appeared the same (Fig. 3B).
FIG. 2.
Mean recovery of C and E genotypes of V. vulnificus from water.
FIG. 3.
Distributions of C and E genotypes of V. vulnificus in oysters. (A) Recovery of C and E genotypes on a per gram of oyster tissue basis, showing disparity in levels of the two types. (B) Recovery of genotypes demonstrating seasonality (note differences in axis units).
Strains of both genotypes were recovered in March from oyster samples, while the C genotype remained unculturable from water samples until April. Further, oysters taken from the same cluster in the oyster reef showed wide variation in the number of V. vulnificus isolates present in their tissue, with some yielding no isolates while adjacent oysters could contain more than 103 CFU/g. Only 2 oysters of the 85 that contained V. vulnificus were found to have populations in which the majority of isolates (61.1% and 66.3%) were of the C genotype, and both were recovered when water temperatures were above 30°C.
Analysis of variance showed no significant difference in the levels of recovery of the C and E genotype strains from water for any of the months in which isolates were detected, while a chi-square analysis of the populations from water and oyster tissue (Fig. 3A) was highly significant (P < 0.001) for the distribution of the two genotypes.
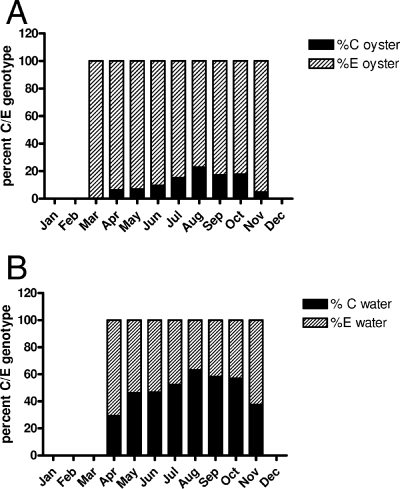
C genotype strains increased as a percentage of the total V. vulnificus population in oyster tissues and water as temperatures increased (Fig. 4A and B). In water samples recovered in April, 70.6% of the isolates were of the E genotype, but by May the ratios were nearly equal. These ratios remained stable until the water temperature reached 30°C (between August and October), when the C genotype became the dominate strain, at more than 60% of the isolates recovered. However, the C genotype strains within oysters never exceeded 23% (August sampling) of the total isolates tested.
FIG. 4.
Ratios of the means of the C and E genotypes (A) in oysters and (B) in water, demonstrating the seasonality of C genotype strains present in these sources.
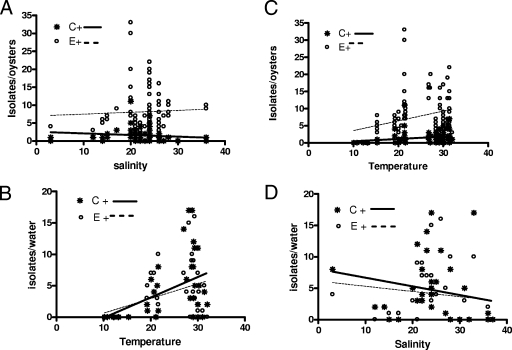
As with previous studies of total V. vulnificus levels, a significant positive correlation (Spearman correlation coefficient) with temperature (+0.31 for the C genotype, P = 0.002, and +0.32 for the E genotype, P = 0.0013) was found in oyster samples (Fig. 5A). Significant positive correlations were similarly observed for C genotype strains (+0.40, P = 0.016) and E genotype strains (+0.35, P = 0.038) and water temperature (Fig. 5B). Significant negative correlations with salinity were observed for the C genotype strains both from oysters (−0.35, P = 0.008; Fig. 5C) and from water (−0.36, P = 0.038; Fig. 5D).
FIG. 5.
Correlations between temperature and salinity and levels of C and E genotypes of V. vulnificus in oysters (A and C) and water (B and D).
In addition to strains of the two genotypes, we isolated 19 strains from oysters and 2 strains from water that amplified both the vcgE and vcgC sequences using our primers. Previous studies have also reported identification of strains possessing both 16S rRNA gene sequence variations (3). Thirteen of the vcgC+ and vcgE+ genotype strains that we isolated were further analyzed to determine their 16S rRNA sequences; 84.6% also amplified both the A and B 16S rRNA sequence types.
