Abstract
The impact of long-term organic and inorganic amendments on the actinobacterial community in soils was studied. Denaturing gradient gel electrophoresis patterns based on the V3 region of 16S rRNA suggested that there was no significant difference between the communities occurring in the different amendments. However, analysis of the clone libraries of the actinobacterial communities by the use of multiple statistical approaches showed that these communities were significantly different from each other. Results showed that long-term organic and inorganic soil amendments did not significantly alter the overall phylogenetic diversity of the actinobacterial communities but did significantly change the community structure.
Actinobacteria, ubiquitously found in terrestrial (7, 9, 20), freshwater (1, 18), and marine (3, 15, 25, 29) ecosystems, are dominant soil bacterial taxa (9, 10) and probably play multiple roles in the environment (8, 14, 17, 19, 27). Applying organic amendments to soil is a common agronomic practice that likely has a significant impact on the diversity and community structure of actinobacteria. Extensive work has been directed toward understanding the effects of soil amendments on general rather than group-specific soil microbial communities. The purpose of the present study was to compare the community structure and diversity of actinobacteria in untreated soil with those of actinobacteria in soil treated with manure for 25 years.
The site of the long-term experiment, established in 1980, is in Suzhou (31°32′45"N; 120°41′57″E), Jiangsu Province, China. The site was initially selected on the basis of its relatively uniform crop growth, the flatness of the soil surface, and the relatively uniform soil fertility, as evidenced by the levels of total nitrogen, phosphorus, and potassium measured in 1980. Rice-wheat rotation, a typical cropping system used for more than 1,500 years in the area (4, 5), was adopted (with only six exceptional crops over 25 years) during the experimental period. The total experimental area was 1,600 m2, which was split into 42 plots (14 treatments in triplicate), each with 20 m2 of effective cropping area. The plots were separated by cement plates extending from 50 cm beneath to 15 cm above the soil surface to limit the mixing of water between plots. Separate ditches for irrigation or drainage were designed to ensure uniform control of the water regime. All field agronomic practices were performed manually. The soil is clay loam in texture. Soil fertility variables measured in 2004 (23) indicated that triplicate plots within each treatment were reasonably uniform. A control and four treatments were included in the present study: (i) CK, in which the soil remained unamended (the control); (ii) NPK, in which the soil received N, P, and K fertilizers in combination; (iii) SN, in which the soil received straw plus N; (iv) M, in which the soil received pig manure only; and (v) MNPK, in which the soil received N, P, and K in addition to pig manure. Additional information on the field plots can be found elsewhere (23). Ten soil cores (15 cm in height by 8 cm in diameter) randomly distributed within each 20-m2 plot were sampled, combined, sieved to remove the roots, and brought on ice to the laboratory, where the total DNA was extracted. The soils were sampled twice during the wheat growing season, on 8 April 2006, when wheat was in the early earing stage, and on 1 July 2006, after the wheat harvest (rice transplanting was delayed in this particular year).
For DNA extraction, 2 g of soil was repeatedly homogenized by vortexing it in 20 ml phosphate-buffered saline and centrifuged at 200 × g for 2 min. Microbial cells in the combined supernatant liquid were collected by centrifugation at 12,000 × g for 10 min, washed three times with TENP buffer (50 mM Tris, 20 mM EDTA, 100 mM NaCl, 1% polyvinylpyrrolidone, pH 10; 1-liter volume), and lysed by bead beating. Briefly, the tubes containing the cells, 0.3 g of 0.1-mm zirconium beads, and 150 μl redistilled phenol (pH 8.0) were agitated on a bead beater (Mini-Bead-Beater-8; Biospec Products) at the highest speed for 80 s and then allowed to sit on ice for 1 min. This was repeated twice more for a total of three runs per sample. Next, 110 μl of sodium dodecyl sulfate (10%) was added and gently mixed, and the sample was incubated on ice for 10 min. After this, 150 μl chloroform-isopropanol (25:1, vol/vol) was added, gently mixed, and then centrifuged at 15,000 × g for 10 min. The supernatant liquid was collected, and 1/10 volume of 3 M sodium acetate and 1 volume of phenol were added, followed by centrifugation at 15,000 × g for 10 min. The supernatant was then extracted twice with chloroform-isopropanol (24:1, vol/vol). Nucleic acids in the supernatant were precipitated with cold ethanol. The size of the extracted DNA, as determined by electrophoresis on a 0.5% agarose gel, was found to be ∼10 to 15 kbp.
