Abstract
To assess the presence of the four main viruses responsible for human acute gastroenteritis in a hydrographic network impacted by a disordered urbanization process, a 1-year study was performed involving water sample collection from streams in the hydrographic basin surrounding the city of Manaus, Amazonas, Brazil. Thirteen surface water sample collection sites, including different areas of human settlement characterized as urban, rural, and primary forest, located in the Tarumã-Açu, São Raimundo, Educandos, and Puraquequara microbasins, were defined with a global positioning system. At least one virus was detected in 59.6% (31/52) of the water samples analyzed, and rotavirus was the most frequent (44.2%), followed by human adenovirus (30.8%), human astrovirus (15.4%), and norovirus (5.8%). The viral contamination observed mainly in the urban streams reflected the presence of a local high-density population and indicated the gastroenteritis burden from pathogenic viruses in the water, principally due to recreational activities such as bathing. The presence of viral genomes in areas where fecal contamination was not demonstrated by bacterial indicators suggests prolonged virus persistence in aquatic environments and emphasizes the enteric virus group as the most reliable for environmental monitoring.
Although water is recognized as the most precious natural resource on our planet, human activities disregard this fact by continually polluting freshwater bodies. Increasing worldwide awareness of the poor quality of potable water has occurred mainly due to the significant increase in human morbidity and mortality. More than 2.2 million people die every year from diseases associated with poor quality water and sanitary conditions, mostly in developing countries. The presence of pathogenic enteric microorganisms in aquatic environments reveals how human health can be affected by contamination from sewage discharge into surface waters. It is estimated that nearly a quarter of all hospital beds in the world are occupied by patients presenting complications arising from infections caused by enteric microorganisms (53, 56).
Water sanitary quality is usually determined by the concentration of fecal indicator bacteria and occasionally by bacteriophages (8, 17). However, numerous investigations have shown that achieving minimum fecal coliform standards does not predict viral contamination (8, 47). Enteric viruses are highly stable in the environment, maintaining their infectivity even after exposure to treatment processes, and are often the most diluted pathogens in water, thus requiring concentration methods for their detection (2, 8, 42, 53).
After replication in the gastrointestinal tract, human enteric pathogenic viruses are excreted in high concentrations in the feces (105 to 1011/g feces) and can enter the environment through the discharge of waste materials from symptomatic or asymptomatic carriers and therefore may be dispersed in environmental waters (2). Difficulties in obtaining viruses from environmental samples have been overcome through the association of virus concentration methods with the use of molecular techniques, such as PCR, which provide rapid, sensitive, and specific detection (2, 15, 16, 32, 35, 46, 48, 54).
Although the presence of viruses in water is underestimated, mainly due to the difficulties associated with the detection of such agents in different matrices, enteric viruses have been implicated in waterborne outbreaks in different countries every year (2, 36, 38, 53). Among these, rotaviruses (RV), noroviruses (NoV), human astroviruses (HAstV), and human adenoviruses (HAdV) are recognized as the most important etiologic agents of acute gastroenteritis and have been considered for environmental monitoring (11, 29, 55).
Diarrhea, a water-related disease, is a global public health problem and is ranked third among the causes of death affecting children under 5 years old, accounting for 17% of all deaths. It is estimated that 1.5 billion episodes occur each year, mostly in developing countries. It is recognized that a significant proportion of diarrhea cases caused by waterborne transmission in such countries is related to water quality. Levels of diarrhea disease differ between communities due to socioeconomic factors such as water availability and hygienic behavior (9, 45).
Despite a significant decrease in diarrhea-related mortality in developed and some developing countries, such as Brazil, diarrhea is still an important cause of morbidity in these countries (37). In the Northern region of Brazil, the city of Manaus reported an increase of 90.5% in the number of diarrhea cases between 1998 and 2000, from 8,878 cases to 16,914 (4).
The goal of this study was to assess viral contamination by the four main viruses responsible for acute gastroenteritis (RV, HAdV, HAstV, and NoV) in the hydrographic network that surrounds Manaus. Investigation and determination of the viruses that are dumped into streams from domestic sewage without prior treatment, as occurs in Manaus, could reveal how rapid population growth associated with a disordered urbanization process represents a threat to human health caused by the increased risk of disease transmission.
MATERIALS AND METHODS
Study area.
The city of Manaus (3°S, 60°W) is the capital of Amazonas, which is the largest Brazilian state located in the northern region of the country. The city has an area of 11,458.5 km2, corresponding to 0.73% of the territory of the State of Amazonas (1,577,820.2 km2). The city of 1,403,796 inhabitants is located 900 miles (1,450 km) inland from the Atlantic coast, in the heart of the Amazon rain forest. It is surrounded by a dense hydrographic network composed by the Tarumã-Açu basin, which is partially situated within the urban area; the Puraquequara basin, which is located in the forest area; and the São Raimundo and Educandos microbasins of the Negro River basin, within the urban area of Manaus.
Thirteen precisely positioned sites located in these different basins were defined with a global positioning system (TREX Legend; Garmin Ltd., Olathe, KS). These sites were chosen for water sample collection and were positioned in different areas of human settlement; three control sites were represented by streams located in primary forest with intact vegetation and free of domestic sewage (sites 11 to 13); two sites were located at decamped areas, where the streams are also not affected by domestic sewage (sites 9 and 10); and eight sites were situated in regions of variable human settlement (sites 1 to 8). The São Raimundo and Educandos basins are characterized as urban areas with the presence of squatter slums without basic services such as sewage and a water supply. Some urban streams present different levels of water degradation processes, caused mainly by the complete or partial removal of riparian vegetation and by pollution from domestic sewage that is dumped into these streams without prior treatment (18).
