Abstract
Staphylococcus aureus and Staphylococcus epidermidis are major human pathogens of increasing importance due to the dissemination of antibiotic-resistant strains. Evidence suggests that the ability to form matrix-encased biofilms contributes to the pathogenesis of S. aureus and S. epidermidis. In this study, we investigated the functions of two staphylococcal biofilm matrix polymers: poly-N-acetylglucosamine surface polysaccharide (PNAG) and extracellular DNA (ecDNA). We measured the ability of a PNAG-degrading enzyme (dispersin B) and DNase I to inhibit biofilm formation, detach preformed biofilms, and sensitize biofilms to killing by the cationic detergent cetylpyridinium chloride (CPC) in a 96-well microtiter plate assay. When added to growth medium, both dispersin B and DNase I inhibited biofilm formation by both S. aureus and S. epidermidis. Dispersin B detached preformed S. epidermidis biofilms but not S. aureus biofilms, whereas DNase I detached S. aureus biofilms but not S. epidermidis biofilms. Similarly, dispersin B sensitized S. epidermidis biofilms to CPC killing, whereas DNase I sensitized S. aureus biofilms to CPC killing. We concluded that PNAG and ecDNA play fundamentally different structural roles in S. aureus and S. epidermidis biofilms.
Staphylococcus aureus and Staphylococcus epidermidis are among the most common bacteria isolated from human infections. S. aureus colonizes mucosal surfaces such as the anterior nares, whereas S. epidermidis is part of the normal microflora of the skin. Infection results when a breach in the mucosal barrier or skin allows bacterial cells access to the underlying tissues or to the bloodstream (36, 39). S. aureus causes numerous infections, ranging from acute skin abscesses to life-threatening bacteremias and endocarditis (36). S. epidermidis is a leading cause of infections associated with implanted medical devices (56). The increasing incidence of antibiotic-resistant strains of S. aureus and S. epidermidis has heightened efforts to find new ways to fight these pathogens (6, 52).
Both S. aureus and S. epidermidis are known for their ability to form biofilms, which are defined as communities of bacteria, encased in a self-synthesized extracellular polymeric matrix, growing attached to a biotic or abiotic surface (15, 19, 42). Biofilms that form on tissues or medical devices are extremely difficult to eradicate because the biofilm mode of growth protects bacterial cells from killing by antibiotics and host defenses (18). Evidence suggests that biofilm formation plays a role in S. aureus wound infections (2) and osteomyelitis (7) and in S. epidermidis catheter infections (10).
Numerous studies have identified and characterized extracellular factors that mediate surface attachment and intercellular adhesion in S. aureus and S. epidermidis biofilms. Both species produce poly-β(1,6)-N-acetyl-d-glucosamine (PNAG), a surface polysaccharide that is sometimes referred to as polysaccharide intercellular adhesin (11, 37). PNAG contributes to biofilm accumulation and immune evasion in both S. aureus and S. epidermidis (10, 35, 57). However, not all strains produce PNAG, and many PNAG-deficient strains exhibit a strong biofilm phenotype (42, 48, 54). Recently, extracellular DNA (ecDNA) has also been shown to comprise a structural component of the S. aureus and S. epidermidis biofilm matrix (13, 46, 47). In addition, proteinaceous adhesins, such as S. aureus biofilm-associated protein (12) and S. epidermidis accumulation-associated protein (24), as well as cell wall teichoic acids (20, 50, 55), have been shown to participate in surface attachment and biofilm cohesion.
The purpose of the present study was to gain better insight into the functional roles of PNAG and ecDNA in S. aureus and S. epidermidis biofilms. By treating biofilms grown in 96-well microtiter plates with a PNAG-degrading enzyme (dispersin B) and DNase I, we obtained evidence that PNAG and ecDNA perform markedly different functions in S. aureus and S. epidermidis biofilms.
MATERIALS AND METHODS
Reagents.
Bovine DNase I, cetylpyridinium chloride (CPC) (hexadecylpyridinium chloride), sodium dodecyl sulfate (SDS), and phosphate-buffered saline (PBS) were purchased from Sigma Chemical Co. (St. Louis, MO). Restriction endonucleases were purchased from Invitrogen (Carlsbad, CA). Dispersin B (31) was obtained from Kane Biotech, Inc. (Winnipeg, Canada). Gram's crystal violet stain (no. 23255960) was purchased from Fisher Scientific (Fair Lawn, NJ).
Bacterial strains, media, and growth conditions.
The bacterial strains used in this study were S. aureus SH1000 (23), 8325 (41), MRSA252 (22), MZ100 (51), and ATCC 6341 (American Type Culture Collection, Manassas, VA), as well as S. epidermidis NJ9709 (32) and 1467 (38). All strains were passaged weekly on blood agar and stored at 4°C. Biofilms were cultured in tryptic soy broth (Becton-Dickinson, Sparks, MD) containing 6 g of yeast extract and 8 g of glucose per liter (TSB). All cultures were incubated at 37°C.
Biofilm formation assay.