The V3 region of the 16S rRNA gene was amplified for denaturing gradient gel electrophoresis (DGGE) analysis using primers P2 and P3 with a 40-bp GC clamp at the 5′ end of P3 (16), as previously described (13). The amplified products were separated with a Dcode system (Bio-Rad, Hercules, CA) in an 8% (wt/vol) polyacrylamide gel containing a linear, 35 to 60% denaturant gradient. Electrophoresis was carried out using 1× Tris-acetate-EDTA buffer at 200 V and 60°C for 200 min. The DNA bands were stained with Sybr green (Amresco, Solon, OH) and photographed with a UVI gel documentation system (UVItec, Cambridge, United Kingdom). The images were analyzed with Quantity One software version 4.4 (Bio-Rad, Hercules, CA). A dendrogram of the bands was constructed based on the Dice similarity coefficient using the unweighted-pair group arithmetic average clustering algorithm.
For clone library construction, actinobacterial 16S rRNA genes were amplified with primers S-C-Act-0235-a-S-20 and S-C-Act-0878-a-A-19 by use of previously described PCR conditions (26). The amplified products were purified and concentrated with the UltraClean 15 purification kit (Mo Bio, Inc.), ligated with T4 DNA ligase into a pGEM-T Easy vector (Promega), and electrotransformed into competent Escherichia coli DH5α cells according to the manufacturer's instructions. The transformed cells were plated onto LB agar containing ampicillin (100 μg ml−1; Amresco), isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 80 μg ml−1). Positive colonies were screened by PCR using the amplifying primers. The plasmids containing the correct inserts were sequenced commercially with an ABI 3730 DNA analyzer (Applied Biosystems) using the S-C-Act-0235-a-S-20 primer (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd.). The sequences were double checked for chimeras by use of both the Chimera Detection program from the Ribosomal Database Project II (http://rdp8.cme.msu.edu/cgis/chimera.cgi?su=SSU) and Mallard version 1.02 (2).
Actinobacterial 16S rRNA gene sequences were aligned using the CLUSTAL X interface (28). Operational taxonomic units (OTUs) were identified with DOTUR 1.53 (21) at a DNA distance cutoff of 0.01. The closest representative for each OTU sequence was identified using BLASTn. Phylogenetic neighbor-joining trees were constructed with MEGA version 3.1 (12) using a Jukes-Cantor model. Libraries were compared using the LIBSHUFF program (24) by treating each cloned sequence as a separate sample. Two nonparametric richness estimators (SACE and SChao1), calculated with web-based software (11), were used to estimate whether the libraries were large enough to yield stable phylotype richness estimates. Nonparametric estimators of the fraction and richness of OTUs shared between two communities were analyzed using SONS software (22). The phylogenetic diversity within each community was estimated using DOTUR 1.53, SPADE v2.1 (A. Chao and T.-J. Shen [http://chao.stat.nthu.edu.tw/softwareCE.html]), and Arlequin v3.01 (6).
