Abstract
Resistance in Monilinia fructicola to demethylation inhibitor (DMI) fungicides is beginning to emerge in North America, but its molecular basis is unknown. Two potential genetic determinants of DMI fungicide resistance including the 14α-demethylase gene (MfCYP51) and the ATP-binding cassette transporter gene MfABC1, were investigated in six resistant (DMI-R) and six sensitive (DMI-S) field isolates. No point mutations leading to an amino acid change were found in the MfCYP51 gene. The constitutive expression of the MfCYP51 gene in DMI-R isolates was significantly higher compared to DMI-S isolates. Gene expression was not induced in mycelium of DMI-R or DMI-S isolates treated with 0.3 μg of propiconazole/ml. A slightly higher average MfCYP51 copy number value was detected in DMI-R isolates (1.35) compared to DMI-S isolates (1.13); however, this difference could not be verified in Southern hybridization experiments or explain the up to 11-fold-increased MfCYP51 mRNA levels in DMI-R isolates. Analysis of the upstream nucleotide sequence of the MfCYP51 gene revealed a unique 65-bp repetitive element at base pair position −117 from the translational start site in DMI-R isolates but not in DMI-S isolates. This repetitive element contained a putative promoter and was named Mona. The link between Mona and the DMI resistance phenotype became even more apparent after studying the genetic diversity between the isolates. In contrast to DMI-S isolates, DMI-R isolates contained an MfCYP51 gene of identical nucleotide sequence associated with Mona. Still, DMI-R isolates were not genetically identical as revealed by Microsatellite-PCR analysis. Also, real-time PCR analysis of genomic DNA indicated that the relative copy number of Mona among DMI-S and DMI-R isolates varied, suggesting its potential for mobility. Interestingly, constitutive expression of the MfABC1 gene in DMI-R isolates was slightly lower than that of DMI-S isolates, but expression of the MfABC1 gene in DMI-R isolates was induced in mycelium after propiconazole treatment. Therefore, the MfABC1 gene may play a minor role in DMI fungicide resistance in M. fructicola. Our results strongly suggest that overexpression of the MfCYP51 gene is an important mechanism in conferring DMI fungicide resistance in M. fructicola field isolates from Georgia and that this overexpression is correlated with Mona located upstream of the MfCYP51 gene.
Brown rot of peach is caused by the ascomycete fungus Monilinia fructicola (G Wint) Honey. The disease is primarily controlled by preharvest applications of fungicides. Among the most effective are the sterol demethylation inhibitor (DMI) fungicides, which are widely used for controlling brown rot in the United States. The first evidence that M. fructicola was able to develop resistance to DMI fungicides was found in laboratory studies (19). However, it took more than two decades after the introduction of DMI fungicides to detect DMI resistance in M. fructicola field populations, and thus far such populations have only been reported in Georgia and New York (21, 27). Isolates with documented in vitro resistance from Georgia were harder to control in detached fruit assays (27) compared to sensitive isolates, and follow-up studies showed that label rates of propiconazole cannot control such isolates in the field (2). Knowledge of the mechanism of DMI resistance in M. fructicola may help in designing antiresistance strategies and may be useful for identifying molecular markers for DMI fungicide resistance.
DMI fungicides specifically bind to the cytochrome P450 lanosterol 14α-demethylase (CYP51), thereby inhibiting the biosynthesis of ergosterol (32), the primary fungal cell membrane sterol that is responsible for maintaining membrane fluidity and stability (22, 25). Molecular mechanisms leading to DMI resistance have been studied in several important plant fungal pathogens. Common mechanisms include mutations in the target gene CYP51 (6, 7), overexpression of the CYP51 gene (10, 14, 30), and energy-dependent drug efflux mechanisms (11, 18, 20, 24).
We recently cloned and sequenced potential genetic determinants for DMI fungicide resistance in M. fructicola. These included the 14α-demethylase gene (designated MfCYP51) and the ATP-binding cassette (ABC) transporter gene 1 (MfABC1) (28, 29). When the MfCYP51 gene was introduced into PDR5::TN5 Saccharomyces cerevisiae, transformants revealed reduced sensitivity to the DMI fungicide myclobutanil (28). This result indicated that overexpression of the MfCYP51 gene is a potential mechanism of DMI fungicide resistance in M. fructicola. In a different study, the expression of the ABC transporter gene MfABC1 was induced by the addition of sublethal concentrations of propiconazole to the mycelium prior to RNA extraction, indicating a possible involvement of this gene in DMI fungicide resistance as well (29). Furthermore, MfABC1 revealed high amino acid homology with atrB gene from Aspergillus nidulans, an ABC transporter gene conferring resistance to different classes of fungicides, including DMI fungicides.
Based on the above-mentioned references, the MfCYP51 and MfABC1 genes were thought to be determinants for DMI fungicide resistance in M. fructicola. The goal of the present study was to substantiate the potential role of the MfCYP51 and MfABC1 genes in conferring DMI fungicide resistance in field isolates with documented resistance to propiconazole. Our specific objectives were to sequence the MfCYP51 gene and its upstream flanking sequence from DMI-resistant (DMI-R) and DMI-sensitive (DMI-S) isolates and to conduct expression analyses of both genes.
MATERIALS AND METHODS
M. fructicola isolates.
Seven DMI-R and nine DMI-S single-spore field isolates of M. fructicola were randomly chosen from our collection for the present study. Their origin and other characteristics are listed in Table 1. All of these isolates were stored at −25°C permanently.
TABLE 1.
