Abstract
The phospholipids of Methylococcus capsulatus, Methylosinus trichosporium, La Paz, and OBT were examined in relation to their qualitative and quantitative composition. M. capsulatus exhibited a phospholipid composition consisting of phosphatidylethanolamine, phosphatidylglycerol, cardiolipin, and phosphatidyl-choline. The esterified fatty acids were predominantly C16:0 and C16:1. M. trichosporium, La Paz, and OBT exhibited an essentially identical phospholipid composition consisting of phosphatidylmonomethylethanolamine, phosphatidyl-dimethylethanolamine, phosphatidylcholine, and phosphatidylglycerol. Only trace amounts (less than 1%) of cardiolipin were found in these organisms. The major esterified fatty acid in these organisms was C18:1 (87 to 90%). The monounsaturated fatty acids from all four organisms consisted of both cis and trans isomers, each of which contained delta8, delta9, delta10, and delta11 double-bond positional isomers.
Full text
PDF
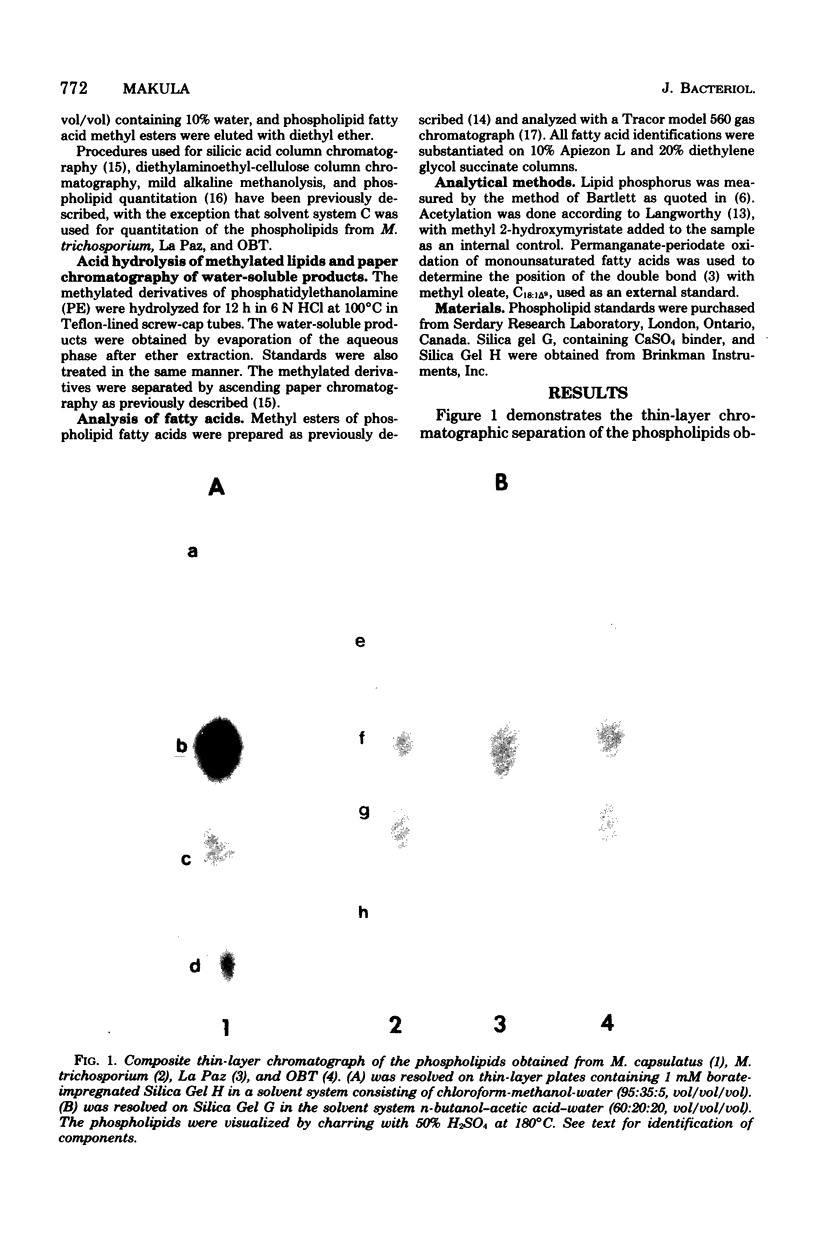

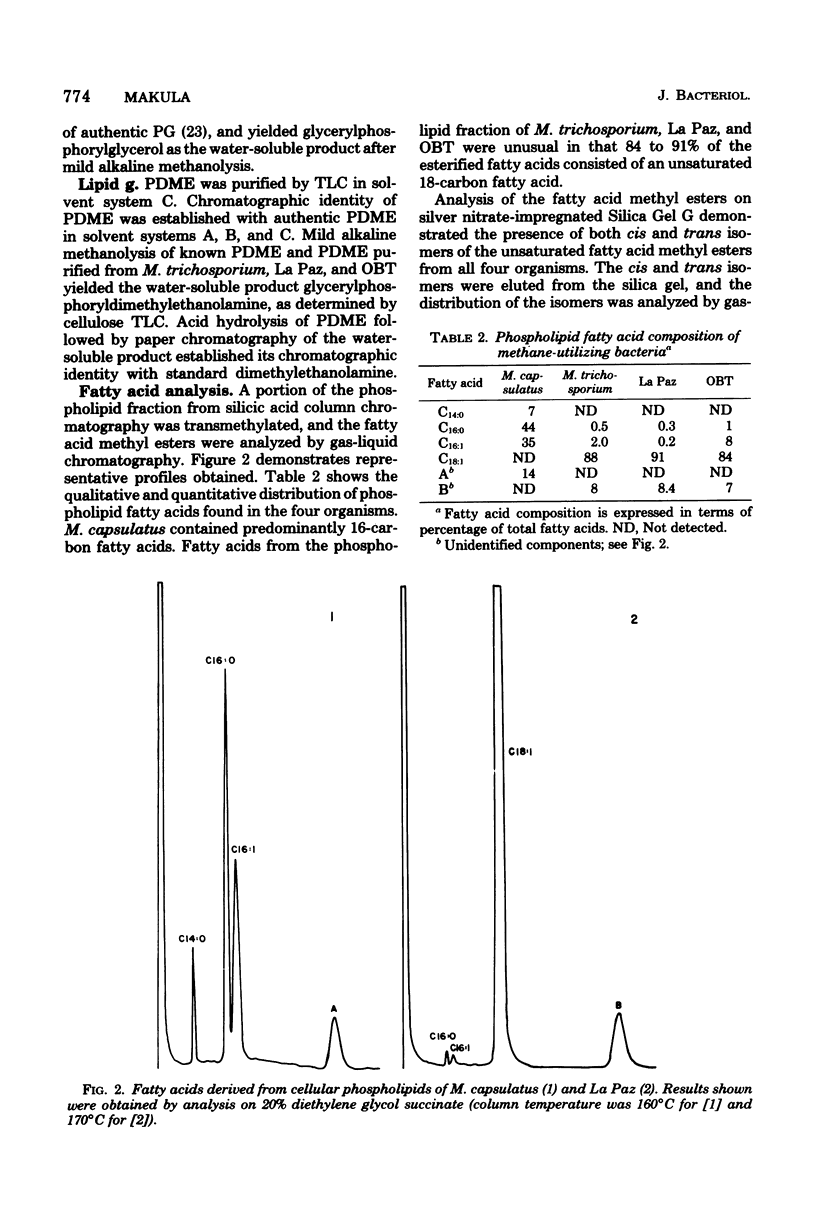
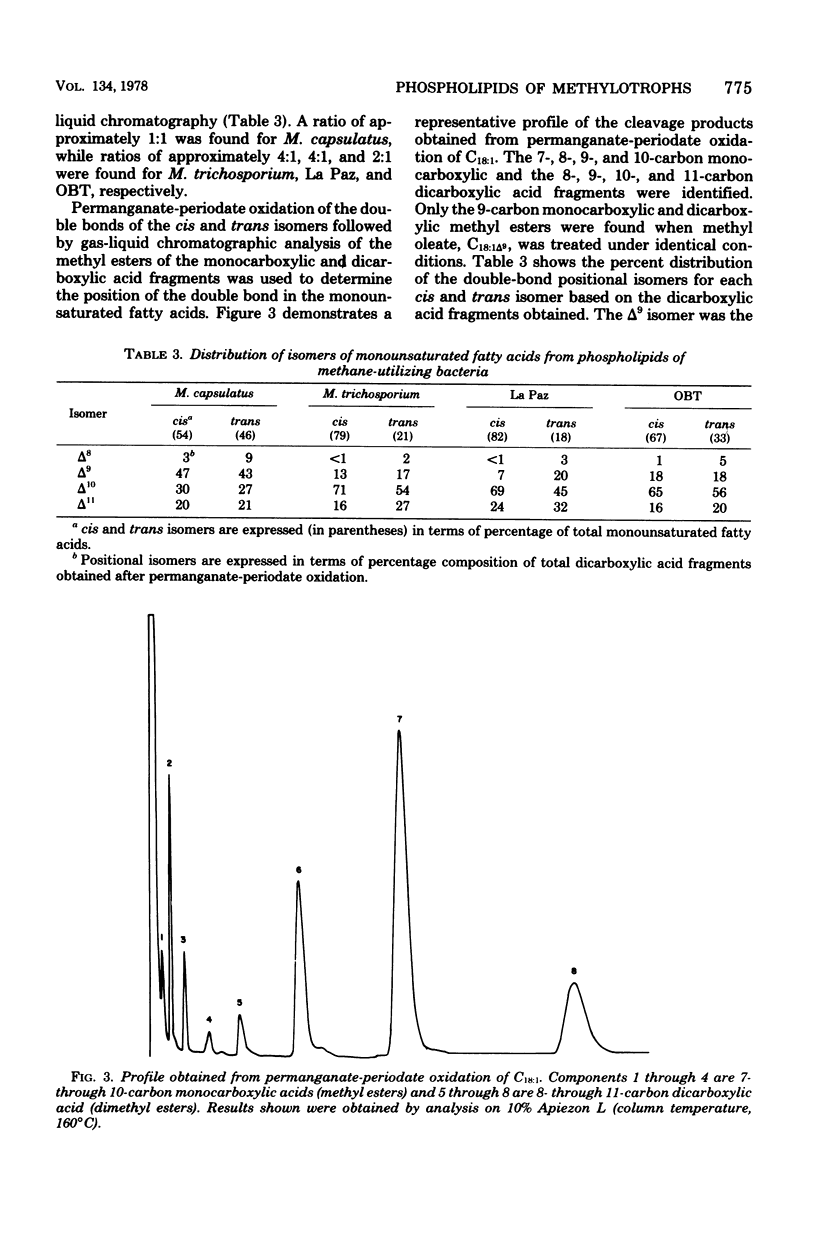