Sampling schedule.
The 1-year environmental surveillance was based on four sample collections at each site, conducted between August 2004 and June 2005, according to the annual fluctuation level of the Negro River, which usually shows a peak flood in June during the wet season. A total of 52 samples were obtained from collections performed in August (beginning of the dry season) and November (dry season), 2004, and in February (beginning of the wet season) and June (wet season), 2005.
Three-liter samples of surface water were collected in sterile bottles and transferred to the laboratory, where they were immediately stored at 4°C for viral and bacteria investigations, while the physicochemical parameters were measured in locum.
Physicochemical parameters.
Temperature (°C), conductivity (μS), dissolved oxygen (DO; mg/liter), and pH were measured at the moment of collection with a YSI model 85 handheld salinity, conductivity, DO, and temperature system (YSI, Incorporated, Yellow Springs, OH) and a portable potentiometer (pH tester 2, waterproof, double junction).
Bacteriology.
Standard multiple-tube fermentation and the membrane filtration technique for determining total and fecal coliforms were performed according to previously described protocols (17).
Virus concentration.
The viral particles present in the samples were concentrated by the adsorption-elution method, with negatively charged membranes with the insertion of an acid rinse step for the removal of cations, as described previously (35). Briefly, the samples were prefiltered through an AP20 membrane (Millipore), and prior to process filtration, 1.2 g of MgCl2 was added to 2 liters of water and the pH was adjusted to 5. The samples were filtered in a type HA negatively charged membrane (Millipore) with a 0.45-μm pore size with a vacuum pump system. The membrane was rinsed with 350 ml of 0.5 mM H2SO4 (pH 3.0), after which 15 ml of 1 mM NaOH (pH 10.8) was used to release the virus from the membrane. To neutralize the solution, 50 μl of 50 mM H2SO4 and 100× TE buffer (pH 8.0) was added. The eluate was filtered by using a Centriprep Concentrator 50 (Millipore) and centrifuged at 1,500 × g for 10 min at 4°C to obtain a final volume of 2 ml. The system was soaked briefly in a 10% bleach solution and rinsed in deionized H2O prior to each use.
RNA and DNA extraction.
Nucleic acid extraction was processed with the QIAamp viral RNA and QIAamp viral DNA kits (Qiagen, Inc., Valencia, CA) according to the manufacturer's instructions.
Reverse transcription (RT) reaction.
cDNA synthesis was carried out by RT with a random primer (PdN6; 50 A260 units; Amersham Biosciences, Chalfont St Giles, Buckinghamshire, United Kingdom) for three groups of enteric viruses, i.e., RV, HAstV, and NoV. Briefly, 2 μl of dimethyl sulfoxide (Sigma, St. Louis, MO) and 10 μl of RNA were mixed, heated at 97°C for 7 min, and chilled on ice for 2 min. The components of the mixture and their final concentrations for a 50-μl RT reaction were as follows: 2.5 mM each deoxynucleoside triphosphate (GIBCO BRL, Life Technologies, Inc., Grand Island, NY), 1.5 mM MgCl2, 200 U of Superscript II reverse transcriptase (Invitrogen), and 1 μl of PdN6. The RT reaction mixture was incubated in a thermal cycler (PTC-100 Programmable Thermal Controller; MJ Research, Inc., Watertown, MA) at 42°C for 60 min and 95°C for 10 min.
Primers and PCR protocols for virus detection.
Primer characteristics and references for the amplification conditions of different PCR and nested PCR protocols used for nucleic acid detection of RV, NoV, HAstV, and HAdV were all described previously (3, 20, 24, 28, 43). To avoid false-positive results, quality control measures were followed as recommended and for each set of amplifications, negative and positive control samples were included. All methodologies were standardized with reference strains of each virus, and for the present study, previously characterized virus strains obtained from fecal samples were used as positive controls. The PCR products were resolved on 1.0% electrophoresis grade agarose gel (GIBCO BRL, Life Technologies, Inc., Grand Island, NY), followed by ethidium bromide staining (0.5 μg/ml), and images were obtained with the image capture system (BioImaging Systems) with the Labworks 4.0 software program.
Molecular characterization of RVs.
Molecular characterization of RV was performed with specific primers routinely used for the binary classification of group A RV into G (VP7) and P (VP4) types, where G stands for glycoprotein and P stands for protease-sensitive protein. All procedures were performed with previously described primers and amplification conditions (20, 28).
Nucleotide sequencing of HAstVs, NoVs, and HAdVs.
The amplicons of HAstV and NoV and the first round of HAdV (primers hex1deg and hex2deg) obtained in the PCR were sequenced to confirm the correct PCR products. The amplicons were purified with the QIAquick gel extraction kit (Qiagen) according to the manufacturer's instructions and quantified by 1% agarose gel electrophoresis with the Low DNA Mass Ladder (Invitrogen) as a molecular pattern. The PCR amplicons were sequenced with an ABI Prism 3100 genetic analyzer and Big Dye Terminator cycle sequencing kit v. 3.1 (PE Applied Biosystems, Foster City, CA) in both directions, with the same primers used in the amplification reactions. CentriSep columns (Princeton Separations, Inc., Adelphia, NJ) were used to purify the sequencing reaction products, according to the manufacturer's recommendations.
Strain characterization and phylogenetic analysis.