Characteristics of M. fructicola isolates used in this study
| Isolate | Origin | Yr isolated | Sensitivity to propiconazolea |
|---|---|---|---|
| Bmpc7 | Byron, GA | 2006 | R |
| Bmpc10 | Byron, GA | 2006 | R |
| Bmpc13 | Byron, GA | 2006 | R |
| GADL129 | Fort Valley, GA | 2004 | R |
| GADL193 | Fort Valley, GA | 2004 | R |
| GAAP5 | Macon, GA | 2003 | R |
| GAAP6 | Macon, GA | 2003 | R |
| BGA25 | Byron, GA | 2004 | S |
| GAAP11 | Macon, GA | 2003 | S |
| GAAP12 | Macon, GA | 2003 | S |
| GADL3 | Fort Valley, GA | 2004 | S |
| GADL161 | Fort Valley, GA | 2004 | S |
| SCDL72 | Anderson, SC | 2004 | S |
| SC99A3 | Edgefield, SC | 2004 | S |
| SC99A4 | Edgefield, SC | 2004 | S |
| Kac-18 | Fresno, CA | 2005 | S |
An isolate was considered resistant (R) when the relative mycelial growth was ≥20% at the discriminatory dose of 0.3 μg of propiconazole/ml; all other isolates were considered sensitive (S).
Determination of propiconazole sensitivity.
The sensitivity of the isolates to DMI fungicide propiconazole (PropiMax EC; Syngenta Crop Protection, Greensboro, NC) was determined by measuring relative mycelium growth on fungicide-amended medium. Briefly, each isolate was grown for 4 days on potato dextrose agar (PDA; EMD Chemicals, Gibbstown, NJ) and mycelium-containing plugs (5 by 5 mm) from the periphery of these cultures served as inoculum. Plugs from the same inoculum plate and from visibly identical areas were placed in triplicates upside down on nonamended or propiconazole-amended PDA at a discriminatory dose of 0.3 μg/ml in petri dishes (90 mm in diameter). At this dose, sensitive isolates are arrested in growth, whereas resistant isolates typically grow between 20 and 70% (3). Colony diameters were measured after 4 days of incubation in the dark at 22°C. Relative growth was calculated by expressing the growth of isolates on amended medium as a percentage of the growth on nonamended medium.
Extraction of genomic DNA.
Each fungal isolate was grown in 40 ml of potato dextrose broth (PDB; Difco Laboratories, Sparks, MD) for 5 days at 22°C on an orbital shaker (120 rpm). Mycelium was separated from the liquid culture by centrifugation at 10,000 rpm for 5 min. Mycelial mats were frozen in liquid nitrogen and ground into fine powder with mortar and pestle. Genomic DNA was isolated by using a DNeasy plant minikit (Qiagen, Inc., Valencia, CA) according to the manufacturer's manual. DNA quality was assessed by electrophoresis on 1.0% agarose gels (Promega Corp., Madison, WI) including ethidium bromide (1 μg/ml) in 0.5× TBE buffer (44.5 mM Tris-borate, 1 mM EDTA [pH 8.0]).
Amplification and sequencing of the MfCYP51 gene.
Gene sequencing analysis was restricted to the MfCYP51 gene because point mutations in ABC transporters are not known to cause fungicide resistance. The primers used to amplify and sequence the MfCYP51 gene are listed in Table 2. The complete MfCYP51 gene was amplified with the primer pair MfCYP51-F and MfCYP51-R designed by Schnabel and Dai (28). PCR volumes were 50 μl containing 1× PCR buffer provided by the manufacturer, 20 ng of genomic DNA, 0.5 μM concentrations of each primer, 200 μM concentrations of each deoxynucleoside triphosphate, 1.25 U of Taq DNA polymerase (New England Biolabs, Ipswich, MA). Amplifications were performed in an iCycler thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA) programmed for 3 min at 94°C, 30 cycles of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C, followed by a final extension step of 5 min at 72°C. The PCR products were separated on 1.0% agarose gels in 0.5× TBE buffer at 100 V for 1 h. Gels were photographed with an electrophoresis documentation and analysis system (Kodak EDAS 290; Eastman Kodak Company, New Haven, CT).
TABLE 2.
Nucleotide sequence and characteristics of primers used in this study
| Primer | Sequence (5′→3′) | Description | Source or reference |
|---|---|---|---|
| MfCYP51-F | GTTTCGCCATGGGTGTTCT | Primer for amplification and partial sequencing of MfCYP51 | 28 |
| MfCYP51-R | TCGTCTCTCCCATGCCACAA | Same as for MfCYP51-F | 28 |
| MfCYP51-5′7 | TTGCAGGGAAAGGAAGTC | Sequencing primer for MfCYP51 | 28 |
| MfCYP51-3′1 | CGGAATGATCAGCAAAGGTGC | Same as for MfCYP51-5′7 | 28 |
| MfCYP51-5′2 | GAGAAGAGCGATCATCATGTTAGCGATCTCT | Same as for MfCYP51-5′7; also used as a gene-specific primer for amplifying the region upstream of MfCYP51 in nested PCR assays | 28 |
| MfCYP51-5′5 | GGGCAGTCGTAAACCACAT | Same as for MfCYP51-5′2 | 28 |
| Micro 1 | GACAGACGGACGGACG | Microsatellite primer for MP-PCR analysis | 15 |
| Micro 4 | AGAGAGAGAGAGAGAGC | Same as for Micro 1 | 15 |
| M13 | GAGGGTGGCGGTTCT | Same as for Micro 1 | 15 |
| RealCYP-F2 | TCTTGGGCGCCGACCTTC | Real-time PCR primer for amplifying a 120-bp fragment of MfCYP51 | This study |
| RealCYP-R2 | CTTTGCGCATGATAGAGTGGAT | Same as for RealCYP-F2 | This study |
| MfABC5-F | CCGATGAAACAAGCTCGACCTTTT | Real-time PCR primer for amplifying a 260-bp fragment of MfABC1 | 29 |
| MfABC5-R | CGGATTTGATTGGGTTGTTAT | Same as for MfABC5-F | 29 |
| MfActin-F | GTGTTGATATGGCCGGTCGTGATT | Real-time PCR primer for amplifying a 85-bp fragment of MfActin, the endogenous control | 28 |
| MfActin-R | TCGGCAGTGGTGGAGAAAGTGTAA | Same as for MfActin-F | 28 |
| AP1 | GGATCCTAATACGACTCACTATAGGGC | Adaptor-specific primer for amplifying the upstream region of MfCYP51 in nested PCR | 31 |
| AP2 | AATAGGGCTCGAGCGGC | Same as for AP1 | 31 |
| UpCYP-1F | AGAGCTACCACCCACGAGGAA | Sequencing primer for the upstream region of MfCYP51 | This study |
| UpCYP-2F | GCCATACCGCAGACCATTGAA | Same as for UpCYP-1F | This study |
| UpCYP-3F | CCATGCACCTGCCACTGACTA | Same as for UpCYP-1F | This study |
| UpCYP-1R | GACCGCTGCGAAATCTCTTGA | Same as for UpCYP-1F | This study |
| UpCYP-2R | GGGAATCCAATCACGAGTTT | Same as for UpCYP-1F | This study |
| UpCYP-3R | CCCTTGCGACGCCTCAGTA | Same as for UpCYP-1F | This study |
| Insert-1F | CACACCGCTCTTTTAGT | Real-time PCR primer for amplifying a 56-bp fragment of Mona | This study |
| Insert-2R | CTCTGCTCCTACTACGGA | Same as for Insert-1F | This study |
PCR products were purified by using the QiaQuick gel extraction kit (Qiagen) and sequenced with BigDye Terminator v3.1 kit (Applied Biosystems, Inc., Foster City, CA). The sequencing primers were the forward primers MfCYP51-F, MfCYP51-5′7, and MfCYP51-3′1 and the reverse primers MfCYP51-R, MfCYP51-5′2, and MfCYP51-5′5. Sequencing was performed at the Clemson University Genomics Institute. The software of DNASTAR (DNASTAR, Inc., Nevada City, CA) was used to assemble and align the MfCYP51 nucleotide sequences.