Sampling of V. vulnificus in July and during one of two sampling trips in August occurred prior to a change in the isolation medium from CPC (17) to CPC+ (30). This resulted in a threefold increase in the recovery of V. vulnificus on CPC+ compared to CPC (data not shown). Thus, the lower number of isolates reported here for those sampling times may not accurately reflect the true number of V. vulnificus cells which were present. Nevertheless, the percentages we observed were consistent using either medium.
A similar disparity in the ratios of the two genotypes was also identified from the limited sampling of water (75 V. vulnificus isolates) and 10 oysters (40 isolates) collected from Cedar Key, FL, in August 2005 and August 2006. Of the water isolates, 62.7% were the C genotype and 37.3% of the E genotype, whereas from oysters 20% were of the C genotype and 80% of the E genotype.
DISCUSSION
The PCR method developed by Rosche et al. (26) allows rapid identification of samples without the need for DNA extraction, and in most cases samples can be processed and the genotype identified within 48 h of collection. The medium employed, CPC+, proved to be a superior medium compared to the commonly employed CPC agar for selecting V. vulnificus from a background of a large number of competing bacteria. Indeed, 80% of presumptive water isolates and 87.3% of presumptive oyster isolates isolated on this medium were confirmed as being V. vulnificus.
While there appears to be little difference in the numbers of the two genotypes in the water column, the distribution of the two genotypes within oysters indicates that oyster uptake or colonization strongly favors the E genotype. This finding was true for the oyster samples taken in North Carolina and in Florida, suggesting that our observations are not limited to one geographical area. Peak recovery from water occurred in June, while recovery from oysters reached the highest levels in October, possibly indicating that colonization of oysters continues at the expense of cells in the water column. The seasonal fluctuations in the ratios of the C and E genotypes appeared to follow natural variations in seasonal water temperature. The National Climatic Data Center recorded hurricane Ophelia on the North Carolina coast between 7 and 17 September 2005, and this storm was associated with 10 to 12 in. of rain. This may have been responsible for the low salinities (20o/oo) recorded in October, when sampling was conducted. Water temperatures had dropped 10°C between September and October, but low salinity may have contributed to the high levels of cells recovered during these studies. There was an increase in the population of both genotypes in conjunction with increasing temperature, but the C genotype strains increased as a percentage of the population as temperatures rose. The recovery of V. vulnificus cells from oysters was found to be the highest in October and occurred following the warmest recorded water temperatures in August and September. This peak was followed by a dramatic decline in recovery of culturable cells beginning in November.
Changing weather patterns may have an impact on the levels of V. vulnificus and contribute to prolonged culturability. Lin et al. (13) reported a sharp decline in the level of recovered cells of both 16S rRNA gene types in October, similar to the decline we observed in November. Increasing surface water temperatures and decreasing salinity relative to rising sea levels are associated with global climate change (8) and have implications for the presence of potentially pathogenic strains of bacteria. Correlations between climate change and infectious bacteria have been reported previously, and both low salinity and warm temperatures are factors favorable to V. vulnificus growth. The emergence of V. vulnificus biotype 3 strains in Israel is stated to have occurred in response to unseasonably high water temperatures (22); a similar pattern has been documented for the emergence of new variants of the pathogens Vibrio cholerae and Vibrio parahaemolyticus (16).
The dichotomy in the V. vulnificus population structure seems to indicate that the E genotype is generally suited to a life within oysters, while the C strains have evolved to cope with the stresses associated with such changing habitats as occur on entry into a human host. This possibility is supported by the higher proportion of C genotype strains that are isolated from clinical cases. Further, the comparatively low numbers of the C genotype within oysters may help explain the low incidence of human infection. The fact that we found only two oysters to have C genotype strains as the dominant strain type further suggests the possibility that only those oysters harboring a larger percentage of this genotype would be likely to be infective to humans and that such oysters are generally rare within the environment. Using 16S rRNA gene sequences to type V. vulnificus, Lin and Schwarz (12) identified similar seasonal fluctuations in V. vulnificus based on 208 pooled oyster and water isolates. The overall percentages of the two populations in their study were similar to those reported here, and they also showed that the number of isolates of the 16S B type (our C genotype) was highest when water temperatures were warmest.