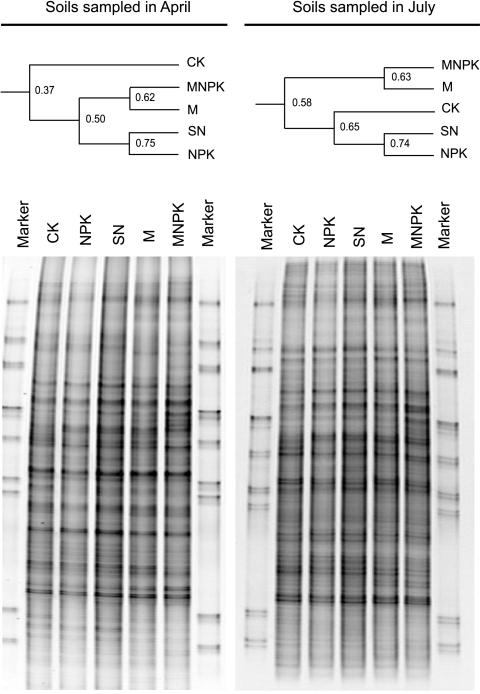
The DGGE patterns of the 16S rRNA amplified from all of the treatments were generally similar (Fig. 1). The most notable differences were the band intensities rather than the band positions. The dendrogram generated from the bands showed that the April soils could be placed into three clusters, representing CK, manure-treated, and non-manure-treated soils. Similarly to the April soils, the July soils also showed similar DGGE patterns among the treatments (Fig. 1). Note that communities from the April and July samples could not be compared directly by band positions because they were from two separate gels. However, analysis of both the April and July gels generated three clusters that represented the same treatments, indicating that there was no substantial community change over time. CK, NPK, and MNPK soils were, therefore, chosen as the representative soils for further clone library analysis. Clone libraries were constructed from the July samples only.
FIG. 1.
DGGE patterns of soil samples treated with CK, NPK, M, NPK, and SN based on the sequence of the V3 region in the 16S rRNA gene. See the text for descriptions of treatments.
Of the 247 clones in total, one was detected as a putative chimera and was set aside for further analysis, one belonged to the genus Gemmatimonas, and the remaining 245 were actinobacterial in origin. The numbers of OTUs derived from the different samples are listed in Table 1. The library size estimated by the method described in reference 11 showed that the SChao1 and SACE richness estimators did not reach an asymptotic maxima but tended to converge into a narrow range that exceeded the unstable estimator-producing stage when the subsample sizes approach the actual library sizes (see Fig. S1 in the supplemental material). SChao1 and SACE richness estimator patterns were, in fact, very similar to the patterns that are generated from libraries not considered to have been exhaustively sampled but that were large enough to yield stable and unbiased estimates of phylotype richness (11). We therefore considered that the actinobacterial libraries were of sufficient size to allow valid comparisons.
TABLE 1.
Comparison of ecological and molecular estimates of sequence diversity for actinobacterial communities in differently treated soils
| Community in soil with indicated treatmenta | No. of unique sequences | No. of OTUs | Value determined by DOTURb
|
HJackknife value determined by SPADEc | Value determined by Arlequind
|
||||
|---|---|---|---|---|---|---|---|---|---|
| HShannon | SChao1 | CACE | Gene diversity | Nucleotide diversity | θ(π) | ||||
| CK | 61 | 46 | 3.7 (∼3.5 to 3.9) | 77 (∼59 to 123) | 89 (∼64 to 146) | 4.29 (0.11) | 1.0 (0.003) | 0.12 (0.06) | 76.1 (36.8) |
| NPK | 84 | 53 | 3.8 (∼3.6 to 3.9) | 101 (∼73 to 170) | 106 (∼77 to 172) | 4.27 (0.11) | 1.0 (0.002) | 0.11 (0.05) | 72.0 (34.7) |
| MNPK | 75 | 49 | 3.7 (∼3.5 to 3.9) | 89 (∼65 to 148) | 96 (∼70 to 154) | 4.16 (0.11) | 1.0 (0.002) | 0.11 (0.05) | 71.6 (34.5) |
| CK+NPK+MNPK | 214 | 106 | 4.3 (∼4.2 to 4.5) | 190 (∼148 to 274) | 194 (∼156 to 263) | 4.68 (0.08) | 1.0 (0.0004) | 0.11 (0.05) | 72.7 (34.7) |
A “+” means that the libraries from the different soils were pooled.