Investigation of genetic diversity between M. fructicola isolates using MP-PCR.
Three microsatellite primers (MPs), Micro 1, Micro 4, and M13, which have been successfully used to characterize M. fructicola isolates by Ma et al. (15), were selected to investigate genetic diversity between isolates used in the present study. PCR was largely performed as described for the amplification of the MfCYP51 gene, except that a single MP primer was used at 1 μM in each reaction and the annealing temperature was 50°C. For each isolate, the presence or absence of polymorphic bands was scored as “1” or “0,” respectively. Dice coefficients were calculated for all pairwise comparisons by using the Windist program (Winboot; International Rice Research Institute, Manila, Philippines) as F = 2Nxy/(Nx + Ny), where Nxy is the number of bands shared by a given pair of isolates, and Nx and Ny are the number of bands found in isolates x and y, respectively. A phenogram was generated based on similarity coefficients by using UPGMA (unweighted pair-group method with arithmetic averages) and the SAHN program of the software package NTSYS-pc 2.1 (Department of Ecology and Evolution, State University of New York).
RNA isolation, cDNA synthesis, and quantification of MfCYP51 and MfABC1 mRNAs.
Mycelia of M. fructicola isolates were transferred into 250-ml flasks containing 40 ml of PDB and nine glass beads (10 mm in diameter) each. The liquid cultures were incubated for 5 days at 22°C and shaken vigorously every 8 h to encourage uniform growth of mycelium in the liquid medium. After 5 days, water or propiconazole (0.3 μg/ml) formulated as PropiMax EC (Syngenta) was added to the flasks. One hour later, total RNA was isolated using an RNeasy plant minikit according to the manufacturer's manual. cDNA was synthesized by using iScript cDNA synthesis kit (Bio-Rad). The absence of genomic DNA contamination was verified by PCR using the primers MfCYP51-F and MfCYP51-5′2, which span introns of the genomic sequence. Genomic DNA would have generated an ∼1,000-bp PCR product, 100 bp longer than from cDNA.
Real-time PCR technology was used to quantify mRNA levels of the MfCYP51 and MfABC1 genes in cDNA. Fragments of the MfCYP51 gene (120 bp) and MfABC1 gene (260 bp) were amplified with the primer pairs RealCYP-F2-RealCYP-R2 and MfABC5-F-MfABC5-R, respectively. The expression of the MfCYP51 and MfABC1 genes was normalized with the expression of the MfActin gene by using the primer pair MfActin-F-MfActin-R. Reactions were performed in a 30-μl volume containing 1× iQ SYBR Green Supermix (Bio-Rad), 0.3 μM concentrations of each primer, and 0.5 μl of cDNA. All PCRs were amplified in triplicates with the program: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 55°C for 45 s and one cycle of 95°C for 1 min and 55°C for 1 min. To confirm that products were amplified correctly in these reactions, a melting curve analysis was performed immediately after amplification for 100 cycles of increasing temperature with 0.4°C increments starting at 55°C. Reactions were performed in an iCycler thermal cycler (Bio-Rad Laboratories). Two biological replications starting with new cultures for RNA extraction were conducted.
The comparative CT method (separate tubes) (1) was used to determine MfCYP51 expression, because efficiencies of target and reference amplifications were nearly equal in validation experiments (slope = 0.048). The relative expression of the MfCYP51 gene from M. fructicola isolates was calibrated to the lowest expressor (isolate Kac-18). The validation experiment yielded unequal efficiencies of the target and reference amplifications (slope = 0.147) for the MfABC1 gene expression analysis; therefore, the “relative standard curve method” (1) was used to analyze the data. Standard curves were made by using the fivefold dilution series of genomic DNA from isolate SC99A3 as templates. The DNA concentration of isolate SC99A3 was determined by using GeneQuantPro (Biochrom, Cambridge, United Kingdom). The relative expression of MfABC1 gene was calculated for each isolate by dividing the amount of normalized MfABC1 mRNA by the amount of normalized MfABC1 mRNA of isolate SC99A3, which had the lowest amount of MfABC1 mRNA among DMI-S isolates.
Determination of relative MfCYP51 gene copy numbers.
The relative copy number of the MfCYP51 gene was determined by using real-time PCR technology for each isolate as described above except genomic DNA was used as a template. The relative copy number (2−ΔΔCT) was calculated by the equation:
 |
where x is one of the isolate samples, and y is SCDL72, which was chosen as a calibrator because it had the highest ΔCT value thus the lowest number MfCYP51 gene copies in the genome compared to all other isolates.
Cloning of the region upstream the MfCYP51 gene.