A loopful of cells from an agar plate was transferred to a polypropylene microcentrifuge tube containing 200 μl of TSB. The cells were crushed with a disposable pellet pestle, vortexed for 30 s, diluted to 1 ml in fresh TSB, and then passed through a 5-μm-pore-size syringe filter to remove large clumps of cells, as previously described (30). Filtered cells were diluted to 103 to 105 CFU/ml in TSB. For inhibition studies, filtered cells were diluted in TSB containing 20 μg/ml of dispersin B or 100 μg/ml of DNase I. Aliquots of cells (200 μl each) were transferred to the wells of a 96-well tissue-culture-treated polystyrene microtiter plate (Falcon no. 324662; Becton-Dickinson), and the plate was incubated for 24 h. Biofilms were washed once with water and then dried. Biofilms were stained for 1 min with 200 μl of Gram's crystal violet and then rinsed with water and dried. The amount of biofilm biomass was quantitated by destaining the biofilms for 10 min with 33% acetic acid (by vol) and then measuring the absorbance of the crystal violet solution at 590 nm (A590).
Biofilm detachment assay.
Biofilms were grown in 96-well microtiter plates as described above. Biofilms were rinsed once with water and then treated with 200 μl of dispersin B (20 μg/ml in PBS), DNase I (100 μg/ml in 150 mM NaCl, 1 mM CaCl2), or various restriction endonucleases (20 U/ml in 10 mM Tris [pH 7.9], 10 mM MgCl2, 50 mM NaCl, 1 mM dithiothreitol). Control wells were treated with 200 μl of the appropriate buffer alone. After 1 h at 37°C, biofilms were rinsed with water and stained with crystal violet as described above.
Biofilm killing assay.
Biofilms were grown in 96-well microtiter plates as described above. Biofilms were rinsed once with water and then treated with 200 μl of TSB containing 20 μg/ml of dispersin B or 100 μg/ml of DNase I. Control wells were treated with 200 μl of TSB alone. Control experiments indicated that dispersin B and DNase I did not affect S. aureus or S. epidermidis growth and viability (data not shown). After 10 min at 37°C, 20 μl of 3% CPC (for S. aureus) or 1% CPC (for S. epidermidis) was added to each well and biofilms were incubated for 5 min at room temperature. Control wells received 20 μl of water. For biofilms treated with TSB alone and for S. aureus biofilms treated with dispersin B and S. epidermidis biofilms treated with DNase I, biofilms were washed four times with PBS to remove the CPC and then treated with 100 μg/ml of DNase I (for S. aureus) or 20 μg/ml of dispersin B (for S. epidermidis) to dissolve the biofilm. These reactions were carried out in the enzyme buffers described above. After 10 min, cells were mixed and then serial dilutions were plated on agar. For S. aureus biofilms treated with DNase I and S. epidermidis biofilms treated with dispersin B, cells were mixed and then a 50-μl aliquot of cells was diluted in 50 ml of PBS. The cells were passed through an analytical test filter funnel (no. 145-2020; Nalgene, Rochester, NY), and the filter was rinsed with 250 ml of sterile water, aseptically removed from the filter unit, and placed on a blood agar plate. Colonies were enumerated after 24 h.
Planktonic cell killing assay.
Cells scraped from an agar plate were resuspended in PBS and filtered as described above. Cells were adjusted to 1 × 107 to 5 × 107 CFU/ml and transferred in 180-μl aliquots to the wells of a 96-well microtiter plate. Twenty microliters of 0.0025 to 0.01% CPC was transferred to each well. Control wells received 20 μl of water. After 5 min at room temperature, cells were diluted and plated on agar. In some experiments, planktonic cells were pretreated for 15 min with 20 μg/ml of dispersin B prior to the addition of CPC.
Total hexosamine assay.
Tissue-culture-treated polystyrene petri dishes (100-mm-diameter; Falcon no. 353003) were filled with 20 ml of TSB containing 104 to 105 CFU/ml and incubated for 24 h. The resulting biofilms were rinsed with PBS and scraped into a small volume of PBS. Aliquots of cells (≈60 mg [wet weight]) were transferred to 1.5-ml polypropylene microcentrifuge tubes and resuspended in 400 μl of 20 μg/ml dispersin B in PBS. Control cells were resuspended in PBS alone. After 1 h at 37°C, cells were pelleted and the amount of total hexosamine in the supernatant was measured by using the Morgan-Elson assay as previously described (53).
Statistics and reproducibility of results.
Biofilm inhibition and killing assays and biofilm detachment assays with dispersin B and DNase I were performed in duplicate wells. Biofilm detachment assays with restriction endonucleases were performed in quadruplicate wells. Replicate wells exhibited 0 to 10% variation in A590 or CFU/well values. The significance of differences between means was calculated using a Student t test. P values of <5% were considered significant. All inhibition and detachment assays were performed on at least three occasions with similarly significant decreases in A590 values. Total hexosamine assays were performed on at least two occasions with similar increases in total hexosamine release. Killing assays were performed on numerous occasions with similar highly significant decreases in CFU/well values.