The OTUs are defined at a DNA distance of 0.01. The numbers in parentheses are the ranges at the 95% confidence interval. HShannon, Shannon diversity index.
The OTUs are defined at a DNA distance of 0.01. The numbers in parentheses are standard errors. HJackknife, Jackknife diversity index.
All clone sequences, each treated as an individual haplotype, were uploaded to the program. The numbers in parentheses are standard errors. θ(π), molecular diversity index calculated on the basis of the number of nucleotide differences between two randomly chosen sequences from a population.
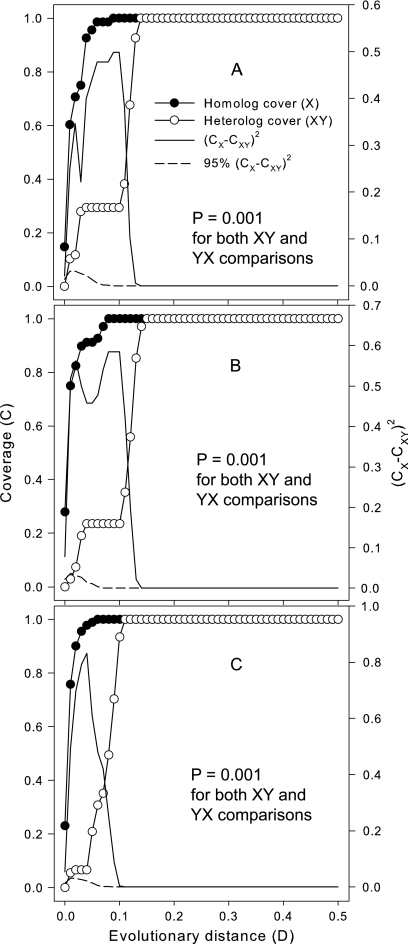
LIBSHUFF (24) analysis of homologous and heterologous coverage (C) curves indicated that the three libraries were significantly different from each other, as the P values produced by both XY and YX comparisons (where X and Y represent two different libraries) were all 0.001, smaller than the critical P value of 0.025 (Fig. 2). These results suggested that most sequences in the libraries were different. Significant differences between (CX − CXY)2 and 95% of (CX − CXY)2 values were found only at a distance (D) of less than 0.10, indicating that the libraries differed greatly at a D of <0.10. At a D of >0.10, both (CX − CXY)2 and 95% of (CX − CXY)2 values were virtually zero, suggesting that deep phylogenetic groups were not found in the libraries (see Fig. S2 in the supplemental material).
FIG. 2.
LIBSHUFF comparison of the homologous and heterogenous coverages between the CK and NPK libraries (A), the CK and MNPK libraries (B), and the NPK and MNPK libraries (C). X represents the CK library in panels A and B and the NPK library in panel C. Y represents the NPK library in panel A and the MNPK library in panels B and C. The fact that the P values produced by both the XY and YX comparisons are smaller than the critical P value of 0.025 indicates that the two libraries being compared are different.
The diversity indices generated for each individual community by DOTUR (21), SPADE, and Arlequin (6) were very similar (Table 1), suggesting that soil amendments did not significantly change the diversity levels of the actinobacterial communities. When the communities were pooled, most of the estimators did not change significantly. Species richness (SChao1) and coverage (CACE) values were the exceptions, since they were significantly higher for the pooled library than for the individual libraries. These results indicated that one library contained a fraction of the species that were not found in the other individual libraries, which is further supported by SONS analysis.
Nonparametric estimates obtained by SONS analysis (22) are listed in Table 2. The Jabund value, defined as the probability that a randomly selected OTU is found in the libraries under comparison, is a measure of community overlap. In our case, all Jabund values between any two individual communities under comparison were low and were significantly different from 1.0 (Table 2), suggesting that the communities were different from each other. This is further supported by low θYC values (Table 2), measures of community structure similarity, as well as by LIBSHUFF results (Fig. 2). When two libraries were pooled and compared with a third one, the resulting estimates of Jabund values were also low and significantly different from 1.0. When CK was compared with NPK, the Jabund value, for example, was 0.48. When CK was pooled with MNPK (CK+MNPK) and compared with NPK again, the Jabund value was 0.63, higher than 0.48 but still significantly different from 1.0. These results indicate that the pooled community did not result in a significant increase in overlap.