Nested PCR with gene-specific and adaptor-specific primers was used to amplify the upstream region of the MfCYP51 gene from PCR libraries (31). PmlI, SmaI, EcoRV, and XmnI PCR libraries were created previously to isolate the MfCYP51 and MfABC1 genes (28, 29). The first PCR amplification was conducted with the adaptor-specific primer AP1 combined with the gene-specific primer MfCYP51-5′2. Nested PCR was then conducted with the adaptor-specific primer AP2 combined with the gene-specific primer MfCYP51-5′5 using a 1:50 dilution of the first PCR product as a template. PCRs were conducted in 50-μl volumes containing 1 μl of template, 1× Ext PCR buffer including 1.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 0.4 μM concentrations of each of adaptor-specific and gene-specific primer, and 2 μl of Ext polymerase (1 U/μl) (Roche Applied Sciences, Indianapolis, IN). The cycle parameters were as described previously (31). PCR products were examined on a 1.0% agarose-ethidium bromide gel and sequenced as described above. Based on the resulting sequence, the primer pair UpCYP-1F and UpCYP-1R was designed to amplify the upstream regions of the MfCYP51 gene from the isolates used in the present study. Sequences were compared to database sequences by using BLAST searches, aligned with DNASTAR software, and analyzed with the promoter predictor software NNPP (Neural Network Promoter Prediction, version 2.2, March 1999; http://www.fruitfly.org/seq_tools/promoter.html).
Determination of the relative copy number of the 65-bp repetitive element (Mona) in M. fructicola field isolates.
The relative copy number of Mona was determined by using primer pair Insert-1F and Insert-2R and the real-time PCR protocol described above, except that the annealing and extension temperature was 50°C. The primer pair amplified a 56-bp internal fragment of Mona.
The relative copy number (2−ΔΔCT) of Mona was calculated by the equation:
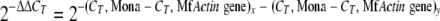 |
where x is one of the field isolates, and y is SCDL72, which was chosen as calibrator because it had the highest ΔCT value and thus the lowest Mona copy number in the genome compared to all other isolates.
RESULTS
Sensitivity of M. fructicola isolates to propiconazole.
Relative growth of DMI-S isolates subjected to 0.3 μg of propiconazole/ml ranged from 0 to 5%. On the other hand, DMI-R isolates revealed relative growth values greater than 20% ranging from 21.8 to 36.3% (Table 3). These data were consistent with the observation that DMI-R isolates typically grew with relative growth ranging from 20 to 70% on 0.3 μg of propiconazole/ml (3).
TABLE 3.
Relative expression of the MfCYP51 and MfABC1 genes in M. fructicola field isolates
| Isolate | Relative growth (%)a | Propiconazole sensitivity phenotypeb | Relative expression ofc:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MfCYP51
|
MfABC1
|
|||||||||
| Untreated
|
Propiconazole treated
|
Untreated
|
Propiconazole treated
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Bmpc7 | 29.7 | R | 11.2 | 0.0 | 10.8 | 1.4 | 1.6 | 1.0 | 1.7 | 0.7 |
| Bmpc10 | 30.8 | R | 7.3 | 1.0 | 7.6 | 1.6 | 1.5 | 1.1 | 2.2 | 0.1 |
| Bmpc13 | 29.5 | R | 10.1 | 0.8 | 9.5 | 1.4 | 1.6 | 0.1 | 2.2 | 0.9 |
| GADL193 | 35.0 | R | 9.1 | 1.6 | 7.5 | 0.9 | 1.2 | 0.1 | 2.1 | 0.0 |
| GAAP5 | 36.3 | R | 10.3 | 1.5 | 10.0 | 0.7 | 0.6 | 0.3 | 1.9 | 0.9 |
| GAAP6 | 21.8 | R | 5.2 | 0.8 | 4.6 | 0.4 | 1.4 | 0.5 | 1.8 | 0.5 |
| GAAP12 | 0 | S | 1.9 | 0.5 | 1.3 | 0.3 | 2.6 | 0.7 | 1.5 | 0.0 |
| GADL3 | 0 | S | 3.4 | 0.2 | 1.9 | 0.4 | 2.8 | 0.3 | 1.1 | 0.6 |
| SCDL72 | 3.1 | S | 1.2 | 0.1 | 1.8 | 0.3 | 2.0 | 0.4 | 1.1 | 0.1 |
| SC99A3 | 5.0 | S | 2.3 | 1.0 | 1.8 | 0.0 | 1.0 | 0.0 | 1.6 | 0.9 |
| SC99A4 | 1.3 | S | 1.0 | 0.1 | 1.6 | 0.5 | 2.0 | 0.4 | 2.5 | 0.4 |
| Kac-18 | 0.4 | S | 1.0 | 0.2 | 0.9 | 0.4 | 2.2 | 0.1 | 2.5 | 0.4 |
Relative growth was calculated by expressing the growth of isolates on medium amended with 0.3 μg of propiconazole/ml as a percentage of the growth on nonamended medium.
An isolate was considered resistant (R) when the relative mycelial growth on medium amended with 0.3 μg of propiconazole/ml was ≥20%; all other isolates were considered sensitive (S).
The expression of the MfCYP51 or MfABC1 genes was normalized using the MfActin gene expression levels and then calibrated to the normalized MfCYP51 or MfABC1 mRNA value from the isolate with the lowest amount of MfCYP51 or MfABC1 mRNA. The mean and standard deviation values indicate the average relative expression between two independent biological experiments. Relative expression was determined in isolates subjected to water or 0.3 μg of propiconazole/ml 1 h before mRNA isolation.
Amplification and sequencing of the MfCYP51 gene.
The entire MfCYP51 gene was amplified with primer pair MfCYP51-F and MfCYP51-R. PCR amplifications yielded a specific product of the expected size (1,685 bp) from all isolates (data not shown). Comparison of the 1,569-bp coding region of the MfCYP51 gene from DMI-R and DMI-S isolates revealed several nucleotide variations at 30 positions (Table 4). All variations were found among eight DMI-S isolates (Table 4). The DMI-R isolates and one DMI-S isolate (SCDL72) had identical coding regions (Table 4). None of these nucleotide sequence variations resulted in a predictable amino acid change.