RESULTS
Inhibition of biofilm formation by the PNAG-degrading enzyme dispersin B.
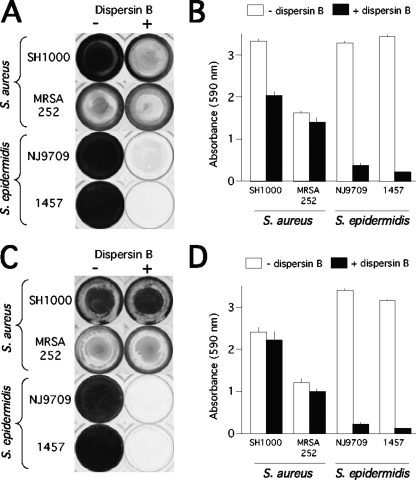
Figure 1A shows S. aureus and S. epidermidis biofilm formation in the wells of a 96-well microtiter plate. Biofilms were stained with crystal violet, which stains bacterial cells and biofilm matrix components but not polystyrene (43). The amount of biofilm biomass was measured by destaining the biofilms and quantitating the amount of bound dye (Fig. 1B). Biofilm formation by S. aureus strain SH1000 and by S. epidermidis strains NJ9709 and 1457 was significantly inhibited in growth medium supplemented with dispersin B compared to biofilm formation in unsupplemented medium. These results are consistent with those of previous studies showing that dispersin B inhibited biofilm formation by 18 out of 18 S. aureus strains and 26 out of 26 PNAG-positive S. epidermidis strains isolated from prosthetic joint infections (48) and by S. epidermidis strain 1457 (25). Biofilm formation by S. aureus strain MRSA252, which produced weaker biofilms than strain SH1000, was not significantly inhibited by dispersin B.
FIG. 1.
Inhibition and detachment of S. aureus and S. epidermidis biofilms by dispersin B in 96-well microtiter plates. (A) The indicated strains were grown for 24 h in unsupplemented TSB medium (−) or TSB supplemented with 20 μg/ml of dispersin B (+). Wells were rinsed and stained with crystal violet. (B) Quantitation of crystal violet staining in panel A. Wells were destained with acetic acid, and the absorbance of the crystal violet solution was measured at 590 nm. Absorbance is proportional to biofilm biomass. Values represent the means for duplicate wells, and error bars indicate range. (C) Biofilms were grown for 24 h in unsupplemented TSB and then rinsed, treated for 1 h with PBS (−) or PBS with dispersin B (+), rinsed, and stained with crystal violet. (D) Quantitation of crystal violet staining in panel C.
Detachment of preformed biofilms by dispersin B.
Fig. 1C and D show 24-h-old S. aureus and S. epidermidis biofilms treated with dispersin B. Dispersin B had no significant effect on the attachment of S. aureus SH1000 and MRSA252 biofilms. In contrast, dispersin B rapidly and efficiently detached S. epidermidis NJ9709 and 1457 biofilms. These findings are consistent with those of previous studies demonstrating that dispersin B detached biofilms produced by various strains of S. epidermidis (9, 25, 32) but not biofilms produced by S. aureus strain 383 (9).
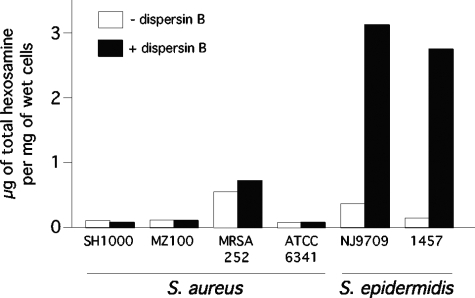
PNAG is not a major matrix component in S. aureus biofilms.
The amount of PNAG present in S. aureus and S. epidermidis biofilms was measured by treating biofilm cells with dispersin B and then quantitating the amount of total hexosamine in the cell supernatant using the Morgan-Elson assay (Fig. 2). Four different S. aureus strains did not release detectable amounts of total hexosamine when treated with dispersin B, whereas S. epidermidis strains NJ9709 and 1457 exhibited large increases in the amount of total hexosamine in the cell supernatant. To rule out the possibility that dispersin B does not penetrate aggregates of S. aureus biofilm cells, we pretreated the cells with DNase I, which disaggregates the cells (see below), prior to treating them with dispersin B. S. aureus biofilm cells treated in this manner still did not release detectable amounts of total hexosamine after dispersin B treatment (data not shown).
FIG. 2.
Quantitation of total hexosamine released by S. aureus and S. epidermidis biofilm cells treated with dispersin B. Control cells (− dispersin B) were treated with PBS alone. Total hexosamine was measured using the Morgan-Elson assay.