Nucleotide sequences were edited and aligned with the BioEdit sequence alignment editor (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The sequences were compared with their respective prototypes and to other sequences available in the GenBank database (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?DB=pubmed). Rooted phylogenetic trees were constructed with the MEGA 2 software (http://www.megasoftware.net/) by the neighbor-joining method, with genetic distance corrected by the Kimura two-parameter model with 1,000 pseudoreplicas.
Statistical analysis.
Statistical results were produced by the software Epi Info, version 3.3, from the Centers for Disease Control and Prevention (http://www.cdc.gov/epiinfo/).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences obtained in this study are DQ464891 to DQ464895 (HAdV), EF100670 (HAstV), and EF107526 (NoV).
RESULTS
Fifty-two river water concentrates were analyzed by PCR assay during this study, and at least one virus was detected in 31 (59.6%) of these. The number of viruses detected in water samples from different sites was variable (Table 1), totaling 50 virus strains, with RV (23 to 44.2%) verified as the most prevalent detected, followed by HAdV (16 to 30.8%), HAstV (8 to 15.4%), and NoV (3 to 5.8%). The different distributions of these viruses according to the collection area revealed that RV, HAstV, and HAdV were detected at sites located in the primary forest and rural areas (Table 2). The urban streams located at the Educandos (sites 7 and 8) and São Raimundo (sites 3 to 6) basins presented a high level of viral contamination, as revealed by the percentages of virus detection per site of 100.0% (8/8) and 81.3% (13/16), respectively. In these basins, all four groups of viruses investigated were detected, with HAdV and RV being the most frequent viruses present in the Educandos basin. Site 4, located in São Raimundo, presented the highest number of viral types detected and the highest variability of virus strains belonging to the four groups tested. This site is an urban stream presenting intact vegetation; however, it routinely receives contamination from sewage discharge originating from the surrounding houses.
TABLE 1.
Sample collection site characteristics and virus groups detected in 52 surface water samples obtained during four collection periods
| Human settlement level/area type | Basin | Site no./stream | Virus(es) collected
|
|||
|---|---|---|---|---|---|---|
| August 2004 | November 2004 | February 2005 | June 2005 | |||
| Low/rural | Tarumã-Açú | 1/estuary | HAstV | HAstV, RV | RV | |
| 2/medium | RV | RV | ||||
| High/urban | São Raimundo | 3/estuary | HAdV, RV | RV | RV | HAdV, HAstV, RV |
| 4/medium | HAstV, AdV | HAdV, HAstV, NoV | HAdV, HAstV, RV, NoV | HAdV, HAstV, RV | ||
| 5/medium | HAdV, RV | HAdV, HAstV, RV | HAdV | |||
| 6/medium | RV | RV | ||||
| High/urban | Educandos | 7/estuary | HAdV | HAdV | NoV, RV | HAdV, RV |
| 8/medium | HAdV, RV | HAdV | HAdV, RV | RV | ||
| Low/rural | Puraquequara | 9/decamped in 2001 | RV | |||
| Tarumã-Açú | 10/decamped in 2002 | |||||
| Very low/primary forest | Puraquequara | 11/Mainã Grande | RV | |||
| Tarumã-Açú | 12/Reserva Ducke | RV | RV | |||
| 13/Reserva Ducke | HAdV | |||||
TABLE 2.
Frequencies of the viruses investigated and fecal coliforms detected in the areas studied
| Pathogen | No. (%) found in:
|
Chi square | P value | |
|---|---|---|---|---|
| Urban area (n = 24) | Rural and forest areas (n = 28) | |||
| Any enteric virus | 21 (87.5) | 10 (35.7) | 14.4 | <0.001 |
| RV | 15 (62.5) | 8 (28.6) | 6.0 | 0.014 |
| NoV | 3 (12.5) | 3.7 | 0.09 | |
| HAstV | 6 (25.0) | 2 (7.1) | 3.2 | 0.08 |
| HAdV | 15 (62.5) | 1 (3.6) | 21.1 | <0.001 |
| Fecal coliforms | 24 (100.0) | 14 (50.0) | 16.4 | <0.001 |
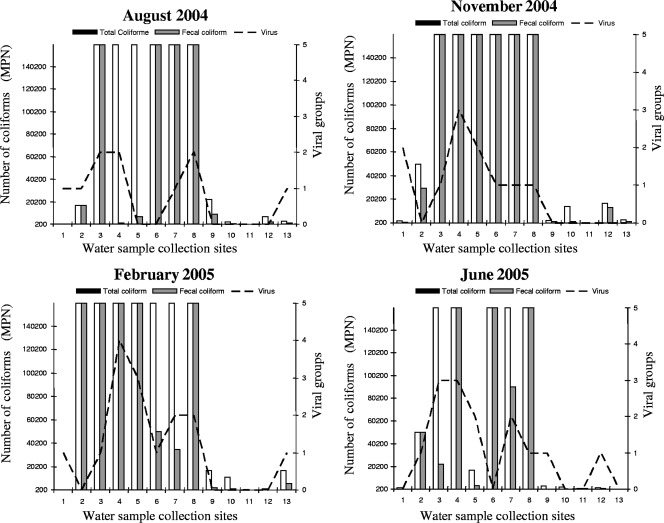
The microbiological analysis results showed that the total and fecal coliform values in the samples exceeded those established by the standard methods used for the examination of water and wastewater guideline for recreational water (most-probable numbers [MPN], 5,000 and 1,000/100 ml) by 67.3% (35/52) and 73.1% (38/52), respectively. Sites that were positive for the presence of fecal coliforms included 6 of the 20 sites located in primary forest and rural areas, 4 out of 8 in the Tarumã-Açu microbasin, and all 14 sites located in the São Raimundo and Educandos microbasins. Only two sites (site 1, the Tarumã-Açu estuary, and site 12, the Ducke Forest Reserve) were negative for fecal contamination in the four water samples obtained during this study. However viruses were detected in the strains obtained from the estuary (Fig. 1). The Tarumã-Açu estuary (site 1) is perpendicular to the Negro River and has a few floating houses. The water is clear, besides being naturally dark and regularly used for recreational bathing.
