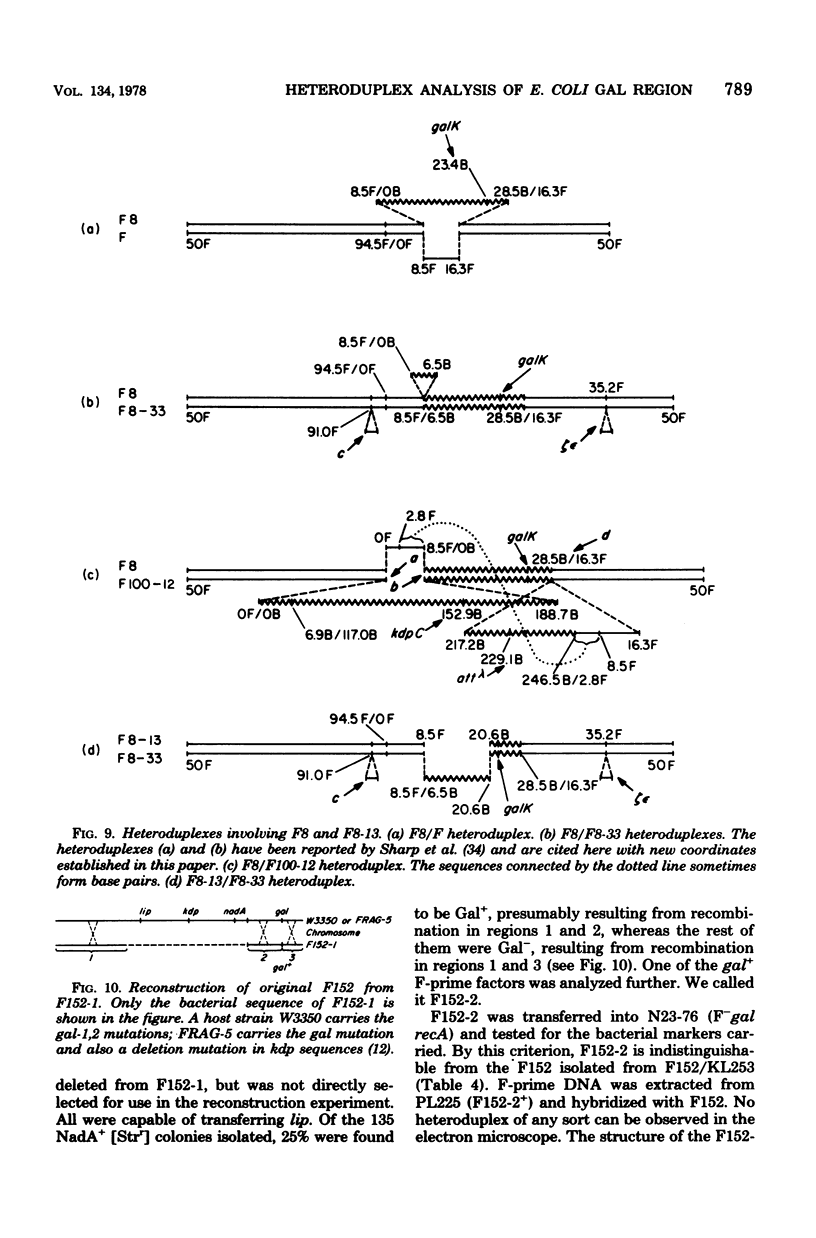
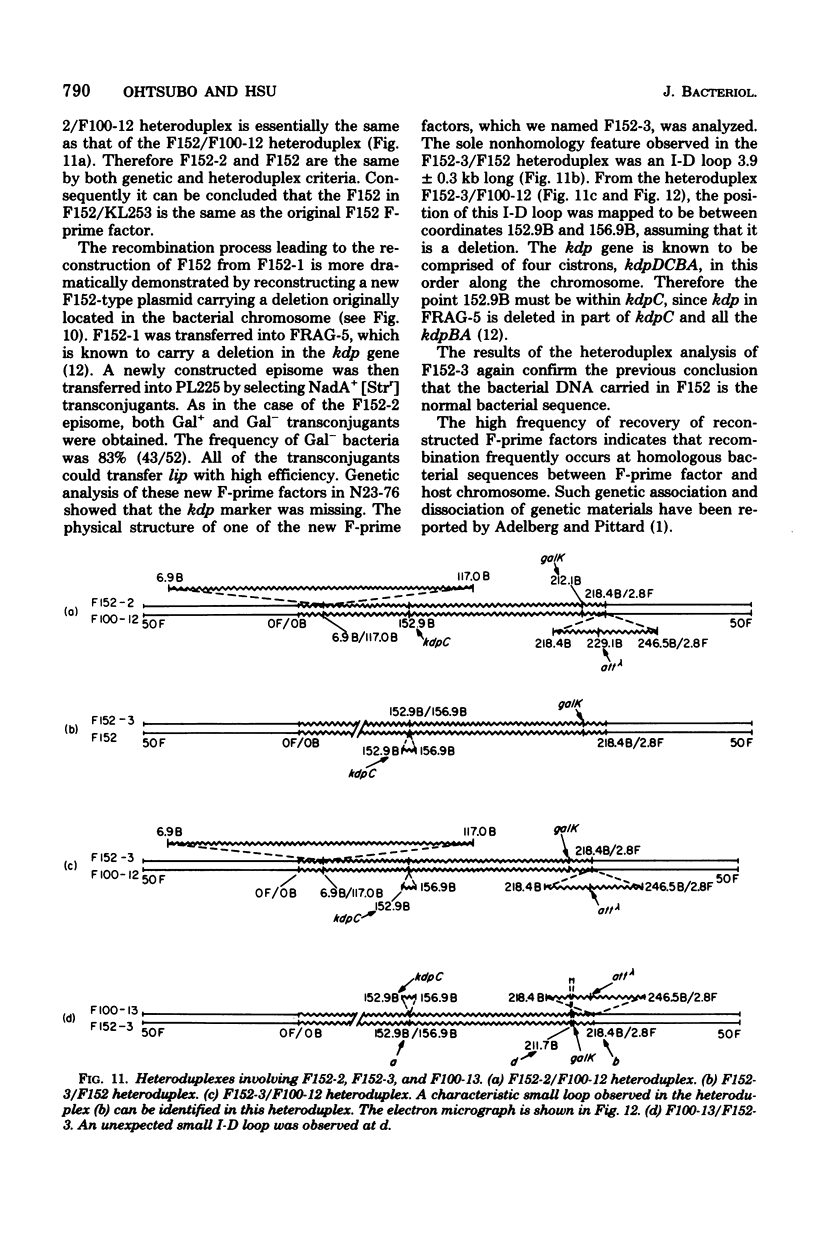
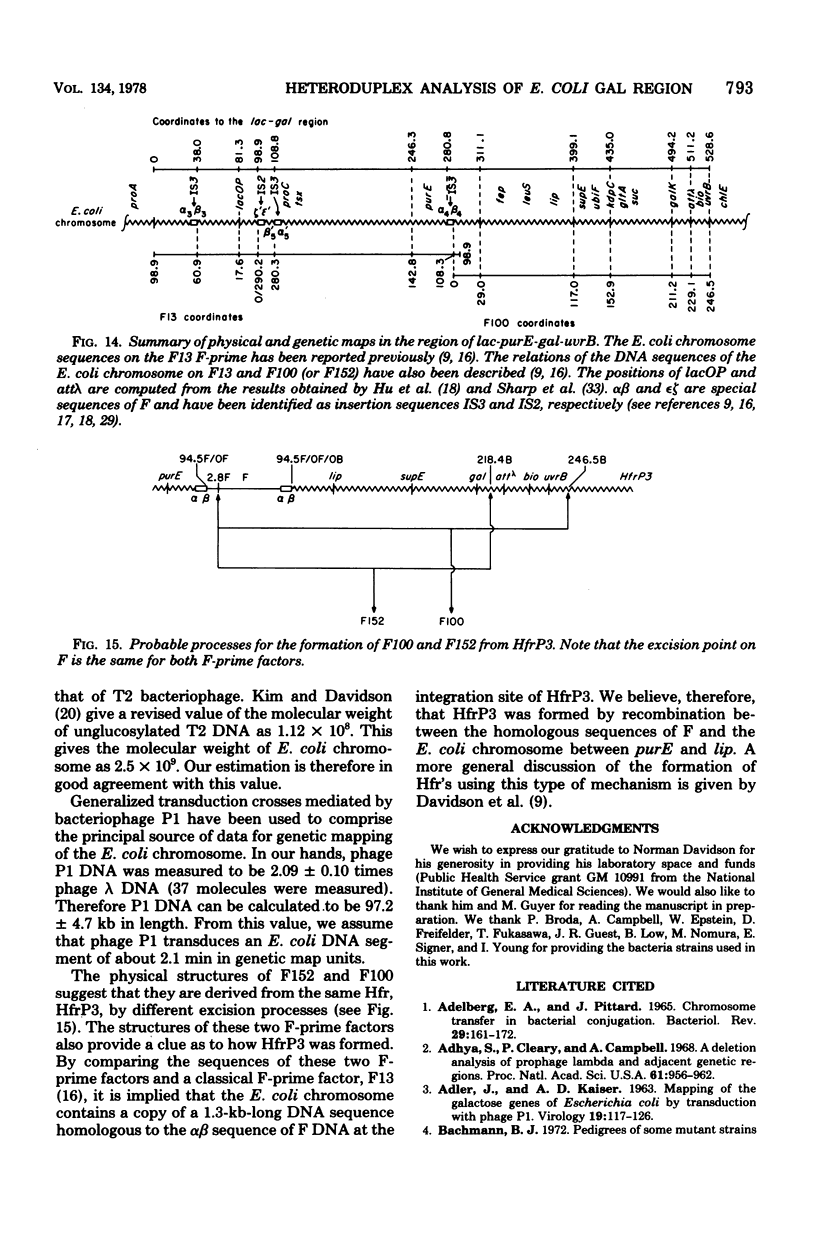
Abstract
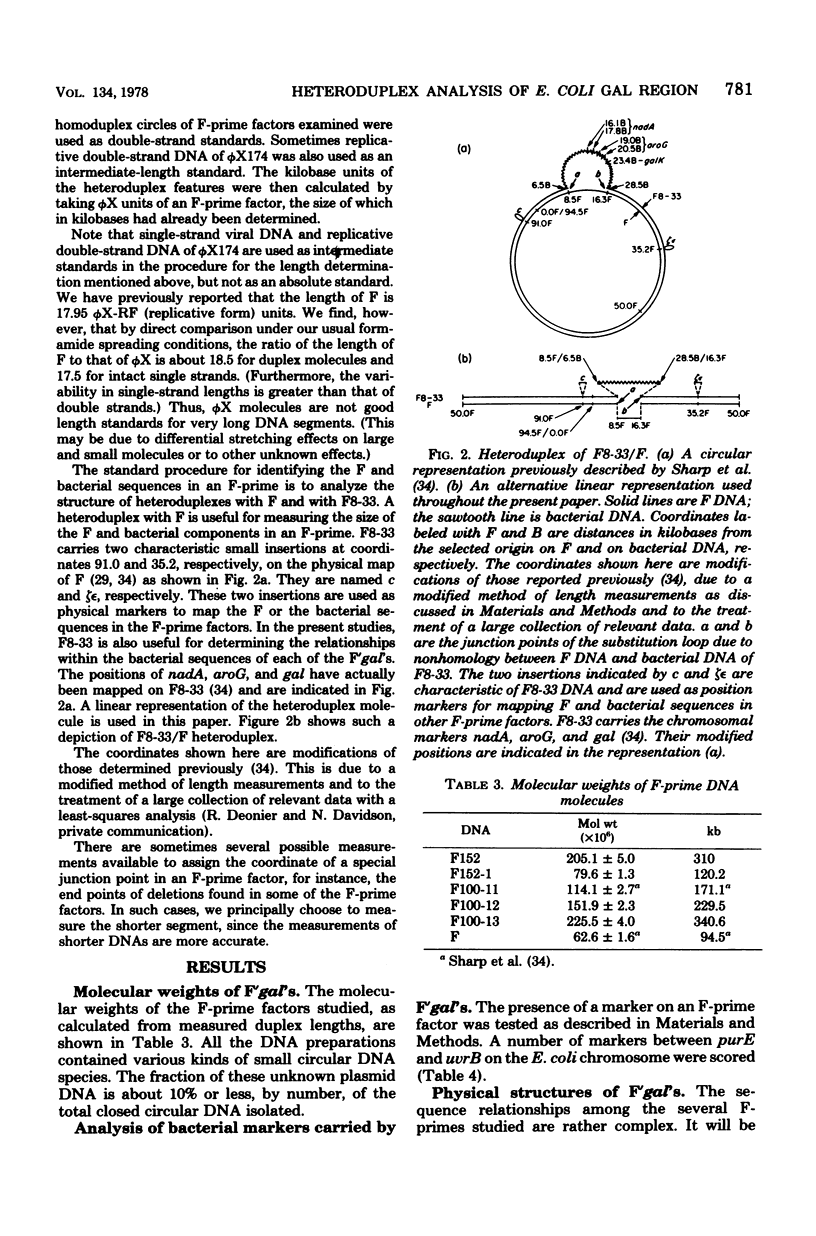
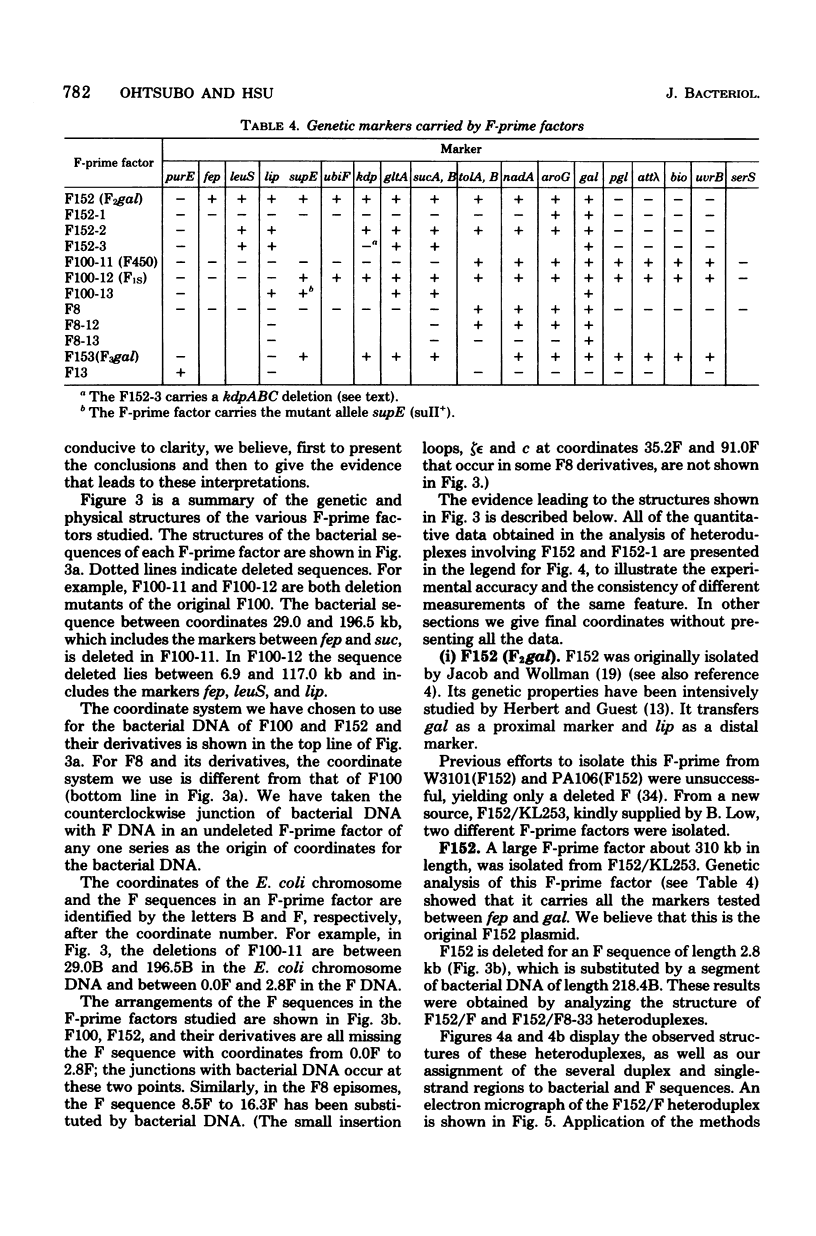
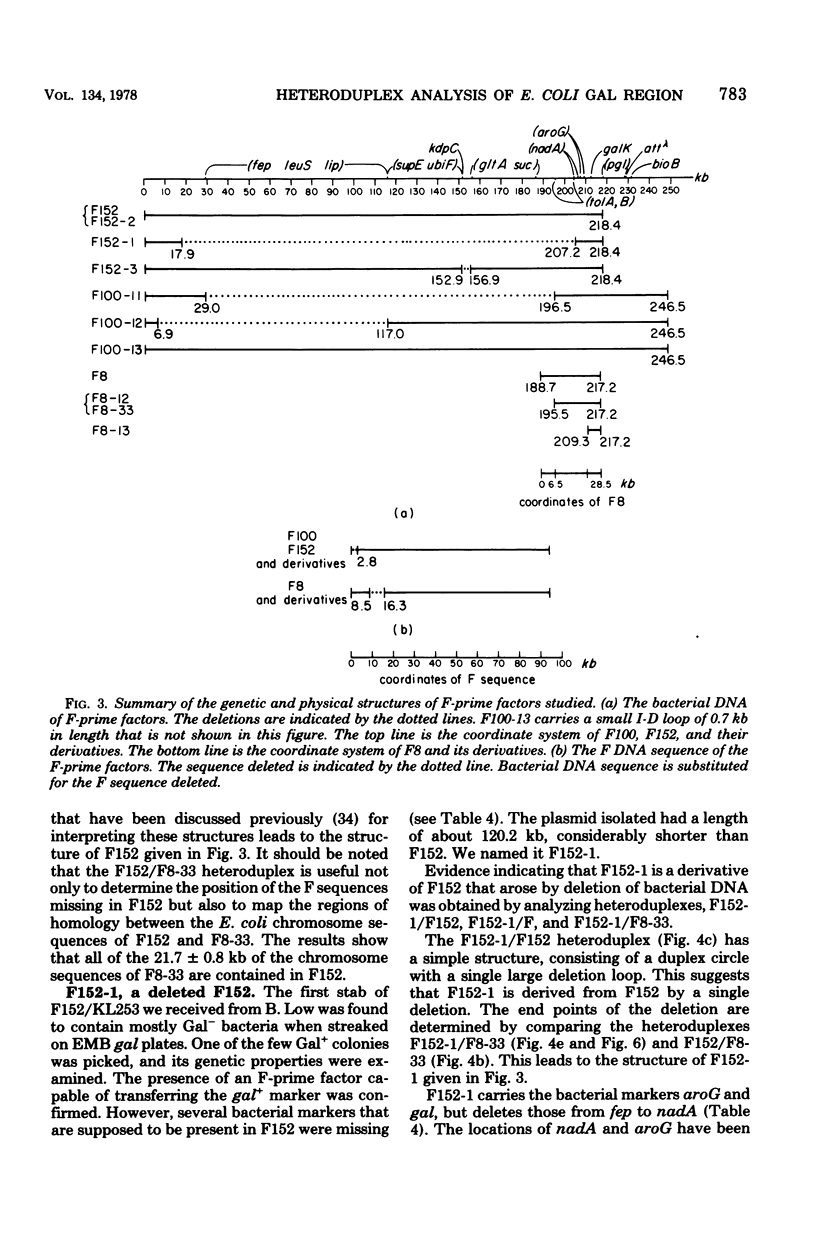
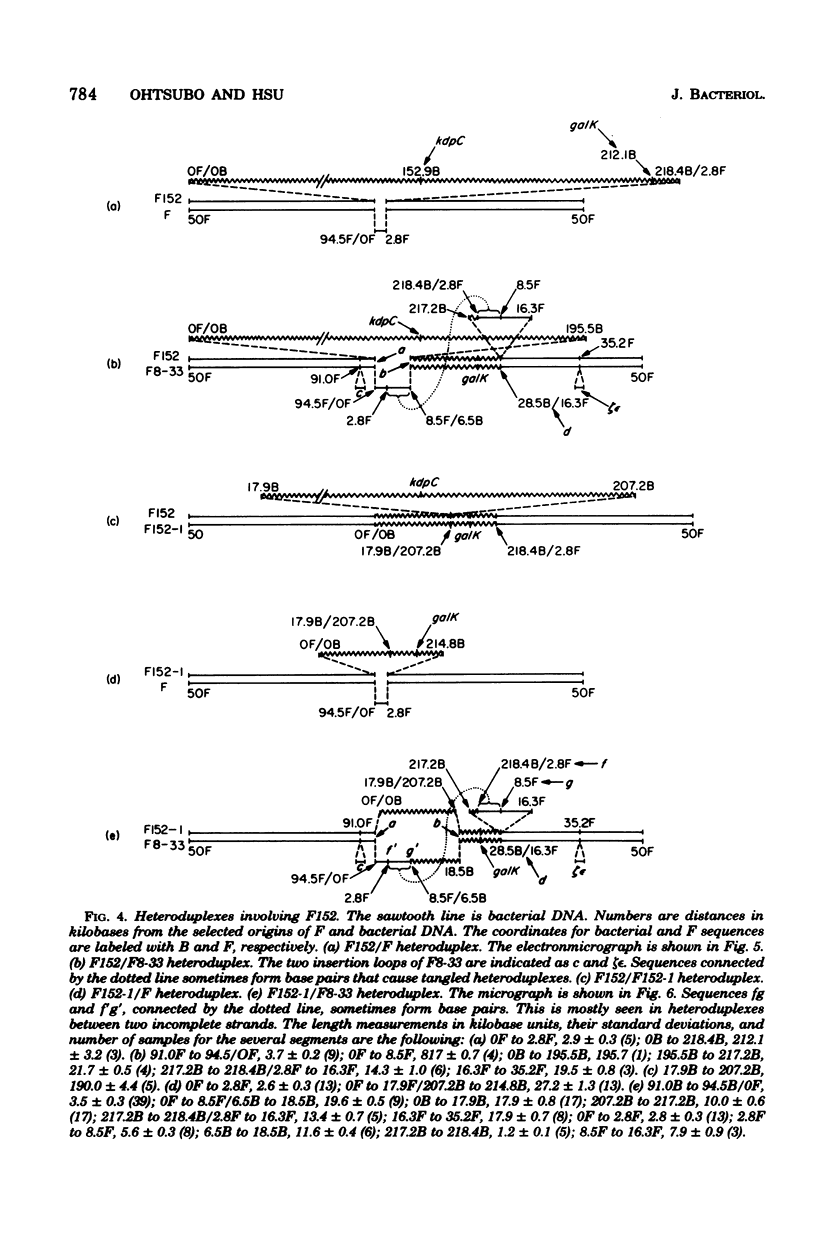
The genetic and physical structures of commonly used F-prime factors carrying the galactose region of the Escherichia coli chromosome were analyzed. Deletions in the chromosomal DNA sequences in the F-prime factors were found to be frequent events. A genetic method was developed to reconstruct the original F-prime factors from deletion