Abstract
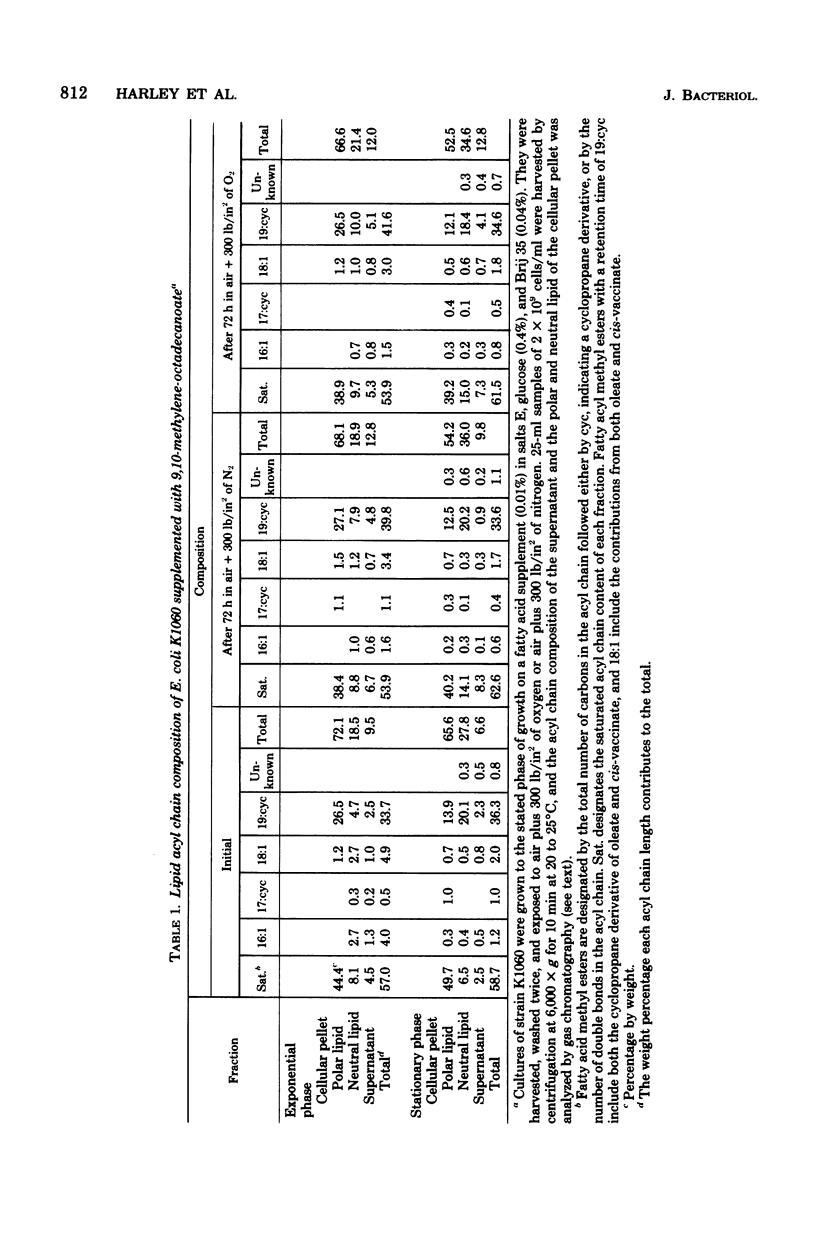
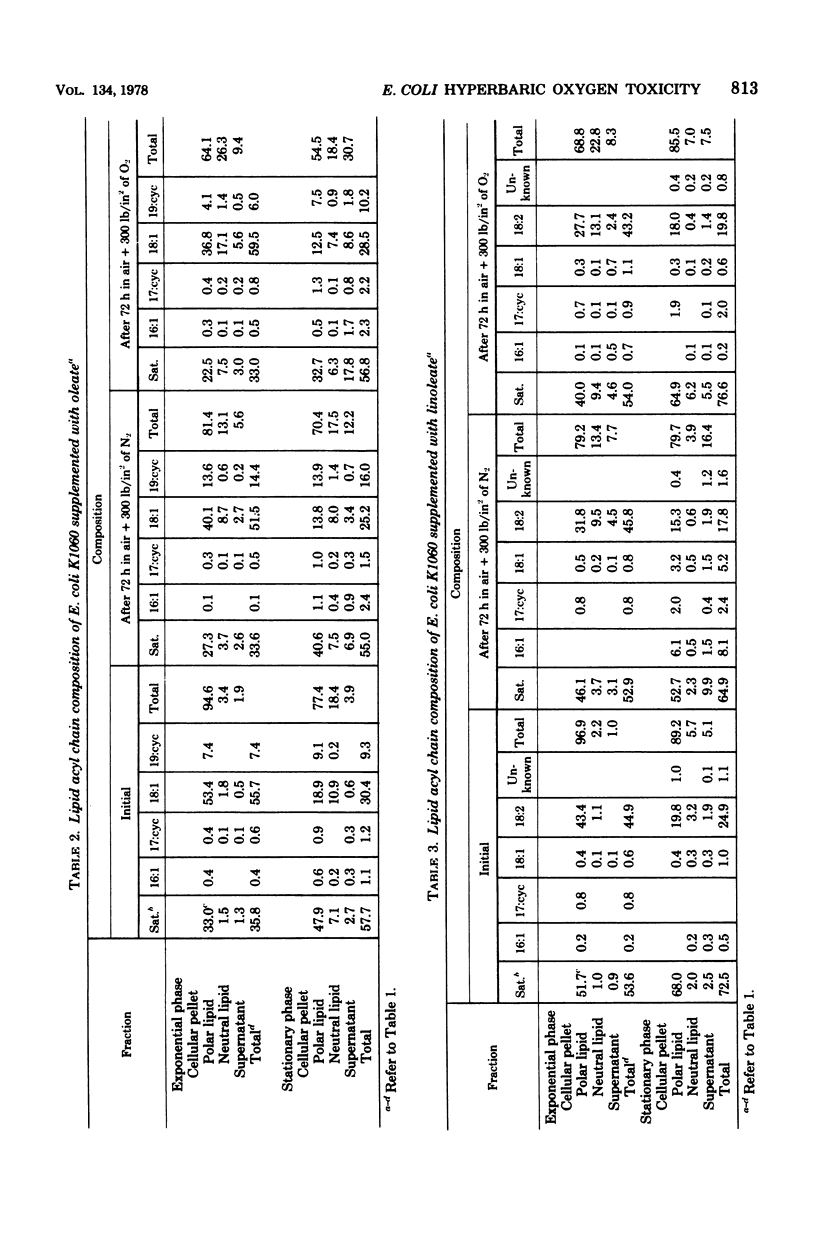
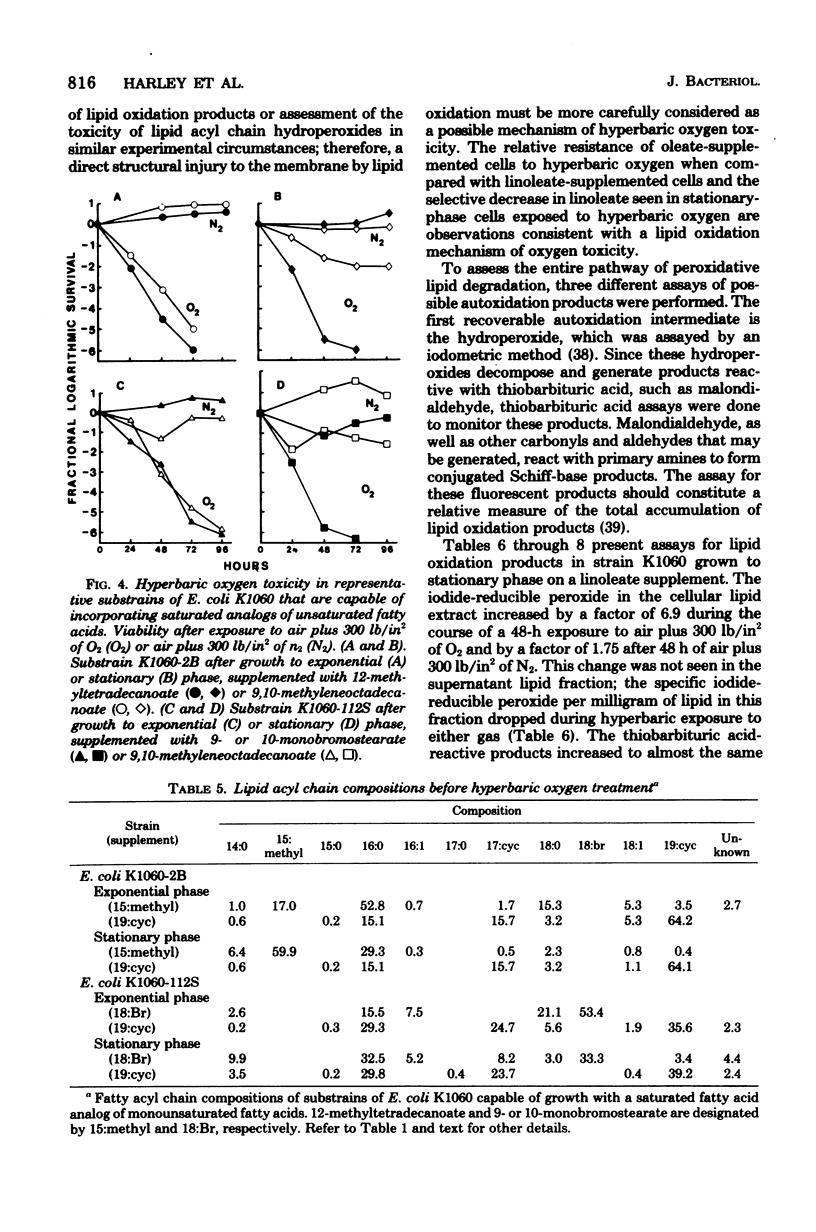
This study examines certain membrane-related aspects of oxygen poisoning in Escherichia coli K1060 (fabB fadE lacI) and its parent strain, K-12 Ymel. Cells were grown to exponential or stationary phase in a minimal medium and exposed to air plus 300 lb/in2 of O2 as a suspension in minimal salts. After an initial lag, both strains lost viability with apparent first-order kinetics. Hypebaric oxygen was more toxic to cells harvested during the exponential phase of growth than to cells harvested from the stationary phase of growth for both strains K-12 Ymel and K1060. Control suspensions exposed to air plus 300 lb/in2 of N2 did not lose viability during a 96-h exposure. The sensitivity of the unsaturated fatty acid auxotroph, strain K1060, to hyperbaric oxygen increased as the degree of unsaturation of the fatty acid supplement increased. Cells grown with a cyclopropane fatty acid (9,10=methylenoctadecanoate) were