FIG. 1.
Microbiological results obtained with water samples collected at 13 sites from streams of the Amazon hydrographic network according to the collection period.
The frequency of sites where the presence of fecal coliforms exceeded an MPN of 1,000/ml and where virus detection was positive characterized significant microbiological contamination in the urban water streams and revealed that HAdV is the most significant marker of human presence (Table 2). Enteric virus detection in samples positive and negative for fecal coliforms was 63.2% (24/38) and 50.0% (7/14), respectively (P = 0.29), showing no correlation between these findings.
Global analysis for the presence of viruses and fecal coliforms by using recreational water parameters (MPN, 1,000/ml) according to the collection period demonstrated a slight increase in the number of strains detected during wet-season rainfall, although the number of positive sites for virus detection remained almost unaltered (data not shown).
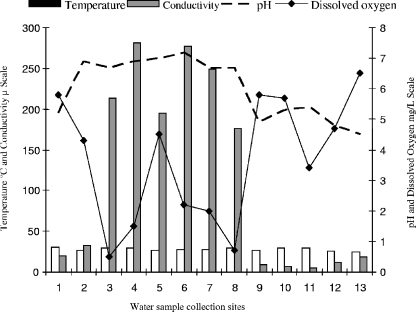
The median values for the physicochemical parameters, including temperature, pH, DO, and conductivity, obtained at each site during the four sample collections are shown in Fig. 2. Observation revealed that the pH, DO, and conductivity generally followed two different patterns, characterizing the two distinct areas studied, i.e., areas with zero or minimal human settlement and areas where human activity clearly affected the local natural conditions. The former areas were characterized by acidic water presenting low electrical conductivity and high DO content. The urban areas (São Raimundo and Educandos) presented higher pH and conductivity values and low DO contents. The temperature of the water samples was the most stable parameter, ranging from 24.5°C to 30.6°C and from 24.0°C to 32.8°C in the respective areas.
FIG. 2.
Means of the physicochemical data obtained from the 52 surface water samples collected during the study period according to the collection sites.
Molecular characterization.
Genotyping of VP4 of group A RV demonstrated that 13 samples were P[8], 1 was a coinfection of P[8] and P[4], and 1 was untypeable. Regarding VP7, 10 samples were characterized as G1 and 1 was untypeable. Only three samples were characterized for both VP4 and VP7 genes, and all of these were P[8],G1. Most of the positive samples were obtained from polluted streams; however, three were collected in rural and forest areas.
For HAdV, HAstV, and NoV genotyping, phylogenetic analysis was performed by comparing the nucleotide sequences obtained with their respective genotypes and with other strains available in the GenBank database representing different countries worldwide. For HAstV, the sequence analysis was performed based on a region of 348 nucleotides in open reading frame 2 of the HAstV genome, which clustered this strain with the HAstV Oxford type 1 prototype (nucleotide identity of 97.4%). Phylogenetic analysis performed to correlate the HAstV sequence with other isolates from different countries worldwide revealed a cluster including strains from Argentina, Colombia, and Brazil. Among the Brazilian strains, the environmental sequence displayed identities varying from 90.2% to 98.9%.
Partial sequencing of one strain was performed with region B primers to determine the NoV genogroups. Phylogenetic analysis revealed that this strain clustered within GII; however, we were unable to determine its genotype with this set of primers. Molecular characterization of HAdV was performed by sequencing 5 out of 16 detected strains. The sequence analysis corresponded to the 253 nucleotides between positions 47 and 299 within the hexon gene. The data generated were compared with the GenBank database and used to construct a phylogenetic tree, which, according to the nucleotide sequence identities (data not shown), showed that all of the strains belonged to species F; two of them were HAdV-40, and three were HAdV-41.
DISCUSSION
According to the Brazilian Institute of Geography and Statistics, Brazil has surface water resources of 168,870 m3/s, representing 50% and 11% of all the water available in Latin America and in the world, respectively. Seventy percent of Brazilian freshwater resources are located in the northern region of the country, in the Amazon hydrographic basin, where only 7% of the Brazilian population lives. Although this is a favorable situation, water quality is declining in most regions of the country, mainly due to rapid population growth without corresponding planning control. Similar to other Brazilian cities, Manaus has undergone rapid population growth in the last 3 decades, exhibiting growth from 300,000 inhabitants in the 1970s to 1,403,796 in the year 2000. Today, the urban population represents 99.4% of the total population (4, 39). This increase is the result of a large population influx to the city due to federal government policies that provide incentives for manufacturing activities initiated during the 1960s (12).