variants. Heteroduplex analysis of the reconstructed F-prime factors confirmed the derivation of the F-prime factors F100 and F152, from the same Hfr, and finally determined the normal E. coli chromosomal sequence in the region between fep and uvrB, containing about 5 min in genetic units and about 246.5 in kilobase units (kb). This sequence could be connected with the DNA sequences of the lac-purE region, which had been physically determined previously. Together they constituted a total of 528.6 kb. From these combined sequences, the distance from lacPO to galK was calculated to be 412.9 kb, which corresponds to 8.8 min in genetic units.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., PITTARD J. CHROMOSOME TRANSFER IN BACTERIAL CONJUGATION. Bacteriol Rev. 1965 Jun;29:161–172. doi: 10.1128/br.29.2.161-172.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER J., KAISER A. D. Mapping of the galactose genes of Escherichia coli by transduction with phage P1. Virology. 1963 Feb;19:117–126. doi: 10.1016/0042-6822(63)90001-1. [DOI] [PubMed] [Google Scholar]
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda P. Modified map positions for lac and the pro markers in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):741–746. doi: 10.1128/jb.117.2.741-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Hirsch H. J., Saedler H., Starlinger P. Insertion mutations in the control region of the galactose operon of E. coli. II. Physical characterization of the mutations. Mol Gen Genet. 1972;115(3):266–276. doi: 10.1007/BF00268890. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Davidson N. Electron microscope heteroduplex study of sequence relations of T2, T4, and T6 bacteriophage DNAs. Virology. 1974 Jan;57(1):93–111. doi: 10.1016/0042-6822(74)90111-1. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lederberg J. Aberrant Heterozygotes in Escherichia