the most resistant; cells grown with a monounsaturated fatty acid (oleate) were intermediate; and those grown with polyunsaturated fatty acids (linoleate and linolenate) were most sensitive to hyperbaric oxygen. The parent strain, K-12 Ymel, lost viability in hyperbaric oxygen most similarly to strain K1060 supplemented with oleate. To determine the relative effect of hyperbaric oxygen on the survival of E. coli with saturated membranes, substrains of K1060 were selected for growth on 12-methyltetrade-canoate or on 9 or 10-monobromostearate. Substrains grown with a saturated fatty acid supplement were equally or more sensitive to hyperbaric oxygen than when the same substrains were grown with a cyclopropane fatty acid supplement. The lipid acyl chain composition was determined in E. coli K1060 before and after exposure to hyperbaric oxygen or hyperbaric nitrogen. The proportion of nonsaturated acyl chain lipid of either the oleate- or the 9,10-methyleneoctade-canoate-supplemented K1060 remained unchanged after hyperbaric gas exposure. In strain K1060 supplemented with linoleate and grown to stationary phase, however, the relative unsaturated acyl chain content after hyperbaric exposure decreased in both gases. This finding prompted an investigation of the role of lipid oxidation in hyperbaric oxygen toxicity. Assays of potential lipid oxidation products were performed with linoleate-grown cells. The lipid hydroperoxide and peroxide content of the lipid extract increased by 6.9 times after 48 h of air plus 300 lb/in2 of O2; malondialdehyde and fluorescent complex lipid oxidation products showed much smaller or no changes. Lipid extracts from hyperbaric oxygen-exposed cells were not toxic to viable E. coli K1060, nor did they increase the rate of loss of viability in cells simultaneously exposed to hyperbaric oxygen. Linoleic acid hydroperoxide at 1.0 mM had no effect on the viability of E. coli K-12 Ymel and only marginally decreased the viability of E. coli K1060 supplemented with linoleate. We conclude that the kinetics of oxygen toxicity in E...