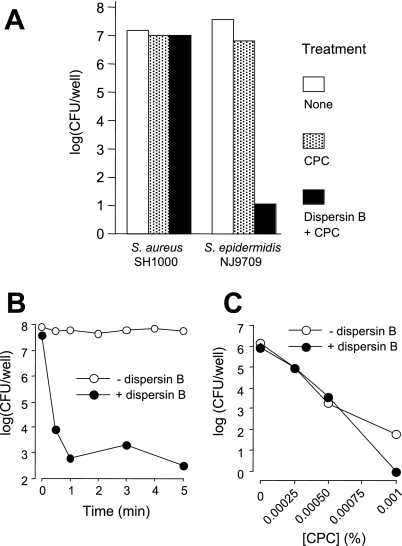
PNAG protects S. epidermidis biofilm cells from detergent killing.
Previous studies showed that dispersin B sensitizes biofilms produced by the gram-negative bacterium Aggregatibacter actinomycetemcomitans to killing by the cationic detergent CPC (27). We tested whether dispersin B could also sensitize S. aureus SH1000 and S. epidermidis NJ9709 biofilms to CPC killing. Control experiments indicated that the minimal bactericidal concentration (>99.999% killing in 5 min) of CPC was 0.0003% against S. aureus SH1000 planktonic cells and 0.001% against S. epidermidis NJ9709 planktonic cells and that S. aureus SH1000 and S. epidermidis NJ9709 biofilms were resistant to killing by CPC concentrations that were 1,000-fold and 100-fold greater, respectively, than their planktonic minimal bactericidal concentrations (data not shown).
Pretreatment of S. aureus SH1000 biofilms with dispersin B did not increase their sensitivity to killing by 0.3% CPC in 5 min (Fig. 3A). In contrast, pretreatment of S. epidermidis NJ9709 biofilms with dispersin B resulted in a 5- to 6-log unit decrease in CFU/well values after treatment with 0.1% CPC for 5 min compared to values for control biofilms that were pretreated with PBS alone. Time course studies showed that killing of dispersin B-treated S. epidermidis biofilms by CPC was complete within 1 min (Fig. 3B). Pretreatment of S. epidermidis planktonic cells with dispersin B did not increase their susceptibility to killing by CPC (Fig. 3C), indicating that PNAG protects S. epidermidis cells from CPC killing at the multicellular level.
FIG. 3.
Pretreatment of S. epidermidis biofilms with dispersin B renders them sensitive to killing by CPC. (A) S. aureus SH1000 and S. epidermidis NJ9709 biofilms grown in 96-well microtiter plates were treated with 0.3% CPC (for S. aureus) or 0.1% CPC (for S. epidermidis) for 5 min. Values show mean numbers of surviving CFU/well, and error bars indicate range. Black bars show CFU/well values for biofilms pretreated with 20 μg/ml of dispersin B for 10 min prior to addition of the CPC. (B) Time course for CPC killing of S. epidermidis NJ9709 biofilms with or without dispersin B pretreatment. (C) Five-minute killing of S. epidermidis NJ9709 planktonic cells by CPC with or without dispersin B pretreatment.
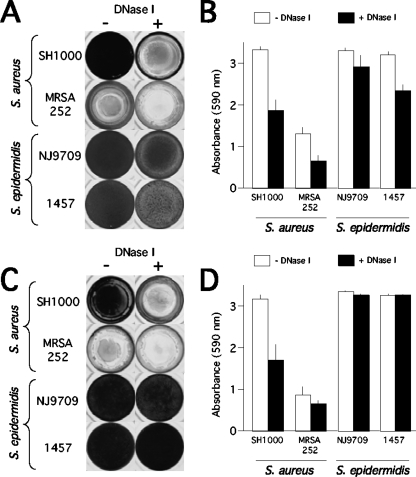
Inhibition of biofilm formation by DNase I.
Figure 4A and B show that biofilm formation by S. aureus strains SH1000 and MRSA252 and by S. epidermidis strain 1457 was significantly inhibited in medium supplemented with DNase I. These findings are consistent with those of previous studies demonstrating that DNase I inhibits biofilm formation by two different S. aureus clinical isolates (13, 47) and six different S. epidermidis reference strains and clinical isolates (46).
FIG. 4.
Inhibition and detachment of S. aureus and S. epidermidis biofilms by DNase I in 96-well microtiter plates. (A) The indicated strains were grown for 24 h in unsupplemented TSB medium (−) or TSB supplemented with 100 μg/ml of DNase I (+). Wells were rinsed and stained with crystal violet. (B) Quantitation of crystal violet staining in panel A as described in the legend to Fig. 1. (C) Biofilms were grown for 24 h in unsupplemented TSB and then rinsed, treated for 1 h with DNase I buffer alone (−) or DNase I buffer with DNase I (+), rinsed, and stained with crystal violet. (D) Quantitation of crystal violet staining shown in panel C.
Detachment of biofilms by DNase I.
Figure 4C and D show 24-h-old S. aureus and S. epidermidis biofilms treated for 1 h with DNase I. DNase I caused significant detachment of S. aureus biofilms from the surface. Microscopic analysis indicated that DNase I rapidly dispersed S. aureus biofilms into uniform suspensions of small cell clusters which did not disperse into single cells upon extended incubation (data not shown). DNase I did not cause significant detachment of biofilms produced by S. epidermidis 1457 or NJ9709. These findings are consistent with those of previous studies demonstrating that mature biofilms produced by six different S. epidermidis reference strains and clinical isolates were resistant to detachment by DNase I (46).