Urban development along the streams has led to a loss of biodiversity and to a decrease in water quality in these ecosystems, due to a series of adverse effects on these water bodies, including the discharge of untreated sewage directly into streams (12, 18, 41). The presence of the four main viruses responsible for acute gastroenteritis observed in the water of urban streams provides evidence that these viruses circulate at relatively high frequencies among the population of Manaus, while also reflecting the effects of increased population densities and anthropogenic activities on freshwater resources. In addition, this information could provide an assessment of the risk of human disease associated with sewage disposal into the streams of Manaus. These findings indicate the gastroenteritis burden of pathogenic viruses present in the water, due to the use of river water for drinking or due to other routes of transmission, such as poor hygiene, lack of sanitation, or even contamination due to recreational activities such as bathing in these areas. Studies showing viral diarrheal illness due to waterborne transmission related to poor water quality have been documented, including the ingestion of contaminated water during body contact recreation (22, 26, 31, 38, 52). In addition, the detection and characterization of HAdV, RV, HAstV, and NoV in environmental water samples may provide potentially useful data for epidemiological studies. The diversity of viruses detected in the water was variable and depends on factors like population density and infection prevalence within a given community, such that the amount of viruses dumped directly into river water in untreated discharge could explain the prevalence and distribution of such viruses.
In the present study, RV was the most prevalent virus detected, with P[8],G1 the only genotype characterized by a seminested, typing-specific PCR, a reflection within the environment concerning the impact of these viruses on the population. RV has been described as the most important virus in cases of acute gastroenteritis, since it is responsible for a third of these cases and RV P[8],G1 has been described as the most common genotype circulating worldwide (44, 49). In a study performed in Germany (47), RV RNA was detected and confirmed in 3 to 24% of the effluent and surface water samples tested.
The high percentage of RV detection in the present samples indicates that this virus should be considered for use as a potential indicator of fecal environmental contamination in developing countries, where their circulation in the environment appears to be higher than HAdVs, which are already considered a molecular index of human virus presence in the environment (7, 13, 46). Recently, an epidemiological surveillance of human enteric viruses of different environmental matrices detected the same viral strain in feces of gastroenteritis cases and in water and suggested both RV and HAdV as reference viruses for risk assessment (11). The stability of RV in environmental waters has been previously described, and its resistance to physiochemical treatment processes used by sewage treatment plants may facilitate its transmission (5).
Single-stranded RNA (ssRNA) virus detection rates were lower, especially for NoV. We believed that the protocol used could affect this result, and it is probable that the percentage of NoV detected would have been higher if a nested (or seminested) PCR assay had been used. The high sensitivity of the nested PCR has been described and for RV could detect double-stranded RNA from as few as 10 to 100 particles (28). Despite the low levels of NoV detected, the emergence of these viruses resulting in outbreaks of gastroenteritis is notable (6, 10, 33). To date, no reliable data regarding the frequency of NoV in Manaus are available and therefore the impact of these infections could not be measured. HAstVs have been recognized as important etiologic agents of viral gastroenteritis, contributing to 2 to 26% of the gastroenteritis cases in developing countries (19, 29, 55). The current rate of HAstV detection in this study is within the 6 to 50% range of surface water, as previously described (15, 29, 47).
The present results, based on direct molecular detection of viruses in river water, were confirmed by direct sequencing of PCR amplicons of one HAstV strain, one NoV strain, and six HAdV strains to ensure detection specificity. Nucleotide sequence analysis revealed the presence of HAstV-1 and NoV genogroup II strains, confirming the wide distribution of these viruses, as previously described (10, 14, 23, 25, 27, 33, 51). The fact that the HAdV detected belongs to species F, serotypes 40 and 41, was also verified. The different types of HAdV detected confirm that the quality control measures adopted throughout these procedures were sufficient to ensure these results. In addition, a study of hepatitis A virus (HAV) carried out with the same samples (21) corroborated these results.
The present study also evaluated the potential of the virus concentration method by negatively charged membrane filtration associated with different PCR protocols routinely used for stool samples. The detection of ssRNA, double-stranded RNA, and DNA viruses revealed that the association of the methods described here is a feasible approach for detecting the main waterborne enteric viruses responsible for gastroenteritis in environmental water samples collected from different grades of pollution. The lower virus detection rate in polluted water samples during the dry season could be explained by the concentration of inhibitors throughout this period. It has been demonstrated that the presence of organic compounds such as humic, fulvic, and tannic acids, proteins, and inorganic compounds such as metals present in the environment is a major obstacle to the routine detection of enteric viruses from environmental waters by PCR (1, 32, 50). In this study, 21 water samples were negative for the presence of HAdV, HAstV, NoV, and RV; however, considering that Torque teno virus and HAV were also investigated in the same water samples (results published elsewhere; 21; L. Diniz-Mendes, unpublished data), the number of water samples negative for all viruses was reduced to 11. Unfortunately, the presence of compounds that could inhibit RT-PCR/PCR was not evaluated. Previously, this method showed average recovery yields of spiked poliovirus of 62% from 1 liter of artificial seawater (35).
Recently, environmental virological studies have emerged worldwide and procedures for concentrating virus in water samples associated with different detection methodologies have been described (13, 30, 40, 48). In this study, we attempted to gain an initial insight into NoV, HAstV, RV, and HAdV occurrence within surface water samples of the Amazon basin. Thus, the PCR approach was useful as an alternative to overcome the limitations of conventional techniques, such as cell culture, since NoV cannot be grown in cell culture and RV, HAstV, HAdV-40, and HAdV-41 are fastidious agents (8, 15, 34). In fact, the characteristics of these viruses were determining factors when selecting the association of a membrane negative charged method with certain PCR methods, since conventional virus concentration procedures that use positive membrane and beef extract as an eluate are known to present certain inhibitory effects on PCR detection for viruses (1, 15, 46).