Coli. Proc Natl Acad Sci U S A. 1949 Apr;35(4):178–184. doi: 10.1073/pnas.35.4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Fukasawa T. Chromosome mobilization in rec-merodiploids of Escherichia coli K12 following infection with bacteriophage lambda. Virology. 1969 Nov;39(3):467–481. doi: 10.1016/0042-6822(69)90095-6. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Ohtsubo E., Hsu M. T. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli: isolation of a new F-prime factor, F80, and its implication for the mechanism of F integration into the chromosome. J Bacteriol. 1978 Jun;134(3):795–800. doi: 10.1128/jb.134.3.795-800.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B., Eisenberg M. A. Genetic and biochemical analysis of the biotin loci of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):515–524. doi: 10.1128/jb.96.2.515-524.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M., Davidson N. Note on the structure of prophage lambda. J Mol Biol. 1972 Nov 14;71(2):499–501. doi: 10.1016/0022-2836(72)90364-6. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Beckwith J. R., Brenner S. Mapping of suppressor loci in Escherichia coli. J Mol Biol. 1965 Nov;14(1):153–166. doi: 10.1016/s0022-2836(65)80237-6. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., de Haan P. G., Nijkamp H. J. Mapping of purine markers in Escherichia coli K 12. Genet Res. 1965 Nov;6(3):442–453. doi: 10.1017/s0016672300004328. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., McCann L. M., Stroobant P., Gibson F. Characterization and genetic analysis of mutant strains of Escherichia coli K-12 accumulating the biquinone precursors 2-octaprenyl-6-methoxy-1,4-benzoquinone and 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone. J Bacteriol. 1971 Mar;105(3):769–778. doi: 10.1128/jb.105.3.769-778.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]