TABLE 4.
Silent nucleotide variations in the coding region of the MfCYP51 gene from seven DMI-R and nine DMI-S isolates
| Isolate(s) | Sensitivity to propiconazole | Nucleotide at positiona:
|
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 105 | 108 | 132 | 177 | 312 | 387 | 540 | 546 | 576 | 588 | 675 | 690 | 702 | 735 | 759 | 793 | 954 | 1017 | 1092 | 1209 | 1230 | 1245 | 1272 | 1278 | 1401 | 1437 | 1470 | 1482 | 1503 | 1533 | ||
| Bmpc7, -10, -13, GAAP5, -6, GADL129, -193 | R | T | T | G | T | C | G | C | C | C | C | G | T | C | T | G | C | C | C | G | C | T | A | A | T | T | C | C | T | T | A |
| SCDL72 | S | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| BGA25 | S | C | C | − | C | − | A | − | − | T | T | C | − | T | − | − | A | − | − | − | − | − | − | G | − | − | − | − | C | G | T |
| GAAP11 | S | C | C | − | C | − | A | − | − | G | − | C | C | T | − | − | A | − | − | − | − | C | G | G | − | − | − | − | C | G | − |
| GAAP12 | S | C | C | − | C | − | − | T | − | T | T | C | − | − | C | − | − | − | − | A | − | − | − | − | − | C | − | − | − | − | − |
| GADL3 | S | C | C | − | C | − | A | − | T | T | − | C | − | T | − | T | A | T | − | − | T | − | − | G | C | − | T | − | C | − | − |
| GADL161 | S | C | C | − | C | A | A | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | T | − | − | − |
| SC99A3 | S | C | C | C | C | − | − | − | − | T | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | C | − | − | − | − | − |
| SC99A4 | S | C | C | C | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Kac-18 | S | C | C | C | C | − | A | − | − | T | − | C | − | T | − | − | − | − | T | − | − | − | − | G | − | − | − | − | C | G | − |
Dashes (-) indicate nucleotide sequences identical to the corresponding positions in the DMI-R isolates.
Genetic diversity between M. fructicola isolates.
Three MPs generated 14 polymorphic bands from 16 isolates of M. fructicola. Analysis of the MP-PCR data revealed a phenogram with two groups of isolates and two ungrouped isolates. DMI-R isolates did not cluster independently from the DMI-S isolates (Fig. 1). Three DMI-S isolates were grouped into group 1 together with five DMI-R isolates. One DMI-R isolate (GAAP6) was grouped together with five DMI-S isolates into group 2. Two isolates, the DMI-R isolate GADL129 and the DMI-S isolate GAAP12, were not part of either group (Fig. 1). Based on the MP-PCR profiles, six DMI-R and six DMI-S isolates representing different genetic backgrounds were selected for further real-time PCR analysis.
FIG. 1.
Phenogram constructed with binary MP-PCR data sets from DMI-R and DMI-S isolates of M. fructicola. The tree was constructed by using UPGMA according to the SAHN clustering procedure of NTSYS-pc 2.1.
Relative expression of the MfCYP51 and MfABC1 genes.
There were no statistical differences in mRNA levels between biological replicates for MfCYP51 (P = 0.624) or MfABC1 (P = 0.565); therefore, the datasets were merged (Table 3). The relative expression values of the MfCYP51 gene in DMI-R and DMI-S isolates were significantly different (P = 0.001) and ranged from 5.2 to 11.2 and from 1.0 to 3.4, respectively. The addition of propiconazole to the mycelia of DMI-R and DMI-S isolates did not affect expression levels of the MfCYP51 gene (P = 0.689 and P = 0.561, respectively; Table 3). A slight but statistically significant difference of relative expression values of the MfABC1 gene was observed between DMI-R and DMI-S isolates (P = 0.041); values ranged from 0.6 to 1.6 and from 1.0 to 2.8, respectively. The addition of propiconazole to mycelium of DMI-R isolates led to a statistically significant increase of MfABC1 expression with average mean values increasing from 1.31 to 1.98 (P = 0.004). A similar increase in MfABC1 mRNA levels was not observed in DMI-S isolates (P = 0.32; Table 3). All P values were determined by using the t test (SigmaStat for Windows version 3.00; Systat Software, Inc., San Jose, CA).
Determination of the relative MfCYP51 copy numbers in DMI-R and DMI-S isolates.
Real-time PCR was used to determine the relative copy number of the MfCYP51 gene in genomic DNAs of DMI-R and DMI-S isolates. The mean 2−ΔΔCT values for DMI-R isolates Bmpc7, Bmpc10, Bmpc13, GADL193, GAAP5, and GAAP6 were 1.40, 1.31, 1.39, 1.31, 1.10, and 1.57, respectively. These values were statistically different (P = 0.014) from the 2−ΔΔCT values of DMI-S isolates, which were 1.19, 1.10, 1.00, 1.11, 1.15, and 1.25 for isolates GAAP12, GADL3, SCDL72 (calibrator isolate), SC99A3, SC99A4, and Kac-18, respectively.
Cloning and sequence analysis of the region upstream the MfCYP51 gene.
Using nested PCR, a fragment of ∼2,500 bp in length was amplified from the XmnI PCR library. This fragment contained 471 bp of the 5′ region of the MfCYP51 gene and 1,872 bp of the 5′ upstream region, excluding the adaptor-specific primer sequence (AP2). Based on this sequence, the primers UpCYP-1F (located at position +71 of the 1,872-bp fragment) and UpCYP-1R (located at position +56 of the MfCYP51 gene) were designed to amplify the majority of the upstream region and a small portion of the 5′ region of the MfCYP51 gene from all isolates. Sequence analysis revealed a 65-bp repetitive element at bp −117 of the MfCYP51 translational start site in each of the DMI-R isolates but not in DMI-S isolates (Fig. 2). This repetitive element was named Mona. A BLAST search of the MfCYP51 upstream region to nucleotide collection database (nr/nt) in GenBank plus EMBL plus DDBJ plus PDB showed no similarity to other sequences. The promoter prediction software identified three and two putative promoter sequences in DMI-R and DMI-S isolates, respectively (Fig. 2). Promoter 2 was located within Mona. The upstream sequences of DMI-R isolate Bmpc7 (identical with the other five DMI-R isolates), DMI-S isolates GAAP12, GADL3, SC99A4, Kac-18, SCDL72, and SC99A3 are available in GenBank under accession numbers EU035301, EU035302, EU035303, EU035304, EU035305, EU035306, and EU035307.
