Abstract
We observed dimethyl sulfide and methanthiol production in pure incubations of the methanogen Methanosarcina acetivorans when carbon monoxide (CO) served as the only electron donor. Energy conservation likely uses sodium ion gradients for ATP synthesis. This novel metabolism permits utilization of CO by the methanogen, resulting in quantitative sulfide methylation.
Rother and Metcalf (18) recently described a CO metabolism for the methanogen Methanosarcina acetivorans. Unlike previously described CO methanogenic pathways (5, 12, 17), this metabolism produces acetate, formate, and methane but not hydrogen. We cultured M. acetivorans C2A by using CO as a growth substrate and observed the additional production of both dimethyl sulfide (DMS) and methanthiol (MeSH). Culturing (33.5°C) occurred under 300 kPa of CO in medium (150 ml per bottle) similar to that described by Moran et al. (16), but with 1 g/liter sodium bicarbonate and no organic substrate in 600-ml bottles. Standard bottles for analyte quantification were made in parallel but lacked the sulfide addition. Cultures were inoculated (2.0 ml) from a CO-grown preculture. At intervals throughout growth, 1.0-ml liquid samples were collected for sulfide analysis (by a technique adopted from Hach water quality test kits [Hach Company, Loveland, CO], which showed no interference with DMS or MeSH), acetate and formate analysis (by ion chromatography; Dionex LC30 and AS18 analytical column), and cell counting. Headspace samples (200 μl) were analyzed for CH4, DMS, and MeSH by using gas chromatography (HP 5890 with a GS-Q column and flame ionization detector), and the analyte was quantified by comparison to standard bottles to determine the total moles of analyte in the bottle (combined dissolved and gaseous fractions).
Our results (Fig. 1) show that during growth, microbial DMS and MeSH production effectively scrubbed out free sulfide in the culture medium to less than 4% of the pregrowth values, suggesting sulfide methylation as the pathway for methyl sulfide formation. Enhanced growth (as measured by increased cell densities and methane production) when cultures were exposed to more sulfide suggests energy conservation by sulfide methylation (Fig. 2). Here, we used four sets of triplicate culture bottles containing preinoculation sulfide totaling 0.00, 0.16, 0.31, and 0.63 mmol per bottle.
FIG. 1.
Metabolic products of M. acetivorans growth on CO. CO is converted to acetate, formate, and methane during growth by M. acetivorans as previously identified (18). We show the additional production of MeSH (▪) and DMS (▴) at the expense of sulfide (•), suggesting sulfide methylation as the production mechanism. Each of the points in the graph represents an average for three parallel cultures having an initial sulfide addition of 0.16 mmol per bottle.
FIG. 2.
Enhanced growth with increasing amounts of sulfide. Increased initial sulfide additions to growth media enhanced MeSH and DMS production (with the sum of the results for DMS and MeSH for each bottle shown [○]), as well as methane production (□) and cell density (▵), suggesting both higher rates of methanogenesis and biomass production with increased levels of sulfide.
At standard state, energy yields for MeSH production are consistent with energy conservation (calculated with data from references 1 and 19):
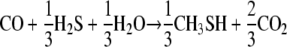 |
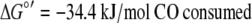 |
Free-energy gains by MeSH production are comparable to those previously estimated (18) for acetate formation from CO, −41.2 kJ/mol CO consumed.
CO is a known methanogenesis inhibitor in both M. acetivorans (18) and other methanogen species (5, 17). This inhibition likely targets the methanogenic pathway at methyl coenzyme M reductase (MCR) and would restrict carbon flow through this enzyme, leading to methyl-CoM accumulation, which would eventually stop energy production by sequestering all CoM.
Methyl sulfide formation provides a low-energy method for regenerating CoM without MCR activity. Working with Methanosarcina barkeri, Tallant et al. (22) demonstrated that direct methylation of MeSH to DMS has a modest energy barrier of only 0.35 kJ per reaction. When MCR is inhibited, the transfer protein (480-kDa corrinoid protein) that normally methylates CoM becomes methylated itself by methyl-CoM (4, 21) and elevated MeSH concentrations promote small-scale methylation of MeSH to DMS (15), suggesting reversibility in the first step of methanogenic DMS consumption. Thus, in instances of MCR inhibition by CO, we hypothesize that methyl sulfide formation is essential for regenerating CoM and maintaining an active metabolism for energy production. Furthermore, this occurrence suggests that the methyltransferase protein identified by Cao and Krzycki (4) is metabolically versatile and can be active in both methanogenic and non-methane-producing energy conservation, depending upon environmental conditions.