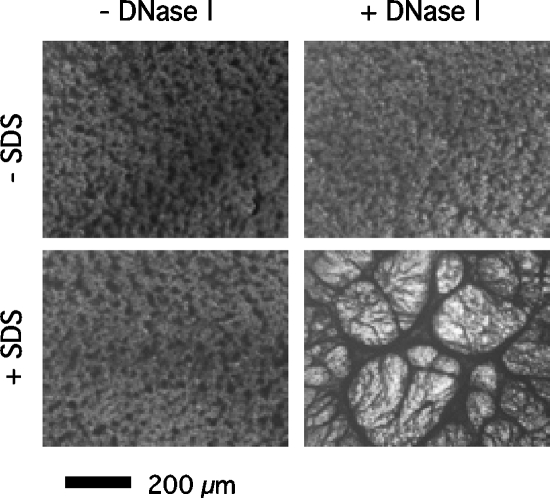
To confirm that ecDNA comprises a structural component of the S. epidermidis biofilm matrix, we treated S. epidermidis NJ9709 biofilms with DNase I and then with a 1% solution of the anionic detergent SDS (Fig. 5). Previous studies showed that Pseudomonas aeruginosa biofilms pretreated with DNase I were sensitized to detachment by SDS (3). DNase I pretreatment did not sensitize S. epidermidis biofilms to SDS detachment. However, SDS caused DNase I-treated S. epidermidis biofilms to condense into long bundles of thick fibrils that covered the entire surface of the well (Fig. 5). Neither DNase I nor SDS had any effect on the viability of S. epidermidis cells under these conditions (data not shown).
FIG. 5.
Close-up view of 24-h-old S. epidermidis NJ9709 biofilms in 96-well microtiter plates. Top left, untreated biofilm; top right, biofilm treated with 100 μg/ml of DNase I for 1 h; bottom left, biofilm treated with 1% SDS for 5 min; bottom right, biofilm treated with DNase I for 1 h and then SDS for 5 min. Biofilms were stained with crystal violet and then viewed and photographed under an Olympus IMT-2 inverted microscope at magnification ×40.
Detachment of S. aureus biofilms by restriction endonucleases.
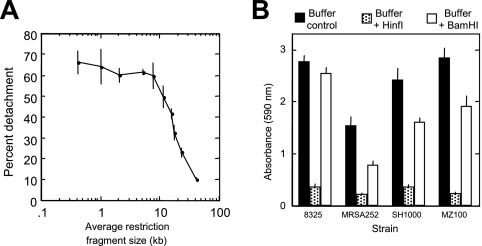
We tested the ability of various restriction endonucleases to detach S. aureus SH1000 biofilms (Fig. 6A). The amount of biofilm detachment depended on the frequency of the enzyme recognition sequence in the S. aureus genome (Fig. 6A). Enzymes that produced restriction fragments with an average size of <10 kb caused efficient biofilm detachment, whereas enzymes that produced restriction fragments with an average size of 11 to 24 kb caused partial detachment. These findings suggest that the fraction of S. aureus ecDNA that mediates intercellular adhesion is composed primarily of genomic DNA. Biofilms produced by three other S. aureus strains (MZ100, MSRA252, and 8325) exhibited similar detachment phenotypes when treated with HinfI (average restriction fragment size, 0.4 kb) or BamHI (average restriction fragment size, 23.9 kb) (Fig. 6B).
FIG. 6.
Detachment of S. aureus biofilms by restriction endonucleases. (A) S. aureus SH1000 biofilms grown in microtiter plates were treated for 1 h with 100 U/ml of various restriction endonucleases and then rinsed and stained with crystal violet. Percent detachment was calculated as 1 − (A595[buffer + enzyme]/A595[buffer alone]) × 100. Values show means and ranges for duplicate wells. The restriction endonucleases used (and average restriction fragment lengths in kilobases) were HinfI (0.4), FokI (1.0), HaeIII (2.1), AlwNI (5.1), ApaLI (7.8), NcoI (11.8), KpnI (16.2), AvaI (17.9), BamHI (23.9), and SstI (41.5). (B) Detachment of S. aureus 8325, MRSA252, SH1000, and MZ100 biofilms by HinfI and BamHI. Biofilms were treated with 100 U/ml of enzyme for 1 h, and then biofilm biomass was quantitated by crystal violet staining. Values show means and standard errors for quadruplicate wells.
ecDNA protects S. aureus biofilm cells from detergent killing.