FIG. 2.
Simplistic model of the upstream region of the MfCYP51 gene in M. fructicola isolates. P1, P2, and P3 indicate promoters 1, 2, and 3, respectively. A 65-bp repetitive element (Mona) was identified at bp −117 of the MfCYP51 gene in DMI-R isolates.
Abundance of Mona in M. fructicola field isolates.
Real-time PCR was used to determine the relative copy number of Mona in genomic DNA of M. fructicola field isolates. For the six DMI-R isolates the mean 2−ΔΔCT values (relative copy numbers to calibrator SCDL72) ranged from 1.3 to 33.9, and for the six DMI-S isolates the mean 2−ΔΔCT values ranged from 1.0 to 85.6 (Table 5). There was no significant difference between the relative copy numbers of DMI-R and DMI-S isolates according to the Mann-Whitney rank sum test (P = 0.485; Table 5). Although we did not determine the absolute copy number, our results do indicate that some of our isolates possess at least 80 copies, assuming that the calibrator isolate contained at least one copy.
TABLE 5.
Relative copy number of Mona in M. fructicola field isolates
| Isolate | Sensitivity to propiconazolea | Relative copy no. of Monab
|
|
|---|---|---|---|
| Mean | SD | ||
| Bmpc7 | R | 7.5 | 0.7 |
| Bmpc10 | R | 6.0 | 1.7 |
| Bmpc13 | R | 5.2 | 1.2 |
| GADL193 | R | 1.3 | 0.3 |
| GAAP5 | R | 1.4 | 0.7 |
| GAAP6 | R | 33.9 | 1.3 |
| GAAP12 | S | 2.1 | 0.7 |
| GADL3 | S | 36.9 | 3.2 |
| SCDL72 | S | 1.0 | 0.2 |
| SC99A3 | S | 85.6 | 5.9 |
| SC99A4 | S | 5.8 | 0.7 |
| Kac-18 | S | 61.1 | 1.2 |
An isolate was considered resistant (R) when the relative mycelial growth was ≥20% at the discriminatory dose of 0.3 μg of propiconazole/ml; all other isolates were considered sensitive (S).
The CT value of Mona was normalized with the MfActin gene CT value (ΔCT) and calibrated to the normalized CT value of the isolate SCDL72 (ΔΔCT), which had the lowest Mona copy number. The relative copy number was calculated by the equation 2−ΔΔCT. Means are average values for three independent experiments.
DISCUSSION
The 14α-demethylase (CYP51) is a key enzyme of fungal sterol biosynthesis and represents the target enzyme for DMI fungicides. Resistance to DMI fungicides can be conferred in fungi by altering the protein structure at key positions, thereby reducing the binding affinity to the fungicide. In plant pathogenic fungi such as Uncinula necator and Erysiphe graminis, a single nucleotide mutation within the substrate recognition site of the CYP51 gene, leading to the substitution of a phenylalanine residue for a tyrosine residue at position 136 in amino acid sequence, has been correlated with resistance (6, 7). Alterations in the sequence of the CYP51 gene in clinical isolates of the yeast Candida albicans have been demonstrated to confer DMI fungicide resistance (9, 13, 16, 17, 26). A point mutation in the CYP51 gene was also reported to contribute to DMI fungicide resistance in clinical isolates of the ascomycete A. fumigatus (8). In the present study, all DMI-R and DMI-S isolates had identical predicted amino acid sequences in the MfCYP51 protein-coding region. Therefore, the DMI resistance in M. fructicola field isolates was not due to changes in the amino acid sequence of the MfCYP51 protein, which is in agreement with a study published previously on DMI fungicide-resistant M. fructicola field isolates from New York (21).
Increased expression of the CYP51 gene is another potential mechanism of DMI fungicide resistance. Overexpression of the CYP51 gene conferred resistance to DMI fungicide fluconazole in clinical isolates of C. glabrata (17). In the filamentous fungi P. digitatum, Venturia inaequalis, and Blumeriella jaapii, the overexpression of the CYP51 gene has been linked to DMI fungicide resistance (10, 14, 30). In the present study, the expression of the MfCYP51 gene in DMI-R isolates was 5- to 11-fold higher than that of DMI-S isolate Kac-18. In plant pathogenic fungi, similar levels of overexpression were reported. In B. jaapii, the expression of the CYP51 gene in DMI-R isolates was 5- to 12-fold higher than in DMI-S isolates (14). The CYP51 gene in DMI-R strains of V. inaequalis containing a sequence inserted upstream the CYP51 gene was up to 18-fold overexpressed compared to DMI-S strains (30). In P. digitatum, the CYP51 gene was estimated to be 100-fold overexpressed in DMI-R strains compared to DMI-S strains (10). The latter, extremely high expression level was estimated by a less quantitative Northern hybridization analysis, whereas expression levels in the other three studies were determined by quantitative real-time PCR.
The driving factors for increased CYP51 mRNA levels can vary. For example, in C. glabrata, the overexpression was based on an increase of CYP51 gene copy numbers (17). In our study, the average MfCYP51 copy number in DMI-R isolates was greater than in DMI-S isolates (1.35 versus 1.13, respectively), but Southern hybridization analysis confirmed the presence of only one MfCYP51 gene copy in DMI-S isolate SCDL72 and in DMI-R isolate Bmpc7 (data not shown). Therefore, gene duplication cannot explain the increase of MfCYP51 mRNA. In plant pathogenic fungi, increased CYP51 mRNA levels have been associated with large inserts in the upstream region of CYP51 (10, 14, 30). In the present study we also found an insert (Mona) in the upstream region of the MfCYP51 gene in DMI-R isolates. However, Mona was smaller than any other insert associated with CYP51 overexpression in plant pathogenic fungi, such as the 126-bp tandem repeat (four repeats) of a transcriptional enhancer in P. digitatum (10), a 553-bp insert in V. inaequalis (30), and >2,000-bp retrotransposons in B. jaapii (14). Interestingly, most of Mona was predicted to be a typical gene promoter sequence (promoter 2). Promoters 1 and 3 were present and identical in both DMI-R and DMI-S isolates; thus, they should not be responsible for the overexpression of the MfCYP51 gene. Except for Mona, we did not find other differences in the upstream regions that correlated with the resistance phenotype.