The mechanism for energy conservation during sulfide methylation is unclear. When generating methane, methanogens rely largely on reduction of a heterodisulfide bond formed by MCR activity for net energy formation (23). The observed methyl sulfide production likely bypasses MCR, suggesting a different pathway for energy conservation. One option is via a sodium gradient (8; J. G. Ferry, personal communication). The Mtr methyltransferase is expressed in M. acetivorans when cultured on CO (14) and is also known to generate a sodium gradient (3) linked to ATP production in another methanogen, Methanococcus voltae (8). The activation of CO2 before reduction, however, consumes some of the sodium gradient produced by Mtr (6), making it unclear how effective ATP synthesis via this route would be during methyl sulfide production by M. acetivorans. Nevertheless, the ability of methanogens to link methyl transfer to a sodium ion gradient may permit energy conservation by sulfide methylation. In contrast, nonenergetic sulfide methylation from methyl transfer is observed in Holophaga foetida (9), a species with no known ability for sodium ion gradient formation.
To the best of our knowledge, the metabolism described here is the first example of both a methanogen producing high concentrations of methyl sulfides and of a CO metabolism resulting in sulfide methylation. M. acetivorans was isolated from marine sediments rich in decaying sea grass and kelp deposits (20). The bladders used to keep kelp upright underwater are filled with up to 12% CO (13), and their decay is a likely CO source. Under realistic marine conditions (500 μM sulfide, 1 μM MeSH, 10 mM dissolved inorganic carbon, pH 8, and 10°C), the free energy of MeSH production remains favorable at low CO concentrations, approaching 10 pM. Potential energy yields at such low CO concentrations suggest that this metabolism could be active in kelp bed sediments. Furthermore, elevated acetate concentrations in organic-rich sediments would thermodynamically disfavor the acetogenic CO metabolism previously reported (18) and therefore confer an energetic advantage toward methyl sulfide formation.
In addition to the potential role of methanogen-mediated CO conversion to methyl sulfides in modern marine environments, this metabolism has implications for sulfur cycling in ancient Earth. Methanogens likely evolved by the late Archean eon or earlier in Earth's history (2, 24) and had a strong influence on global climate regulation (10). The atmosphere at that time may have contained elevated CO concentrations (11), permitting sulfide methylation by the metabolism described herein. The quest for life outside our planet depends on searching for chemical signatures of life (7). If similar, early-evolving organisms are present on other planets, then methyl sulfides provide a valuable target in the search for extraterrestrial microbial life.
Acknowledgments
We thank B. Thomas for technical assistance and many useful discussions in preparing this work.
Graduate support for this project was provided by the Penn State Biogeochemical Research Initiative for Education (BRIE) funded by NSF (IGERT) grant DGE-9972759. This work was also funded by the Penn State Astrobiology Research Center (through the National Astrobiology Institute), NOAA-NURP (UAF 05-0132), and the National Science Foundation (MCB-0348492).
Footnotes
Published ahead of print on 16 November 2007.