TABLE 2.
Nonparametric estimates of actinobacterial library comparisons using SONS at an OTU of 0.01
| Libraries from soils with indicated treatment compareda
|
Estimated valueb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | Uest | Vest | SA,B Chao | Jclas | Ashared | Bshared | Jabund | θYC |
| CK | NPK | 0.74 (0.16) | 0.57 (0.11) | 39 | 0.24 | 0.46 | 0.36 | 0.48 (0.10) | 0.39 (0.09) |
| CK | MNPK | 0.47 (0.15) | 0.47 (0.12) | 27 | 0.20 | 0.34 | 0.32 | 0.48 (0.09) | 0.24 (0.07) |
| NPK | MNPK | 0.50 (0.11) | 0.85 (0.15) | 46 | 0.25 | 0.38 | 0.42 | 0.48 (0.12) | 0.38 (0.08) |
| CK+NPK | MNPK | 0.50 (0.11) | 0.84 (0.12) | 49 | 0.25 | 0.33 | 0.54 | 0.45 (0.11) | 0.36 (0.07) |
| CK+MNPK | NPK | 0.81 (0.14) | 0.74 (0.11) | 63 | 0.29 | 0.38 | 0.55 | 0.63 (0.12) | 0.49 (0.08) |
| CK | NPK+MNPK | 0.77 (0.12) | 0.54 (0.11) | 42 | 0.25 | 0.55 | 0.31 | 0.46 (0.09) | 0.36 (0.08) |
A “+” means that the libraries from different soils were pooled.
The numbers in parentheses are standard errors. The detailed definitions and calculations of the estimates are described by Schloss and Handelsman (22). Uest and Vest, the fractions of sequences from libraries A and B, respectively, that belong to a shared OTU; Jclas, the classic Jaccard similarity index; Ashared, the ratio of shared OTUs to the total number of OTUs in library A; Bshared, the ratio of shared OTUs to the total number of OTUs in library B.
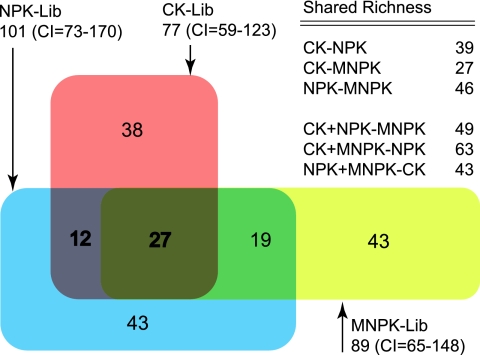
By combining the SA,B Chao values in Tables 1 and 2 (where SA,B Chao is the richness estimator of the number of shared OTUs between libraries A and B), a Venn diagram was generated (Fig. 3). The numbers in each section or intersection of the diagram are nonparametric SA,B Chao values. The fraction of SA,B Chao values in each intersection over the SChao1 value of a specific library could be roughly viewed as the fraction of shared OTUs between the two libraries concerned. The CK library, for example, contained approximately 49% (38/77) unique OTU sequences, 16% (12/77) shared with the NPK library, 0% shared with MNPK, and 35% (27/77) shared with pooled NPK and MNPK libraries. The central intersection in Fig. 3 labeled “27” accounted for roughly 30% of the mean SA,B Chao value of the three libraries (27/[(77 + 101 + 89)/3]). The fraction of OTUs that were not shared with any other communities are probably the OTUs that could be changed easily in response to soil amendments. In contrast, the fraction of OTUs shared by all three communities are probably the OTUs that are unaffected by amendments.
FIG. 3.