The fact that DMI-S isolate SCDL72 shares the same MfCYP51 sequence with the DMI-R isolates but does not contain Mona upstream the MfCYP51 gene strongly supports the linkage of Mona with the resistance phenotype. However, whether Mona is indeed driving the MfCYP51 gene overexpression could not be verified in the present study. Such analyses would have required promoter deletion studies that are difficult to conduct in a multinucleate fungus, such as M. fructicola. Agrobacterium-mediated transformation of M. fructicola has been achieved recently; however, the same study pointed out difficulties with obtaining homokaryotic mutants (12). Alternatively, heterologous expression of the MfCYP51 gene in yeast (28) with or without Mona could be conducted to confirm its role in MfCYP51 overexpression. The association between Mona and the MfCYP51 overexpression may also be confirmed in the future when more DMI-R isolates from other regions in GA or even from other states are discovered and analyzed.
The variability in relative copy numbers of Mona between isolates suggests that it is a mobile genetic element. Filamentous fungi harbor a large variety of mobile genetic elements, including introns, circular and linear plasmids, transposons, and mobile elements not associated with introns, plasmids, or transposons (23). Except for class I transposon SINEs (for short interspersed nuclear elements) and class II DNA transposon MITEs (for miniature inverted-repeat transposable elements), almost all of the mobile elements are able to encode proteins involving transposition (23). Mona is too short to encode proteins; thus, it should be a SINE-like or MITE-like element. However, Mona does not have the typical features of a SINE either, i.e., the presence of an internal binding site for RNA polymerase III and adenine-rich 3′ ends (23). Also, Mona lacks the terminal inverted repeats that are commonly described in class II transposons, including MITEs (4). The unique feature of Mona suggests that it may be a new type of repetitive genetic element.
It appears that if the DMI-R isolates arose recently as a consequence of fungicide selection, these isolates should be closely genetically related, possibly only differing by the presence of Mona upstream MfCYP51. In the present study, all DMI-R isolates revealed a MfCYP51 gene identical in nucleotide sequence. In addition, Mona was located at the same position upstream of the MfCYP51 gene, and no nucleotide variation was detected in Mona or in the surrounding upstream region. On the other hand, the MfCYP51 gene in DMI-S isolates did reveal ample nucleotide diversity. Still, DMI-R isolates were not genetically identical as determined by MP-PCR analysis. Furthermore, DMI-R isolates revealed various numbers of MfCYP51 copies. A possible explanation for these results is that the insertion event may have taken place some time ago, perhaps soon after the introduction of DMI fungicides in the 1970s and 1980s.
High expression of two A. nidulans genes (atrA and atrB) and a P. digitatum gene (PMR1), which encode ABC transporters, were linked to DMI fungicide resistance (5, 18). Likewise, Palani and Lalithakumari (20) reported energy-dependent efflux activity in penconazole-resistant laboratory mutants of V. inaequalis. The energy-dependent efflux transporter activity has been linked to epoxiconazole resistance in wheat tan spot, Pyrenophora tritici-repentis, and the application of the inhibitor of energy-dependent fungicide efflux transporters can shift the fungicide resistance isolates back to normal sensitivity levels (24). Interestingly, in the present study the constitutive expression of the MfABC1 gene was lower in DMI-R isolates than in DMI-S isolates. However, the mRNA levels of MfABC1 increased in DMI-R isolates after the mycelium was subjected to a nonlethal dose of propiconazole. These results indicate that the MfABC1 gene may be a minor genetic determinant for DMI resistance.
In conclusion, DMI resistance in M. fructicola isolates from Georgia is likely a result of increased synthesis of 14-α demethylase due to MfCYP51 gene overexpression. The resistance phenotype is also correlated with a novel 65-bp repetitive element (Mona) located near the translational start site. Mona will be a useful molecular marker for rapid detection of DMI-resistant isolates in field populations from Georgia and maybe elsewhere.
Acknowledgments
We thank M. Kusaba of Saga University (Japan) for constructing the MP-PCR phenogram.
This material is based upon work supported by grant 2004-04014 of the USDA-CSREES RAMP (Risk Avoidance and Mitigation) program, grant 2006-34103-17007 of the U.S. Department of Agriculture S-RIPM grant program, and CSREES/USDA under project no. SC-1000642.
Footnotes
Published ahead of print on 16 November 2007.
Technical contribution no. 5351 of the Clemson University Experiment Station.
REFERENCES
- 1.Applied Biosystems. 1997. (updated 2001). User bulletin 2. ABI Prism 7700 sequence detection systems. Applied Biosystems, Inc., Foster City, CA.