Although the method used for detecting enteric viruses cannot distinguish between infectious and noninfectious virions, the detection of an ssRNA genome in the environment suggests the presence of an infective virus since this molecule is not very stable under environmental conditions (40). Further studies concerning virus viability in these water samples should be performed by cell culture or cell culture associated with PCR, as previously described (15, 47).
The presence of viral genomes in areas showing low levels of fecal contamination by bacterial indicators suggests the prolonged persistence of these viruses in the environment and indicates that the enteric virus group is more reliable for environmental monitoring than bacterial indicators. It has been recognized that these viruses are more stable than bacteria in water and sewage, constituting not only a potential hazard but also good indicators of fecal pollution, as well as the potential presence of other viruses (8).
The anthropogenic influence on streams within the urban area of the municipality of Manaus was also notable due to the high quantities of total and fecal coliforms present in the water samples and the physicochemical analyses that corroborated previous findings, characterizing the São Raimundo and Educandos microbasins with an increased pH, high conductivity, and a low DO content (41). Monitoring of these streams revealed that viral contamination could be derived from infected residents of the São Raimundo and Educandos microbasins and reinforces the need to make improvements in water supply, sewage disposal, garbage collection, and urban drainage services, as suggested in previous reports (12, 41). The flooding events that occur annually in these areas may increase the number of waterborne disease exposure scenarios (4). Additionally, a previous study performed in the city of Manaus by using macroinvertebrates as bioindicators demonstrated that 80% of the streams within the urban area are impacted, such that their abiotic characteristics have been modified by deforestation and water pollution (18).
In Brazil, a lack of studies regarding viral contamination monitoring in surface water exists, and to the best of our knowledge, no investigation to date has evaluated the presence of viruses in river water of the hydrographic basin of Amazonas. Data concerning HAV obtained in the same study were published elsewhere (21). The viral contamination detected in this study provides a better assessment of human disease risk associated with sewage disposal into river water, increases our knowledge regarding this subject, and assists in the development of more efficient public health actions. The possibility of detecting human enteric viruses in a given water source will facilitate the provision of appropriate advice to public and responsible authorities regarding the use and treatment of water. Continuous viral contamination monitoring is useful for preventing waterborne disease outbreaks and for understanding the impact caused by human occupation and the use of territories that contain freshwater resources.
Acknowledgments
This work was supported by the Vice-Presidência de Serviços de Referência e Ambiente, Fiocruz, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 303539/2004-6).
We thank Ana Maria Coimbra Gaspar, Josino Costa Moreira, and Ary Carvalho de Miranda for their collaboration in planning this project; Matías Victória and Filipe Aníbal da Costa for nucleotide sequencing and statistic analysis, respectively; and the staff of the Centro de Pesquisas Leônidas e Maria Deane (CPqLMD), Fiocruz, Amazonas, the Instituto Nacional de Pesquisas do Amazonas (INPA), and the Centro de Pesquisas Eco-Naturais (CEPEN), especially Neusa Hamada and Sheyla Couceiro, who provided the infrastructure for realizing the sample collections.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Abbaszadegan, M., M. S. Huber, C. P. Gerba, and I. L. Pepper. 1993. Detection of enteroviruses in groundwater with the PCR. Appl. Environ. Microbiol. 59:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbaszadegan, M. 2001. Advanced detection of viruses and protozoan parasites in water. Rev. Biol. Biotechnol. 2:21-26. [Google Scholar]
- 3.Allard, A., B. Albinsson, and G. Wadell. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 2002. Projeto geo cidades: relatório ambiental urbano integrado: informe GEO: Manaus. Consórcio Parceria, Rio de Janeiro, Brazil.
- 5.Ansari, S. A., V. S. Springthorpe, and S. A. Sattar. 1991. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev. Infect. Dis. 13:448-461. [DOI] [PubMed] [Google Scholar]
- 6.Blanton, L. H., S. M. Adams, R. S. Beard, G. Wei, S. N. Bulens, M. A. Widdowson, R. I. Glass, and S. S. Monroe. 2006. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000-2004. J. Infect. Dis. 193:413-421. [DOI] [PubMed] [Google Scholar]
- 7.Bofill-Mas, S., N. Albinana-Gimenez, P. Clemente-Casares, A. Hundesa, J. Rodriguez-Manzano, A. Allard, M. Calvo, and R. Girones. 2006. Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl. Environ. Microbiol. 72:7894-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch, A. 1998. Human enteric viruses in the water environment: a minireview. Int. Microbiol. 3:191-196. [PubMed] [Google Scholar]
- 9.Bryce, J., C. Boschi-Pinto, K. Shibuya, and R. E. Black. 2005. WHO estimates of the causes of death in children. Lancet 365:1147-1152. [DOI] [PubMed] [Google Scholar]
- 10.Bull, R. A., E. T. Tu, C. J. McIver, W. D. Rawlinson, and P. A. White. 2006. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J. Clin. Microbiol. 44:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carducci, A., M. Verani, R. Battistini, F. Pizzi, E. Rovini, E. Andreoli, and B. Casini. 2006. Epidemiological surveillance of human enteric viruses by monitoring of different environmental matrices. Water Sci. Technol. 54:239-244. [DOI] [PubMed] [Google Scholar]
- 12.Casey, J. F., J. R. Kahn, and A. Rivas. 2006. Willingness to pay for improved water service in Manaus, Amazonas, Brazil. Ecol. Econ. 58:365-372. [Google Scholar]
- 13.Castignolles, N., F. Petit, I. Mendel, L. Simon, L. Cattolico, and C. Buffet-Janvresse. 1998. Detection of adenovirus in the waters of the Seine River estuary by nested-PCR. Mol. Cell. Probes 12:175-180. [DOI] [PubMed] [Google Scholar]
- 14.Castilho, J. G., V. Munford, H. R. Resque, U. Fagundes-Neto, J. Vinjé, and M. L. Racz. 2006. Genetic diversity of norovirus among children with gastroenteritis in Sao Paulo State, Brazil. J. Clin. Microbiol. 44:3947-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho, H. B., S. H. Lee, J. C. Cho, and S. J. Kim. 2000. Detection of adenoviruses and enteroviruses in tap water and river water by reverse transcription multiplex PCR. Can. J. Microbiol. 46:417-424. [PubMed] [Google Scholar]
- 17.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th edition, p. 906. American Public Health Association, Washington, DC.