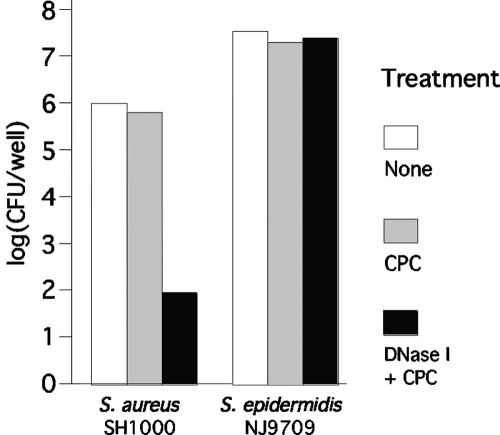
We tested the ability of DNase I to render S. aureus SH1000 and S. epidermidis NJ9709 biofilm cells sensitive to killing by CPC (Fig. 7). Pretreatment of S. aureus SH1000 biofilms with DNase I resulted in a 4-log-unit decrease in CFU/well values after treatment with 0.3% CPC for 5 min compared to results for control biofilms that were pretreated with DNase I buffer alone. DNase I had an identical effect on killing of S. aureus MRSA252 and 8325 biofilm cells by CPC (data not shown). DNase I did not sensitize S. epidermidis NJ9709 biofilms to killing by 0.01% CPC in 5 min (Fig. 7).
FIG. 7.
Pretreatment of S. aureus biofilms with DNase I renders them sensitive to killing by CPC. (A) S. aureus SH1000 and S. epidermidis NJ9709 biofilms grown in microtiter plates were treated with 0.3% CPC (for S. aureus) or 0.1% CPC (for S. epidermidis) for 5 min. Values show mean numbers of surviving CFU/well for duplicate wells. Black bars show CFU/well values for biofilms pretreated with 100 μg/ml of DNase I for 10 min prior to addition of the CPC.
DISCUSSION
Biochemical studies have confirmed that PNAG is produced by both S. aureus and S. epidermidis (29, 37). Homologues of the icaADBC locus, which encodes the production of PNAG in S. aureus and S. epidermidis, have been identified in various other staphylococci, including S. caprae, S. capitis, S. cohnii, S. lugdunensis, S. pasteuri, and S. saprophyticus (4, 16, 17, 40). However, PNAG production has not been directly demonstrated in these species. A polysaccharide that is nearly identical to staphylococcal PNAG is produced by various gram-negative Proteobacteria, including Escherichia coli, Pseudomonas fluorescens, Yersinia pestis, Actinobacillus pleuropneumoniae, Aggregatibacter (Actinobacillus) actinomycetemcomitans, and Bordetella spp. (25, 33, 44). Functions ascribed to proteobacterial PNAG include abiotic surface attachment, intercellular adhesion, biofilm formation, detergent resistance, antibiotic resistance, and epithelial cell attachment (1, 25-27, 45).
Several observations suggest that PNAG performs different functions in S. aureus and S. epidermidis biofilms. For example, S. aureus PNAG mutants can still form biofilms in vitro and in vivo, whereas S. epidermidis PNAG mutants exhibit a severely reduced biofilm phenotype (5, 21). Also, nearly all strains of S. aureus carry the icaADBC locus (42), whereas ica genes are absent in up to half of S. epidermidis strains isolated from device-associated infections (8) and in nearly all skin and mucosal isolates (58). In addition, S. epidermidis mutant strains deficient in PNAG production exhibit decreased virulence in animal models of both systemic and device-associated infection, whereas S. aureus PNAG-deficient strains exhibit reduced virulence in animal models of systemic but not device-associated infection (34, 35, 49). In the present study, we showed that the PNAG-degrading enzyme dispersin B was able to detach preformed biofilms produced by S. epidermidis and render them sensitive to detergent killing whereas dispersin B had no effect on the attachment of preformed S. aureus biofilms or their sensitivity to detergent killing (Fig. 1C and 3). In addition, only small quantities of hexosamine were released from S. aureus biofilms treated with dispersin B compared to the amount released by S. epidermidis biofilms (Fig. 2). Taken together, these findings suggest that PNAG is a major matrix adhesin in S. epidermidis biofilms and a minor component of S. aureus biofilms. The role of PNAG in S. aureus biofilms may be similar to its role in S. lugdunensis biofilms, which are also resistant to detachment by dispersin B (17).
When added to the culture medium at the time of inoculation, dispersin B inhibited biofilm formation by S. aureus strain SH1000 (Fig. 1A). The fact that mature S. aureus biofilms were resistant to detachment by dispersin B suggests that PNAG may function in the early stages of S. aureus biofilm development.
In the present study, we also examined the role of ecDNA in S. aureus and S. epidermidis biofilm formation. Our data confirm that ecDNA is a structural component of the biofilm matrix in both species (Fig. 4 and 5) (13, 46, 47). However, our findings suggest that ecDNA is a major structural component in the S. aureus biofilm matrix and a minor structural component in the S. epidermidis biofilm matrix. This hypothesis is based on the fact that DNase I inhibited S. aureus biofilm formation, detached preformed S. aureus biofilms, and rendered preformed S. aureus biofilms sensitive to detergent killing whereas DNase I had no effect on S. epidermidis biofilm formation, attachment, or detergent sensitivity (Fig. 4 and 7). Previous studies showed that S. aureus ecDNA may be comprised of genomic DNA released from lysed biofilm cells (47). Consistent with this hypothesis, we found that the ability of various restriction endonucleases to detach preformed S. aureus biofilms depended on the frequency of their recognition sequence in the S. aureus genome (Fig. 6). The results of these studies suggest that S. aureus ecDNA fragments of >11 kb in size can function as intercellular adhesins. Interestingly, we found that micrococcal nuclease rapidly and efficiently detached S. aureus biofilms (unpublished data), suggesting that this enzyme may play a role in biofilm cell detachment and biofilm dispersal.