A Venn diagram comparing the OTU0.01 (OTU assigned at a DNA distance of 0.01) memberships found in the CK (n = 68), NPK (n = 91), and MNPK (n = 89) libraries. Below each library's name are the Chao1 richness estimate and the 95% confidence interval estimated by DOTUR for the respective community. The richness of the overlapping regions was estimated based on the pairwise SA,B Chao richness estimates shared by the three communities and by pooling two communities and estimating the fraction shared with a third community. The Chao1 richness estimate for the three libraries pooled together was 190 (confidence interval, 148 to 274), and the sum of the richness estimates for the individual sectors in the diagram was 182.
In summary, our results showed that DGGE patterns based on the V3 region of 16S rRNA gene fingerprints were remarkably similar among the soils receiving long-term amendments. However, actinobacterium-specific clone libraries showed that the communities were significantly different from each other. With the aid of multiple statistical approaches, it was shown that long-term organic and inorganic soil amendments did not significantly alter the phylogenetic diversity of the actinobacterial communities but did significantly change the community structure. These results demonstrated the advantage of group-specific community analysis over general community analysis in revealing the impact of soil amendments on highly complex soil communities. Our results also demonstrated the usefulness of multiple statistical approaches in revealing community structure and diversity.
Nucleotide sequence accession numbers.
The nucleotide sequences derived from this study have been deposited in the GenBank database under accession numbers EF134967 to EF135072.
Supplementary Material
Acknowledgments
We are grateful to M. X. Shen and his group for maintaining the long-term field plots and the sampling. S. Y. thanks James P. Shapleigh for his comments and help in revising the text. The comments from two anonymous reviewers are appreciated.
Financial support from the National Science Foundation of China (40471072), the National Key Basic Research and Development Program of China (2005CB121108), and the Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-YW-407) is acknowledged.
Footnotes
Published ahead of print on 12 October 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allgaier, M., and H.-P. Grossart. 2006. Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl. Environ. Microbiol. 72:3489-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashelford, K. E., N. A. Chuzhanova, J. C. Fry, A. J. Jones, and A. J. Weightman. 2006. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl. Environ. Microbiol. 72:5734-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bull, A. T., J. E. Stach, A. C. Ward, and M. Goodfellow. 2005. Marine actinobacteria: perspectives, challenges, future directions. Antonie van Leeuwenhoek 87:65-79. [PubMed] [Google Scholar]
- 4.Cao, Z. H., J. L. Ding, Z. Y. Hu, H. Knicker, I. Kogel-Knabner, L. Z. Yang, R. Yin, X. G. Lin, and Y. H. Dong. 2006. Ancient paddy soils from the Neolithic age in China's Yangtze River Delta. Naturwissenschaften 93:232-236. [DOI] [PubMed] [Google Scholar]
- 5.Ellis, E. C., and S. M. Wang. 1997. Sustainable traditional agriculture in the Tai Lake region of China. Agric. Ecosyst. Environ. 61:177-193. [Google Scholar]
- 6.Excoffier, L., G. Laval, and S. Schneider. 2005. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1:47-50. [PMC free article] [PubMed] [Google Scholar]
- 7.Felske, A., H. Rheims, A. Wolterink, E. Stackebrandt, and A. D. Akkermans. 1997. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143:2983-2989. [DOI] [PubMed] [Google Scholar]
- 8.Getha, K., S. Vikineswary, W. H. Wong, T. Seki, A. Ward, and M. Goodfellow. 2005. Evaluation of Streptomyces sp. strain g10 for suppression of Fusarium wilt and rhizosphere colonization in pot-grown banana plantlets. J. Ind. Microbiol. Biotechnol. 32:24-32. [DOI] [PubMed] [Google Scholar]