- 18.Couceiro, S. R. M., N. Hamada, S. L. B. Luz, B. R. Forsberg, and T. P. Pimentel. 2007. Deforestation and sewage effects on aquatic macroinvertebrates in urban streams in Manaus, Amazonas, Brazil. Hydrobiology 575:271-284. [Google Scholar]
- 19.Cunliffe, N. A., W. Dove, J. S. Gondwe, B. D. Thindwa, J. Greensill, J. L. Holmes, J. S. Bresee, S. S. Monroe, R. I. Glass, R. L. Broadhead, M. E. Molyneux, and C. A. Hart. 2002. Detection and characterization of human astroviruses in children with acute gastroenteritis in Blantyre, Malawi. J. Med. Virol. 67:563-566. [DOI] [PubMed] [Google Scholar]
- 20.Das, B. K., J. R. Gentsch, H. G. Cicirello, P. A. Woods, A. Gupta, M. Ramachandran, R. Kumar, M. K. Bhan, and R. I. Glass. 1994. Characterization of rotavirus strains from newborns in New Delhi, India. J. Clin. Microbiol. 32:1820-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Paula, V. S., L. Diniz-Mendes, L. M. Villar, S. B. Luz, M. S. Jesus, N. M. V. S. da Silva, and A. M. C. Gaspar. 2007. Hepatitis A virus in environmental water samples from the Amazon basin. Water Res. 41:1169-1176. [DOI] [PubMed] [Google Scholar]
- 22.Divizia, M., R. Gabrieli, D. Donia, A. Macaluso, A. Bosch, S. Guix, G. Sánchez, C. Villena, R. M. Pintó, L. Palombi, E. Buonuomo, F. Cenko, L. Leno, D. Bebeci, and S. Bino. 2004. Waterborne gastroenteritis outbreak in Albania. Water Sci. Technol. 50:57-61. [PubMed] [Google Scholar]
- 23.Espul, C., N. Martinez, J. S. Noel, H. Cuello, C. Abrile, S. Grucci, R. Glass, T. Berke, and D. O. Matson. 2004. Prevalence and characterization of astroviruses in Argentinean children with acute gastroenteritis. J. Med. Virol. 72:75-82. [DOI] [PubMed] [Google Scholar]
- 24.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Galdiero, E., A. Marinelli, M. G. Pisciotta, I. Pagliara, E. S. Di Monteforte, and G. Liguori. 2005. Reverse transcriptase-PCR for the detection of astrovirus in children with nosocomial acute diarrhoea in Naples, Italy. Med. Mal. Infect. 35:213-217. [DOI] [PubMed] [Google Scholar]
- 26.Gallay, A., H. De Valk, M. Cournot, B. Ladeuil, C. Hemery, C. Castor, F. Bon, F. Megraud, P. Le Cann, and J. C. Desenclos. 2006. A large multi-pathogen waterborne community outbreak linked to faecal contamination of a groundwater system, France, 2000. Clin. Microbiol. Infect. 12:561-570. [DOI] [PubMed] [Google Scholar]
- 27.Gallimore, C. I., M. A. Barreiros, D. W. Brown, J. P. Nascimento, and J. P. G. Leite. 2004. Noroviruses associated with acute gastroenteritis in a children's day care facility in Rio de Janeiro, Brazil. Braz. J. Med. Biol. Res. 37:321-326. [DOI] [PubMed] [Google Scholar]
- 28.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gofti-Laroche, L., B. Gratacap-Cavallier, D. Demanse, O. Genoulaz, J. M. Seigneurin, and D. Zmirou. 2003. Are waterborne astrovirus implicated in acute digestive morbidity (E.MI.R.A. study)? J. Clin. Virol. 27:74-82. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez, M. F., M. V. Alvarado, E. Martinez, and N. J. Ajami. 2007. Presence of viral proteins in drinkable water—sufficient condition to consider water a vector of viral transmission? Water Res. 41:373-378. [DOI] [PubMed] [Google Scholar]
- 31.Hoebe, C. J., H. Vennema, A. M. de Roda Husman, and Y. T. van Duynhoven. 2004. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J. Infect. Dis. 189:699-705. [DOI] [PubMed] [Google Scholar]
- 32.Ijzerman, M. M., D. R. Dahling, and G. S. Fout. 1997. A method to remove environmental inhibitors prior to the detection of waterborne enteric viruses by reverse transcription-polymerase chain reaction. J. Virol. Methods 63:145-153. [DOI] [PubMed] [Google Scholar]
- 33.Ike, A. C., S. O. Brockmann, K. Hartelt, R. E. Marschang, M. Contzen, and R. M. Oehme. 2006. Molecular epidemiology of norovirus in outbreaks of gastroenteritis in southwest Germany from 2001 to 2004. J. Clin. Microbiol. 44:1262-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapikian, A. Z., and R. M. Chanock. 2001. Rotaviruses, p. 1657-1708. In B. N. Fields, D. M. Knipe, and P.M. Howley (ed.), Virology, vol. 2. Lippincott-Raven Press, Philadelphia, PA. [Google Scholar]
- 35.Katayama, H., A. Shimasaki, and S. Ohgaki. 2002. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 68:1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, S. H., D. S. Cheon, J. H. Kim, D. H. Lee, W. H. Jheong, Y. J. Heo, H. M. Chung, Y. Jee, and J. S. Lee. 2005. Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J. Clin. Microbiol. 43:4836-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosek, M., C. Bern, and R. L. Guerrant. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. W.H.O. 81:197-201. [PMC free article] [PubMed] [Google Scholar]
- 38.Kukkula, M., P. Arstila, M. L. Klossner, L. Maunula, C. H. Bonsdorff, and P. Jaatinen. 1997. Waterborne outbreak of viral gastroenteritis. Scand. J. Infect. Dis. 29:415-418. [DOI] [PubMed] [Google Scholar]
- 39.Magalhães, F., and E. Rojas. 2005. The re-urbanization of the city center of Manaus, Brazil—facing the challenges of informal settlements. 41st ISOCaRP Congress.