REFERENCES
- 1.Amend, J. P., and E. L. Shock. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175-243. [DOI] [PubMed] [Google Scholar]
- 2.Battistuzzi, F. U., A. Feijao, and S. B. Hedges. 2004. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and colonization of land. BMC Evol. Biol. 4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becher, B., V. Müller, and G. Gottschalk. 1992. N5-Methyl-tetrahydromethanopterin:coenzyme M methyltransferase of Methanosarcina strain Gö1 is an Na+-translocating membrane protein. J. Bacteriol. 174:7656-7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, X. J., and J. A. Krzycki. 1991. Acetate-dependent methylation of two corrinoid proteins in extracts of Methanosarcina barkeri. J. Bacteriol. 173:5439-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels, L., G. Fuchs, R. K. Thauer, and J. G. Zeikus. 1977. Carbon monoxide oxidation by methanogenic bacteria. J. Bacteriol. 132:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Poorter, L. M. I., W. G. Geerts, A. P. R. Theuvenet, and J. T. Keltjens. 2003. Bioenergetics of the formyl-methanofuran dehydrogenase and heterodisulfide reducatase in Methanothermobacter thermautotrophicus. Eur. J. Biochem. 270:66-75. [DOI] [PubMed] [Google Scholar]
- 7.Des Marais, D. J., M. O. Harwit, K. W. Jucks, J. F. Kasting, D. N. C. Lin, J. I. Lunine, J. Schneider, S. Seager, W. A. Traub, and N. J. Woolf. 2002. Remote sensing of planetary properties and biosignatures on extrasolar terrestrial planets. Astrobiology 2:153-181. [DOI] [PubMed] [Google Scholar]
- 8.Dybas, M., and J. Konisky. 1992. Energy transduction in the methanogen Methanococcus voltae is based on a sodium current. J. Bacteriol. 174:5575-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappler, O., P. H. Janssen, J. U. Kreft, and B. Schink. 1997. Effects of alternative methyl group acceptors on the growth energetics of the O-demethylating anaerobe Holophaga foetida. Microbiology 143:1105-1114. [DOI] [PubMed] [Google Scholar]
- 10.Kasting, J. F., and J. L. Siefert. 2002. Life and the evolution of Earth's atmosphere. Science 296:1066-1068. [DOI] [PubMed] [Google Scholar]
- 11.Kharecha, P., J. Kasting, and J. Siefert. 2005. A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology 3:53-76. [Google Scholar]
- 12.Kluyver, A. J., and C. G. T. P. Schnellen. 1947. On the fermentation of carbon monoxide by pure cultures of methane bacteria. Arch. Microbiol. 14:57-70. [PubMed] [Google Scholar]
- 13.Langdon, S. C. 1917. Carbon monoxide, occurrence free in kelp. J. Am. Chem. Soc. 39:149-156. [Google Scholar]
- 14.Lessner, D. J., L. Li, Q. Li, T. Rejtar, V. P. Andreev, M. Reichlen, K. Hill, J. J. Moran, B. L. Karger, and J. G. Ferry. 2006. An unconventional pathway for reduction of CO2 to methane in CO-grown Methanosarcina acetivorans revealed by proteomics. Proc. Natl. Acad. Sci. USA 103:17921-17926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomans, B. P., R. Maas, R. Luderer, H. J. M. Op den Camp, A. Pol, C. van der Drift, and G. D. Vogels. 1999. Isolation and characterization of Methanomethylovorans hollandica gen. nov., sp. nov., isolated from freshwater sediment, a methylotrophic methanogen able to grow on dimethyl sulfide and methanethiol. Appl. Environ. Microbiol. 65:3641-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran, J. J., C. H. House, K. H. Freeman, and J. G. Ferry. 2005. Trace methane oxidation studied in several Euryarchaeota under diverse conditions. Archaea 1:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien, J. M., R. H. Wolkin, T. T. Moench, J. B. Morgan, and J. G. Zeikus. 1984. Association of hydrogen metabolism with unitrophic or mixotrophic growth of Methanosarcina barkeri on carbon monoxide. J. Bacteriol. 158:373-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rother, M., and W. W. Metcalf. 2004. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. USA 101:16929-16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulte, M. D., and K. L. Rogers. 2004. Thiols in hydrothermal solution: standard partial molal properties and their role in the organic geochemistry of hydrothermal environments. Geochim. Cosmochim. Acta 68:1087-1097. [Google Scholar]
- 20.Sowers, K. R., S. F. Baron, and J. G. Ferry. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tallant, T. C., and J. A. Krzycki. 1996. Coenzyme M methylase activity of the 480-kilodalton corrinoid protein from Methanosarcina barkeri. J. Bacteriol. 178:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tallant, T. C., L. Paul, and J. A. Krzycki. 2001. The MtsA subunit of the methylthiol:coenzyme M methyltransferase of Methanosarcina barkeri catalyses both half reactions of corrinoid-dependent dimethylsulfide: coenzyme M methyl transfer. J. Biol. Chem. 276:4485-4493. [DOI] [PubMed] [Google Scholar]
- 23.Thauer, R. K. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology 144:2377-2406. [DOI] [PubMed] [Google Scholar]
- 24.Ueno, Y., K. Yamada, N. Yoshida, S. Maruyama, and Y. Isozaki. 2006. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 440:516-519. [DOI] [PubMed] [Google Scholar]