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Rasmussen H. The effects of oxygen on cellular metabolism. Int Z Klin Pharmakol Ther Toxikol. 1971 Aug;5(1):26–33. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Silbert D. F. Studies on the uridine diphosphate-galactose: lipopolysaccharide galactosyltransferase reaction using a fatty acid mutant of Escherichia coli. J Biol Chem. 1973 Aug 10;248(15):5310–5318. [PubMed] [Google Scholar]
- Cortesi R., Privett O. S. Toxicity of fatty ozonides and peroxides. Lipids. 1972 Nov;7(11):715–721. doi: 10.1007/BF02533120. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products of lipid peroxidation of mitochondria and microsomes. Lipids. 1971 Oct;6(10):715–721. doi: 10.1007/BF02531296. [DOI] [PubMed] [Google Scholar]
- Dolev A., Rohwedder W. K., Dutton H. J. Mechanism of lipoxidase reaction. I. Specificity of hydroperoxidation of linoleic acid. Lipids. 1967 Jan;2(1):28–32. doi: 10.1007/BF02531996. [DOI] [PubMed] [Google Scholar]
- FRANZ J., COLE B. T. Effects of ultraviolet-irradiated methyl linolenate on cell division and respiration in Saccharomyces cerevisiae. Arch Biochem Biophys. 1962 Feb;96:382–385. doi: 10.1016/0003-9861(62)90424-1. [DOI] [PubMed] [Google Scholar]
- Ferguson K. A., Glaser M., Bayer W. H., Vagelos P. R. Alteration of fatty acid composition of LM cells by lipid supplementation and temperature. Biochemistry. 1975 Jan 14;14(1):146–151. doi: 10.1021/bi00672a025. [DOI] [PubMed] [Google Scholar]
- Findlay G. M., Draper H. H., Bergan J. G. Metabolism of 1-14C-methyl linoleate hydroperoxide in the rabbit. Lipids. 1970 Dec;5(12):970–975. doi: 10.1007/BF02533199. [DOI] [PubMed] [Google Scholar]
- Finn G. J., Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):570–579. doi: 10.1128/jb.123.2.570-579.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Law J. H., Tsukagoshi N., Wilson G. A density label for membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):598–605. doi: 10.1073/pnas.67.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHMAN R., GILBERT D. L., NYE S. W., DWYER P., FENN W. O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954 May 7;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPKINSON W. I., TOWERS A. G. EFFECTS OF HYPERBARIC OXYGEN ON SOME COMMON PATHOGENIC BACTERIA. Lancet. 1963 Dec 28;2(7322):1361–1362. doi: 10.1016/s0140-6736(63)90741-4. [DOI] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jungkind D. L., Wood R. C. Physiological differences between cyclopropane fatty acid-deficient mutants and the parent strain of Streptococcus faecalis. Biochim Biophys Acta. 1974 Feb 25;337(2):298–310. doi: 10.1016/0005-2760(74)90211-2. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- ROSE C. S., GYORGY P. Specificity of hemolytic reaction in vitamin E-deficient erythrocytes. Am J Physiol. 1952 Feb;168(2):414–420. doi: 10.1152/ajplegacy.1952.168.2.414. [DOI] [PubMed] [Google Scholar]
- SHUSTER C. W. Effects of oxidized fatty acids on ascites tumor metabolism. Proc Soc Exp Biol Med. 1955 Nov;90(2):423–426. doi: 10.3181/00379727-90-22054. [DOI] [PubMed] [Google Scholar]
- STUART B., GERSCHMAN R., STANNARD J. N. Effect of high oxygen tension on potassium retentivity and colony formation of baker's yeast. J Gen Physiol. 1962 Jul;45:1019–1030. doi: 10.1085/jgp.45.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauenstein E. Autoxidation of polyunsaturated esters in water: chemical structure and biological activity of the products. J Lipid Res. 1967 Sep;8(5):417–428. [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Stees J. L., Brown O. R. Susceptibilities of intracellular and surface sulphydryl groups of Escherichia coli to oxidation by hyperoxia. Microbios. 1973 Apr-May;7(28):257–266. [PubMed] [Google Scholar]
- TSEN C. C., COLLIER H. B. The protective action of tocopherol against hemolysis of rat erythrocytes by dialuric acid. Can J Biochem Physiol. 1960 Sep;38:957–964. [PubMed] [Google Scholar]
- Taylor F., Cronan J. E., Jr Selection and properties of Escherichia coli mutants defective in the synthesis of cyclopropane fatty acids. J Bacteriol. 1976 Feb;125(2):518–523. doi: 10.1128/jb.125.2.518-523.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaysen A. C. Preliminary note on the action of gases under pressure on the growth of micro-organisms: Action of oxygen under pressure at various temperatures. Biochem J. 1934;28(4):1330–1335. doi: 10.1042/bj0281330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vaughan G. L. Oxygen toxicity in a fission yeast. J Cell Physiol. 1971 Jun;77(3):363–372. doi: 10.1002/jcp.1040770310. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WILBUR K. M., WOLFSON N., KENASTON C. B., OTTOLENGHI A., GAULDEN M. E., BERNHEIM F. Inhibition of cell division by ultraviolet irradiated unsaturated fatty acid. Exp Cell Res. 1957 Dec;13(3):503–509. doi: 10.1016/0014-4827(57)90079-4. [DOI] [PubMed] [Google Scholar]
- Yatvin M. B. Evidence that survival of gamma-irradiated Escherichia col is influenced by membrane fluidity. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Dec;30(6):571–575. doi: 10.1080/09553007614551441. [DOI] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. Superoxide and hydrogen peroxide in oxygen damage. Arch Biochem Biophys. 1976 Aug;175(2):514–519. doi: 10.1016/0003-9861(76)90539-7. [DOI] [PubMed] [Google Scholar]