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R., Makula R. A. Microbial lipid metabolism. CRC Crit Rev Microbiol. 1975 Oct;4(1):1–40. doi: 10.3109/10408417509105485. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. The biosynthesis of unsaturated fatty acids by bacilli. I. Temperature induction of the desaturation reaction. J Biol Chem. 1969 Feb 10;244(3):889–895. [PubMed] [Google Scholar]
- Fulco A. J. The effect of temperature on the formation of delta 5-unsaturated fatty acids by bacilli. Biochim Biophys Acta. 1967 Dec 5;144(3):701–703. doi: 10.1016/0005-2760(67)90065-3. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Jensen A. P. Phospholipid and fatty acid composition of methanol-utilizing bacteria. J Bacteriol. 1977 Apr;130(1):535–537. doi: 10.1128/jb.130.1.535-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A. Long-chain diglycerol tetraethers from Thermoplasma acidophilum. Biochim Biophys Acta. 1977 Apr 26;487(1):37–50. doi: 10.1016/0005-2760(77)90042-x. [DOI] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Microbial assimilation of hydrocarbons: identification of phospholipids. J Bacteriol. 1970 Aug;103(2):348–355. doi: 10.1128/jb.103.2.348-355.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Phospholipid composition of Desulfovibrio species. J Bacteriol. 