Abstract
Nucleosome arrays undergo salt-dependent self-association into large oligomers in a process thought to recapitulate essential aspects of higher-order tertiary chromatin structure formation. Lysine acetylation within the core histone tail domains inhibits self-association, an effect likely related to its role in facilitating transcription. As acetylation of specific tail domains may encode distinct functions, we investigated biochemical and self-association properties of model nucleosome arrays containing combinations of native and mutant core histones with lysine-to-glutamine substitutions to mimic acetylation. Acetylation mimics within the tail domains of H2B and H4 caused the largest inhibition of array self-association, while modification of the H3 tail uniquely affected the stability of DNA wrapping within individual nucleosomes. In addition, the effect of acetylation mimics on array self-association is inconsistent with a simple charge neutralization mechanism. For example, acetylation mimics within the H2A tail can have either a positive or negative effect on self-association, dependent upon the acetylation state of the other tails and nucleosomal repeat length. Finally, we demonstrate that glutamine substitutions and lysine acetylation within the H4 tail domain have identical effects on nucleosome array self-association. Our results indicate that acetylation of specific tail domains plays distinct roles in the regulation of chromatin structure.
Decondensation of highly compacted higher-order chromatin structures is thought to be a critical step in increasing the accessibility of genomic DNA to facilitate transcription, and posttranslational acetylation of the core histones likely plays a central role in this process (22, 24, 30, 35, 40). For example, heterochromatin within eukaryotic genomes typically contains hypoacetylated histones, while transcriptionally active regions of the genome are typically hyperacetylated due to the recruitment of histone acetyltransferases (22, 29, 35). Acetylation of the core histone tail domains in conjunction with gene activation affects chromatin structure via direct and indirect mechanisms; acetylation may function as an epigenetic mark recognized by specific secondary factors (3, 19, 31) or can directly affect chromatin structure, likely by altering histone tail interactions with both DNA and protein targets (30, 42).
Many features of chromatin structure can be recapitulated in vitro with reconstituted nucleosome arrays containing only DNA and the four core histones (13, 14). Reconstituted nucleosome arrays exhibit salt-dependent folding into secondary chromatin structures resembling the 30-nm chromatin fiber and self-association into large oligomerized complexes that mimic essential elements of higher-order tertiary-structure formation in native chromatin (2, 13, 27). The formation of these tertiary structures likely involves long-range fiber-fiber interactions mediated by the histone tail domains (18, 26, 27). Nucleosome arrays lacking all core histone tail domains do not oligomerize (26), and arrays lacking various combinations of the core histone tail domains require higher Mg2+ concentrations to oligomerize than do the wild-type arrays, indicating that all four core histone N-terminal tails contribute to array oligomerization, with the H3 and H4 tails individually providing the largest contribution to oligomerization (11, 33).
Each core histone N-terminal tail domain contains multiple highly conserved lysine residues, a subset of which are acetylated in vivo (29, 36). Importantly, acetylation of the core histones drastically reduces the propensity of nucleosome arrays to undergo salt-dependent self-association into tertiary structures, suggesting that acetylation at least partially abrogates tail function (34). In order to assess the effects of acetylation of individual tail domains on array oligomerization, we mutated all acetylatable lysines to glutamines in each core histone tail domain independently or in combination. Glutamine is an effective mimic of acetylated lysine due to the similarity of charge and chemical structure. Indeed, glutamine can function as acetylated lysine in vivo. For example, acetylation of p53 strongly inhibits its ubiquitination mediated by Mdm2, an effect that can be mimicked by lysine-to-glutamine substitution (20). With regard to core histones, it has been shown that the repression of many genes affected by the overexpressed deacetylase Rpd3p can be overcome by glutamine substitution for lysine at sites of acetylation in either the H3 or the H4 N-terminal tail domains (7, 25). We reconstituted nucleosome arrays using all combinations of wild-type histones and mutant core histones containing K→Q substitutions as mimics of acetylation and assessed the abilities of the arrays to undergo MgCl2-dependent self-association. Our results indicate that acetylation of the H2B or H4 tail domain results in the largest abrogation of tertiary-structure formation and suggest that acetylation has effects beyond simple elimination of the charge associated with lysine residues.
MATERIALS AND METHODS
Xenopus core histone purification.