- 40.Meleg, E., F. Jakab, B. Kocsis, K. Bányai, B. Melegh, and G. Szucs. 2006. Human astroviruses in raw sewage samples in Hungary. J. Appl. Microbiol. 101:1123-1129. [DOI] [PubMed] [Google Scholar]
- 41.Melo, E. G. F., M. S. R. Silva, and S. A. F. Miranda. 2005. Antropic influence on the water of streams in the city of Manaus. Caminhos Geogr. 5:40-47. [Google Scholar]
- 42.Metcalf, T. G., and, X. Jiang. 1988. Detection of hepatitis A virus in estuarine samples by gene probe assay. Microbiol. Sci. 5:296-300. [PubMed] [Google Scholar]
- 43.Noel, J., T. W. Lee, J. B. Kurtz, R. I Glass, and S. S. Monroe. 1995. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 33:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood. Emerg. Infect. Dis. 12:304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payment, P., and P. H. Hunter. 2001. Endemic and epidemic infectious intestinal disease and its relationship to drinking water, p. 61-88. In L. Fewtrell and J. Bartram (ed.), Water quality: guidelines, standards and health. Assessment of risk and risk management for water-related infectious disease. IWA Publishing, London, United Kingdom.
- 46.Puig, M., J. Jofre, F. Lucena, A. Allard, G. Wadell, and R. Girones. 1994. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 60:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pusch, D., D.-Y. Oh, S. Wolf, R. Dumke, U. Schroter-Bobsin, M. Hohne, I. Roske, and E. Schreier. 2005. Detection of enteric viruses and bacterial indicators in German environmental waters. Arch. Virol. 150:929-947. [DOI] [PubMed] [Google Scholar]
- 48.Rose, M. A., A. K. Dhar, H. A. Brooks, F. Zecchini, and R. M. Gersberg. 2006. Quantification of hepatitis A virus and enterovirus levels in the lagoon canals and Lido beach of Venice, Italy using real time RT-PCR. Water Res. 40:2378-2396. [DOI] [PubMed] [Google Scholar]
- 49.Santos, N., and Y. Hoshino. 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 15:29-56. [DOI] [PubMed] [Google Scholar]
- 50.Shieh, Y. S., D. Wait, L. Tai, and M. D. Sobsey. 1995. Methods to remove inhibitors in sewage and other fecal wastes for enterovirus detection by the polymerase chain reaction. J. Virol. Methods 54:51-66. [DOI] [PubMed] [Google Scholar]
- 51.Silva, P. A., D. D. Cardoso, and E. Schreier. 2006. Molecular characterization of human astroviruses isolated in Brazil, including the complete sequences of astrovirus genotypes 4 and 5. Arch. Virol. 151:1405-1417. [DOI] [PubMed] [Google Scholar]
- 52.Standish-Lee, P., and E. Loboschefsky. 2006. Protecting public health from the impact of body-contact recreation. Water Sci. Technol. 53:201-207. [DOI] [PubMed] [Google Scholar]
- 53.Straub, T. M., and D. P. Chandler. 2003. Towards a unified system for detecting waterborne pathogens. J. Microbiol. Methods 53:185-197. [DOI] [PubMed] [Google Scholar]
- 54.Villar, L. M., V. S. de Paula, L. Diniz-Mendes, E. Lampe, and A. M. Gaspar. 2006. Evaluation of methods used to concentrate and detect hepatitis A virus in water samples. J. Virol. Methods 137:169-179. [DOI] [PubMed] [Google Scholar]
- 55.Wilhelmi, I., E. Roman, and A. Sánchez-Fauquier. 2003. Viruses causing gastroenteritis. Clin. Microbiol. Infect. 9:247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyn-Jones, A. P., and J. Sellwood. 2001. Enteric viruses in the aquatic environment. J. Appl. Microbiol. 91:945-962. [DOI] [PubMed] [Google Scholar]