Our findings indicate that depolymerization of either S. aureus ecDNA or S. epidermidis PNAG sensitizes the respective biofilm cells to killing by the cationic detergent CPC (Fig. 3 and 7). Previous studies showed that depolymerization of PNAG sensitizes A. actinomycetemcomitans biofilm cells to killing by the anionic detergent SDS (28). In all cases, detergent sensitivity is associated with dissolution and detachment of the biofilm cells. Taken together, these findings suggest that matrix polymers such as ecDNA and PNAG can act as general diffusion barriers that prevent access of detergents to the biofilm cells. However, previous studies also showed that exogenously added S. epidermidis PNAG can interfere with the antimicrobial activity of glycopeptide antibiotics, including vancomycin and teicoplanin (14). These findings suggest that PNAG may also form physical complexes with some antimicrobial agents, thereby sequestering the agents within the polymeric matrix.
Acknowledgments
We thank Barry Kreiswirth (Public Health Research Institute) and Rob Shanks (University of Pittsburgh Medical Center) for providing S. aureus strains, Deepa Rupani (New Jersey Dental School) for technical assistance, and Barry Kreiswirth, Kevin Fennelly (New Jersey Medical School), and Daniel Kadouri (New Jersey Dental School) for helpful discussions.
This work was supported by Public Health Service award DE15124 (to J.B.K.).
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Agladze, K., X. Wang, and T. Romeo. 2005. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J. Bacteriol. 187:8237-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama, H., H. Kanzaki, J. Tada, and J. Arata. 1996. Staphylococcus aureus infection on cut wounds in the mouse skin: experimental staphylococcal botryomycosis. J. Dermatol. Sci. 11:234-238. [DOI] [PubMed] [Google Scholar]
- 3.Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114-1128. [DOI] [PubMed] [Google Scholar]
- 4.Allignet, J., S. Aubert, K. G. Dyke, and N. El Solh. 2001. Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: a gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect. Immun. 69:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biavasco, F., C. Vignaroli, and P. E. Varaldo. 2000. Glycopeptide resistance in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. 19:403-417. [DOI] [PubMed] [Google Scholar]
- 7.Buxton, T. B., J. Horner, A. Hinton, and J. P. Rissing. 1987. In vivo glycocalyx expression by Staphylococcus aureus phage type 52/52A/80 in S. aureus osteomyelitis. J. Infect. Dis. 156:942-946. [DOI] [PubMed] [Google Scholar]
- 8.Cafiso, V., T. Bertuccio, M. Santagati, F. Campanile, G. Amicosante, M. G. Perilli, L. Selan, M. Artini, G. Nicoletti, and S. Stefani. 2004. Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin. Microbiol. Infect. 10:1081-1088. [DOI] [PubMed] [Google Scholar]
- 9.Chaignon, P., I. Sadovskaya, C. Ragunath, N. Ramasubbu, J. B. Kaplan, and S. Jabbouri. 2007. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 75:125-132. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penadés. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckhart, L., H. Fischer, K. B. Barken, T. Tolker-Nielsen, and E. Tschachler. 2007. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 156:1342-1345. [DOI] [PubMed] [Google Scholar]
- 14.Farber, B. F., M. H. Kaplan, and A. G. Clogston. 1990. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J. Infect. Dis. 161:37-40. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick, F., H. Humphreys, and J. P. O'Gara. 2005. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin. Microbiol. Infect. 11:967-973. [DOI] [PubMed] [Google Scholar]
- 16.Frank, K. L., A. D. Hanssen, and R. Patel. 2004. icaA is not a useful diagnostic marker for prosthetic joint infection. J. Clin. Microbiol. 42:4846-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank. K. L., and R. Patel. 2007. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect. Immun. 75:4728-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 19.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 20.Gross, M., S. E. Cramton, F. Götz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 22.Holden, M. T. G., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. J. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Sprat, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 101:9786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsburgh, M., J. Aish, I. White, L. Shaw, J. Lithgow, and S. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain, C., M. Herrmann, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh, Y., X. Wang, B. J. Hinnebusch, J. F. Preston III, and T. Romeo. 2005. Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol. 187:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izano, E. A., I. Sadovskaya, E. Vinogradov, M. H. Mulks, K. Velliyagounder, C. Ragunath, W. B. Kher, N. Ramasubbu, S. Jabbouri, M. B. Perry, and J. B. Kaplan. 2007. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb. Pathog. 43:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izano, E. A., I. Sadovskaya, H. Wang, E. Vinogradov, C. Ragunath, N. Ramasubbu, S. Jabbouri, M. B. Perry, and J. B. Kaplan. 2008. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb. Pathog. 44:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izano, E. A., H. Wang, C. Ragunath, N. Ramasubbu, and J. B. Kaplan. 2007. Detachment and killing of Aggregatibacter actinomycetemcomitans biofilms by dispersin B and SDS. J. Dent. Res. 86:618-622. [DOI] [PubMed] [Google Scholar]