- 9.Gremion, F., A. Chatzinotas, and H. Harms. 2003. Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environ. Microbiol. 5:896-907. [DOI] [PubMed] [Google Scholar]
- 10.Janssen, P. H. 2006. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 72:1719-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp, P. F., and J. Y. Aller. 2004. Estimating prokaryotic diversity: when are 16S rDNA libraries large enough? Limnol. Oceanogr. Methods 2:114-125. [Google Scholar]
- 12.Kumar, S., K. Tamura, and N. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 13.Liu, B. B., F. Zhang, X. X. Feng, Y. D. Liu, X. Yan, X. J. Zhang, L. H. Wang, and L. P. Zhao. 2006. Thauera and Azoarcus as functionally important genera in a denitrifying quinoline-removal bioreactor as revealed by microbial community structure comparison. FEMS Microbiol. Ecol. 55:274-286. [DOI] [PubMed] [Google Scholar]
- 14.Loria, R., J. Kers, and M. Joshi. 2006. Evolution of plant pathogenicity in Streptomyces. Annu. Rev. Phytopathol. 44:469-487. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado, L. A., J. E. Stach, W. Pathom-aree, A. C. Ward, A. T. Bull, and M. Goodfellow. 2005. Diversity of cultivable actinobacteria in geographically widespread marine sediments. Antonie van Leeuwenhoek 87:11-18. [DOI] [PubMed] [Google Scholar]
- 16.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatsu, C. H., N. Carmosini, B. Baldwin, F. Beasley, P. Kourtev, and A. Konopka. 2005. Soil microbial community responses to additions of organic carbon substrates and heavy metals (Pb and Cr). Appl. Environ. Microbiol. 71:7679-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, J. L., C. Klausen, P. H. Nielsen, M. Burford, and N. O. Jorgensen. 2006. Detection of activity among uncultured Actinobacteria in a drinking water reservoir. FEMS Microbiol. Ecol. 55:432-438. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowski, K., and A. Sirrenberg. 2003. Symbiosis between Frankia and actinorhizal plants: root nodules of non-legumes. Indian J. Exp. Biol. 41:1165-1183. [PubMed] [Google Scholar]
- 20.Rheims, H., A. Felske, S. Seufert, and E. Stackebrandt. 1999. Molecular monitoring of an uncultured group of the class Actinobacteria in two terrestrial environments. J. Microbiol. Methods 36:65-75. [DOI] [PubMed] [Google Scholar]
- 21.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloss, P. D., and J. Handelsman. 2006. Introducing SONS, a tool for operational taxonomic unit-based comparisons of microbial community memberships and structures. Appl. Environ. Microbiol. 72:6773-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen, M.-X., L.-Z. Yang, Y.-M. Yao, D.-D. Wu, J. Wang, R. Guo, and S. Yin. 2007. Long-term effects of fertilizer managements on crop yields and organic carbon storage of a typical rice-wheat agroecosystem of China. Biol. Fertil. Soils 44:187-200. [Google Scholar]
- 24.Singleton, D. R., M. A. Furlong, S. L. Rathbun, and W. B. Whitman. 2001. Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl. Environ. Microbiol. 67:4374-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stach, J. E., and A. T. Bull. 2005. Estimating and comparing the diversity of marine actinobacteria. Antonie van Leeuwenhoek 87:3-9. [DOI] [PubMed] [Google Scholar]
- 26.Stach, J. E. M., L. A. Maldonado, A. C. Ward, M. Goodfellow, and A. T. Bull. 2003. New primers for the class Actinobacteria: application to marine and terrestrial environments. Environ. Microbiol. 5:828-841. [DOI] [PubMed] [Google Scholar]
- 27.Thirup, L., A. Johansen, and A. Winding. 2003. Microbial succession in the rhizosphere of live and decomposing barley roots as affected by the antagonistic strain Pseudomonas fluorescens DR54-BN14 or the fungicide imazalil. FEMS Microbiol. Ecol. 43:383-392. [DOI] [PubMed] [Google Scholar]
- 28.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward, A. C., and N. Bora. 2006. Diversity and biogeography of marine actinobacteria. Curr. Opin. Microbiol. 9:279-286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.