- 2.Brannen, P. M., M. Hotchkiss, C. C. Reilly, and G. Schnabel. 2005. Evaluation of fungicide programs to manage a DMI-resistant Monilinia fructicola population in a Georgia peach research block, 2005. F&N Tests 61:STF001. [Google Scholar]
- 3.Cox, D. K., K. P. Bryson, and G. Schnabel. 2007. Instability of propiconazole resistance and fitness in Monilinia fructicola. Phytopathology 97:448-453. [DOI] [PubMed] [Google Scholar]
- 4.Daboussi, M. J., and P. Capy. 2003. Transposable elements in filamentous fungi. Annu. Rev. Microbiol. 57:275-299. [DOI] [PubMed] [Google Scholar]
- 5.Del Sorbo, G., A. C. Andrade, J. G. M. van Nistelrooy, J. A. L. van Kan, E. Balzi, and M. A. de Waard. 1997. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol. Gen. Genet. 254:417-426. [DOI] [PubMed] [Google Scholar]
- 6.Delye, C., L. Bousset, and M. F. Corio-Costet. 1998. PCR cloning and detection of point mutations in the eburicol 14α-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr. Genet. 34:399-403. [DOI] [PubMed] [Google Scholar]
- 7.Delye, C., F. Laigret, and M.-F. Corio-Costet. 1997. A mutation in the 14α-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl. Environ. Microbiol. 63:2966-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2003. A point mutation in the 14α-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favre, B., M. Didmon, and N. S. Ryder. 1999. Multiple amino acid substitutions in lanosterol 14α-sterol demethylase contribute to azole resistance in Candida albicans. Microbiology 145:2715-2725. [DOI] [PubMed] [Google Scholar]
- 10.Hamamoto, H., K. Hasegawa, R. Nakaune, Y. J. Lee, Y. Makizumi, K. Akutsu, and T. Hibi. 2000. Tandem repeat of a transcriptional enhancer upstream of the sterol 14 alpha-demethylase gene (CYP51A1) in Penicillium digitatum. Appl. Environ. Microbiol. 66:3421-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi, K., H. J. Schoonbeek, and M. A. De Waard. 2003. Modulators of membrane drug transporters potentiate the activity of the DMI fungicide oxpoconazole against Botrytis cinerea. Pest Manag. Sci. 59:294-302. [DOI] [PubMed] [Google Scholar]
- 12.Lee, M. H., and R. M. Bostock. 2006. Agrobacterium T-DNA-mediated integration and gene replacement in the brown rot pathogen Monilinia fructicola. Curr. Genet. 49:309-322. [DOI] [PubMed] [Google Scholar]
- 13.Loffler, J., S. L. Kelly, H. Hebart, U. Schumacher, C. Lass Florl, and H. Einsele. 1997. Molecular analysis of CYP51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol. Lett. 151:263-268. [DOI] [PubMed] [Google Scholar]
- 14.Ma, Z., T. J. Proffer, J. L. Jacobs, and G. W. Sundin. 2006. Overexpression of the 14α-demethylase target gene (cyp51) mediates fungicide resistance in Blumeriella jaapii. Appl. Environ. Microbiol. 72:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma, Z. H., M. A. Yoshimura, and T. J. Michailides. 2003. Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Appl. Environ. Microbiol. 69:7145-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. S. Ramaekers, F. C. Odds, and H. V. Bossche. 1999. Contribution of mutations in the cytochrome P450 14α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145:2701-2713. [DOI] [PubMed] [Google Scholar]
- 17.Marichal, P., H. van den Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. van Broeckhoven, S. Fay, and P. Mose Larsen. 1997. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakaune, R., K. Adachi, O. Nawata, M. Tomiyama, K. Akutsu, and T. Hibi. 1998. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl. Environ. Microbiol. 64:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuninger-Ney, C., F. J. Schwinn, and T. Staub. 1989. In vitro selection of sterol-biosynthesis-inhibitor (SBI) resistant mutants in Monilinia fructicola (Wint.) Honey. Netherlands J. Plant Pathol. (Suppl.95 1):137-150. [Google Scholar]
- 20.Palani, P. V., and D. Lalithakumari. 1999. Resistance of Venturia inaequalis to the sterol biosynthesis-inhibiting fungicide, penconazole [1-(2-(2,4-dichlorophenyl)pentyl)-1H-1,2,4-triazole]. Mycol. Res. 9:1157-1164. [Google Scholar]
- 21.Parker, D. M., N. Zhang, C. D. Smart, and W. D. Koller. 2006. Polymorphism of 14 alpha-demethylase gene (CYP51) in brown rot pathogen Monilinia fructicola from a resistant orchard in New York State. Phytopathology 96:S90. [Google Scholar]
- 22.Parks, L. W., and W. M. Casey. 1995. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49:95-116. [DOI] [PubMed] [Google Scholar]
- 23.Poggeler, S., and F. Kempken. 2004. Mobile genetic elements in mycelial fungi, 2nd ed. Springer-Verlag, Berlin, Germany.
- 24.Reimann, S., and H. B. Deising. 2005. Inhibition of efflux transporter-mediated fungicide resistance in Pyrenophora tritici-repentis by a derivative of 4γ-hydroxyflavone and enhancement of fungicide activity. Appl. Environ. Microbiol. 71:3269-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez, R. J., C. Low, C. D. K. Bottema, and L. W. Parks. 1985. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim. Biophys. Acta 837:336-343. [DOI] [PubMed] [Google Scholar]
- 26.Sanglard, D., F. Ischner, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14-alpha demethylase (CYP51A1) from azole resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnabel, G., K. P. Bryson, W. Bridges, and P. M. Brannen. 2004. Reduced sensitivity in Monilinia fructicola to propiconazole in Georgia and implications for disease management. Plant Dis. 88:1000-1004. [DOI] [PubMed] [Google Scholar]
- 28.Schnabel, G., and Q. Dai. 2004. Heterologous expression of the 14-alpha demethylase gene from Monilinia fructicola reduces sensitivity to some but not all DMI fungicides. Pestic. Biochem. Physiol. 88:162-166. [Google Scholar]
- 29.Schnabel, G., Q. Dai, and M. R. Paradkar. 2003. Cloning and expression analysis of the ATP binding cassette transporter MFABC1 gene and the alternative oxidase gene MfAOX1 from Monilinia fructicola. Pest Manag. Sci. 59:1143-1151. [DOI] [PubMed] [Google Scholar]
- 30.Schnabel, G., and A. L. Jones. 2001. The 14α-demethylase (CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology 91:102-110. [DOI] [PubMed] [Google Scholar]
- 31.Siebert, P. D., A. Chenchik, D. E. Kellogg, K. A. Lukyanov, and S. A. Lukyanov. 1995. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 23:1087-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, Y. 1993. Lanosterol 14α-demethylase (cytochrome P45014DM). Springer-Verlag, Berlin, Germany.