Our studies support the presence of a relatively small number of V. vulnificus strains that possess both versions of the 16S rRNA and vcgE and vcgC genes. The significance of these strains, if any, has yet to be determined however.
Our data raise numerous questions regarding the apparent selective ability of the E genotype to be taken up by and/or to colonize oysters. Research reported by Tall et al. (29) demonstrated that a serine protease (ECP) secreted by Perkinsus marinus may suppress the vibriocidal activity of hemocytes, which may be a factor in excluding or favoring one genotype. While we were not able to identify a correlation between the disease state of the oyster and the total V. vulnificus population (27), it is possible that the population structure and the level of C genotype strains may be influenced by the disease state of the oyster and result in a reverse in the C/E ratios, since the existence of these two genotypes was not known at the time of these previous studies.
In summary, our studies found that, while the levels of C and E genotypes of V. vulnificus in estuarine waters were approximately equal and were equally affected by water temperature, the levels within oysters were greatly skewed toward the E genotype. Prior studies that evaluated the two genotype ratios analyzed strains from multiple sources, and the pooled samples provided a general description of their distribution. Our data suggest that there is a great deal of variability even between individual oysters and that the selective advantage for strains of the E genotype may offer some explanation as to why susceptible oyster consumers only rarely develop V. vulnificus infections; numbers of the more infectious C type of the pathogen may be sufficiently low in most oysters to preclude their causing disease. In this regard, it may be especially significant that, of the 85 oysters harboring V. vulnificus, only two oysters had more strains of the C genotype than of the E genotype.
Acknowledgments
We thank Tom Rosche, Alan Buck, Brett Froelich, Ryan Bogard, Rebecca Powell, and Ashley Lakner for their assistance in collecting and processing oyster and water samples. We also thank Melissa Jones for helpful discussions during the preparation of the manuscript.
This report was prepared under award NA05N054781244 from NOAA, U.S. Department of Commerce.
The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of NOAA or the U.S. Department of Commerce.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Amaro, C., L.-I. Hor, E. Marco-Noales, T. Bosque, B. Fouz, and E. Alcaide. 1999. Isolation of Vibrio vulnificus serovar E from aquatic habitats in Taiwan. Appl. Environ. Microbiol. 65:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aznar, R., W. Ludwig, R. I. Amann, and K. H. Schleifer. 1994. Sequence determination of rRNA gene of pathogenic Vibrio species and whole-cell identification of Vibrio vulnificus with rRNA targeted oligonucleotide probes. Int. J. Syst. Bacteriol. 44:330-337. [DOI] [PubMed] [Google Scholar]
- 3.Chatzidaki-Livanis, M., M. A. Hubbard, K. Gordon, V. J. Harwood, and A. C. Wright. 2006. Genetic distinctions among clinical and environmental strains of Vibrio vulnificus. Appl. Environ. Microbiol. 72:6136-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzidaki-Livanis, M., M. K. Jones, and A. C. Wright. 2006. Genetic variation in the Vibrio vulnificus capsular polysaccharide operon. J. Bacteriol. 188:1987-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima, H., and S. Ryotaro. 2004. Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol. Ecol. 48:221-229. [DOI] [PubMed] [Google Scholar]
- 6.Gulig, P., K. L. Bourdage, and A. M. Starks. 2005. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43:118-131. [PubMed] [Google Scholar]
- 7.Gutacker, M., N. Coza, C. Benagli, A. Pedroli, M. V. Bernasconi, R. Aznar, and J.-C. Piffaretti. 2003. Population genetics of Vibrio vulnificus: identification of two divisions and a distinct eel-pathogenic clone. Appl. Environ. Microbiol. 69:3203-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296:2158-2162. [DOI] [PubMed] [Google Scholar]