- 29.Joyce, J., G. C. Abeygunawardana, Q. Xu, J. C. Cook, R. Hepler, C. T. Przysiecki, K. M. Grimm, K. Roper, C. C. Yu Ip, L. Cope, D. Montgomery, M. Chang, S. Campie, M. Brown, T. B. McNeely, J. Zorman, T. Maira-Litran, G. B. Pier, P. M. Keller, K. U. Jansen, and G. E. Mark III. 2003. Isolation, structural characterization, and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr. Res. 338:903-922. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan, J. B., and D. H. Fine. 2002. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl. Environ. Microbiol. 68:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan, J. B., C. Ragunath, N. Ramasubbu, and D. H. Fine. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J. Bacteriol. 185:4693-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan, J. B., C. Ragunath, K. Velliyagounder, D. H. Fine, and N. Ramasubbu. 2004. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 48:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan, J. B., K. Velliyagounder, C. Ragunath, H. Rohde, D. Mack, J. K. Knobloch, and N. Ramasubbu. 2004. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 186:8213-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristian, S. A., T. Golda, F. Ferracin, S. E. Cramton, B. Neumeister, A. Peschel, F. Götz, and R. Landmann. 2004. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb. Pathog. 36:237-245. [DOI] [PubMed] [Google Scholar]
- 35.Kropec, A., T. Maira-Litran, K. K. Jefferson, M. Grout, S. E. Crampton, F. Götz, D. A. Goldmann, and G. B. Pier. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73:6868-6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 37.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmenn, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massey, R. C., M. J. Horsburgh, G. Lina, M. Höök, and M. Recker. 2006. The evolution and maintenance of virulence in Staphylococcus aureus: a role for host-to-host transmission? Nat. Rev. Microbiol. 4:953-958. [DOI] [PubMed] [Google Scholar]
- 40.Moretro, T., L. Hermansen, A. L. Holck, M. S. Sidhu, K. Rudi, and S. Langsrud. 2003. Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food processing environments. Appl. Environ. Microbiol. 69:5648-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 42.O'Gara, J. P. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270:179-188. [DOI] [PubMed] [Google Scholar]
- 43.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 44.Parise, G., M. Mishra, Y. Itoh, T. Romeo, and R. Deora. 2007. Role of a putative polysaccharide locus in Bordetella biofilm development. J. Bacteriol. 189:750-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parise Sloan, G., C. F. Love, N. Sukumar, M. Mishra, and R. Deora. 2007. The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract. J. Bacteriol. 189:8270-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin, Z., Y. Ou, L. Yang, Y. Zhu, T. Tolker-Nielsen, S. Molin, and D. Qu. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083-2092. [DOI] [PubMed] [Google Scholar]
- 47.Rice, K. C., E. E. Mann, J. L. Endres, E. C. Weiss, J. E. Cassat, M. S. Smeltzer, and K. W. Bayles. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 104:8113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohde, H., E. C. Burandt, N. Siemssen, L. Frommelt, C. Burdelski, S. Wurster, S. Scherpe, A. P. Davies, L. G. Harris, M. A. Horstkotte, J. K. M. Knobloch, C. Ragunath, J. B. Kaplan, and D. Mack. 2007. Polysaccharide intercellular adhesin and protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28:1711-1720. [DOI] [PubMed] [Google Scholar]
- 49.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadovskaya, I., E. Vinogradov, J. Li, and S. Jabbouri. 2004. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 339:1467-1473. [DOI] [PubMed] [Google Scholar]
- 51.Shanks, R. M. Q., N. P. Donegan, M. L. Graber, S. E. Buckingham, M. E. Zegans, A. L. Cheung, and G. A. O'Toole. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens, D. L. 2003. Community-acquired Staphylococcus aureus infections: increasing virulence and emerging methicillin resistance in the new millennium. Curr. Opin. Infect. Dis. 16:189-191. [DOI] [PubMed] [Google Scholar]
- 53.Strominger, J. L., J. T. Park, and R. E. Thompson. 1959. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J. Biol. Chem. 234:3263-3268. [PubMed] [Google Scholar]
- 54.Toledo-Arana, A., N. Merino, M. Vergara-Irigaray, M. Débarbouillé, J. R. Penadés, and I. Lasa. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 187:5318-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinogradov, E., I. Sadovskaya, J. Li, and S. Jabbouri. 2006. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr. Res. 341:738-743. [DOI] [PubMed] [Google Scholar]
- 56.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4:481-489. [DOI] [PubMed] [Google Scholar]
- 57.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. DeLeo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269-275. [DOI] [PubMed] [Google Scholar]
- 58.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]