1974 Dec;120(3):1279–1283. doi: 10.1128/jb.120.3.1279-1283.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. I. Fatty acids derived from normal alkanes. J Bacteriol. 1968 Jun;95(6):2102–2107. doi: 10.1128/jb.95.6.2102-2107.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Makula R. A., Finnerty W. R. Metabolism of the alkane analogue n-dioctyl ether by Acinetobacter species. J Bacteriol. 1977 Jul;131(1):92–97. doi: 10.1128/jb.131.1.92-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senff L. M., Wegener W. S., Brooks G. F., Finnerty W. R., Makula R. A. Phospholipid composition and phospholipase A activity of Neisseria gonorrhoeae. J Bacteriol. 1976 Aug;127(2):874–880. doi: 10.1128/jb.127.2.874-880.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa R. E., Makula R. A., Finnerty W. R. Characterization of lysocardiolipin from Acinetobacter sp. HO1-N. J Bacteriol. 1977 Aug;131(2):486–492. doi: 10.1128/jb.131.2.486-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Weaver T. L., Patrick M. A., Dugan P. R. Whole-cell and membrane lipids of the methylotrophic bacterium Methylosinus trichosporium. J Bacteriol. 1975 Nov;124(2):602–605. doi: 10.1128/jb.124.2.602-605.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]