- 9.Hoi, L., J. L. Larsen, I. Dalsgaard, and A. Dalsgaard. 1998. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl. Environ. Microbiol. 64:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, J. K., R. L. Murphree, and M. Tamplin. 1997. Evidence that mortality from Vibrio vulnificus infection results from single strains among heterogeneous populations in shellfish. J. Clin. Microbiol. 35:2098-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, M. S., and H. D. Jeong. 2001. Development of 16S rRNA targeted PCR methods for the detection and differentiation of Vibrio vulnificus in marine environments. Aquaculture 193:199-211. [Google Scholar]
- 12.Lin, M., and J. R. Schwarz. 2003. Seasonal shifts in population structure of Vibrio vulnificus in an estuarine environment as reveled by partial 16S ribosomal DNA sequencing. FEMS Microbiol. Ecol. 43:23-27. [DOI] [PubMed] [Google Scholar]
- 13.Lin, M., D. A. Payne, and J. R. Schwarz. 2003. Intraspecific diversity of Vibrio vulnificus in Galveston Bay water and oysters as determined by randomly amplified polymorphic DNA PCR. Appl. Environ. Microbiol. 69:3170-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, M., and J. R. Schwarz. 2003. Seasonal shifts in population structure of Vibrio vulnificus in an estuarine environment as revealed by partial 16S ribosomal DNA sequencing. FEMS Microbiol. Ecol. 45:23-27. [DOI] [PubMed] [Google Scholar]
- 15.Linkous, D., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 16.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate change on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massad, G., and J. D. Oliver. 1987. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl. Environ. Microbiol. 53:2262-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson, W. B., R. Paranjype, A. DePola, and M. S. Strom. 2003. Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J. Clin. Microbiol. 41:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver, J. D. 1995. The viable but non-culturable state in the human pathogen Vibrio vulnificus. FEMS Microbiol. Lett. 133:203-208. [DOI] [PubMed] [Google Scholar]
- 20.Oliver, J. D. 2006. Vibrio vulnificus, p. 349-366. In B. A. F. L. Thompson and J. Swings (ed.), The biology of Vibrios. ASM Press, Washington, DC.
- 21.Parvathi, A., H. S. Kumar, I. Karunasagar, and I. Karunasagar. 2004. Detection and enumeration of Vibrio vulnificus in oysters from two estuaries along the southwest coast of India, using molecular methods. Appl. Environ. Microbiol. 70:6909-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paz, S., N. Bisharat, E. Paz, O. Kidar, and D. Cohen. 2006. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ. Res. 103:390-396. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer, C. S., M. F. Hite, and J. D. Oliver. 2003. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 69:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pleis, J. R., and M. Leithbridge-Cejku. 2006. Summary health statistics for U.S. adults: national health interview survey, 2005. Centers for Disease Control and Prevention, Atlanta, GA.
- 25.Randa, M. A., M. F. Polz, and E. Lim. 2004. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 70:5469-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosche, T. M., Y. Yano, and J. D. Oliver. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 49:381-389. [DOI] [PubMed] [Google Scholar]
- 27.Sokolova, I. M., L. Leamy, M. Harrison, and J. D. Oliver. 2005. Intrapopulational variation in Vibrio vulnificus levels in Crassostrea virginica (Gmelin 1971) is associated with the host size but not with disease status or developmental stability. J. Shellfish Res. 24:502-508. [Google Scholar]
- 28.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 29.Tall, B. D., J. F. La Peyre, J. W. Bier, M. D. Miliotis, D. E. Hanes, M. H. Kothary, D. B. Shah, and M. Faisal. 1999. Perkinsus marinus extracellular protease modulates survival of Vibrio vulnificus in eastern oyster (Crassostrea virginica) hemocytes. Appl. Environ. Microbiol. 65:4261-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner, E., and J. D. Oliver. 2007. Refined medium for direct isolation of Vibrio vulnificus from oyster tissue and seawater. Appl. Environ. Microbiol. 73:3098-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright, A. C., R. T. Hill, J. A. Johnson, M.-C. Roghman, R. R. Cowell, and J. Glenn Morris. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]