Coding sequences for specific lysine-to-glutamine substitution mutants of the Xenopus core histones H2A, H2B, H3, and H4 were obtained based on the wild-type core histone sequences using the Stratagene QuikChange site-directed mutagenesis kit. Escherichia coli BL21(DE3) cells were transformed with the pET expression plasmids harboring the different wild-type core histones and glutamine mutants and cultured separately. H2A/H2B dimers and H3/H4 tetramers were purified as described previously (16, 32) with minor modifications. Briefly, bacteria were pelleted after expression and then resuspended in 10 mM EDTA, 0.5% Triton X-100, and 150 μg/ml lysozyme. The mixture was kept on ice for 30 min and was then acidified to a final concentration of 0.4 M HCl. After an additional incubation for 30 min, the lysate was centrifuged at 13,000 rpm for 20 min. Supernatants contained H2A, H2B, and H3 after the centrifugation, while most of the H4 was found in the pellet at this stage. H4 was further purified as described previously (32). Partially purified H3 and H4 were mixed in a 1:1 molar ratio and then dialyzed into 10 mM Tris, pH 8.0, and 2.0 M NaCl. The NaCl concentration of the mixture was adjusted to 0.5 M, and the tetramer was purified using Bio-Rex 70 ion-exchange resin as described previously (32), except that 0.6 M NaCl in 10 mM Tris, pH 8.0, 0.25 mM EDTA (TE) was used for the wash step prior to the elution of the tetramer. H2A and H2B supernatants were mixed together and purified as described previously (16). The quality of dimers and tetramers was analyzed by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and concentrations were determined using an A230 of 4.2 for 1 mg/ml (6).
208-12, 207-12, and 177-12 DNA purification.
Plasmids containing 208-12, 207-12, or 177-12 DNA sequences were transformed into E. coli DH5α cells. The cells were grown to stationary phase, and plasmid DNA was purified using the Qiagen Giga plasmid purification kit. Array template DNAs were obtained as described previously (5), except that 208-12 and 177-12 DNA plasmids were digested overnight at 37°C with HhaI and EcoRV, respectively, using 0.1 U enzyme/μg of DNA. Plasmids bearing the 207-12 template were digested with HindIII, XbaI, and DraI in the same manner.
Nucleosome array reconstitution.
Saturated and subsaturated nucleosome arrays were reconstituted with 12-mer DNA templates and core histones by standard salt dialysis methods (15), and all dialysis buffers contained TE, 10 mM fresh β-mercaptoethanol, and decreasing concentrations of NaCl as follows: 1 M for 4 h, 0.75 M for 4 h, and no salt for 4 h. The final dialysis was carried out overnight against TE buffer containing 10 mM β-mercaptoethanol. Reconstituted nucleosome arrays were stored at 4°C.
Restriction enzyme digestion analysis.
Levels of array saturation were analyzed by cutting reconstituted nucleosome arrays with the appropriate restriction enzymes with cleavage sites in the predicted linker regions between nucleosomes. 208-12 arrays were cut with EcoRI as previously described (5). 207-12 and 177-12 arrays were treated with EcoRV and ScaI. The number of nucleosomes per template was determined according to the method of Carruthers et al. (5).
Micrococcal-nuclease digestion.
Nucleosome arrays (200 μl; 80 μg/ml DNA) in 10 mM Tris, pH 8.0, and 0.25 mM EDTA were digested by mixing them with equal volumes of reaction solution containing 10 mM Tris, pH 8.0, 20 U/ml of micrococcal nuclease, and 0.5 mM CaCl2 at room temperature. The digestions were stopped at different times by removing 30 μl of reaction mixture and then adding 10 μl of stopping solution (50 mM EDTA, pH 8.0, and 0.4% SDS). Samples (8 μl) were run on a 1.8% agarose gel and were stained with ethidium bromide.
In vitro acetylation of nucleosome arrays.
Components of the Piccolo v55 histone acetyltransferase (HAT) complex were coexpressed from the vector pST44-HISNyEpl1t3x3-Yng2t1x3-yEsa1x3 (a generous gift from Song Tan). The complex was purified with Ni-nitrilotriacetic acid resin, and the elution was stored at −20°C. Arrays were acetylated in vitro following the method described previously (12, 28). One-milliliter (∼1 nmol H4) reconstituted nucleosome arrays were mixed with 20 μl purified Piccolo v55 HAT complex and ∼10 nmol acetyl-coenzyme A (CoA) in HAT buffer (25 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 10 mM sodium butyrate) at 30°C for 30 min. The reaction mixtures were supplemented one or two times by adding the same amounts of fresh Piccolo v55 HAT complex and acetyl-CoA. Acetylated histones were precipitated with trichloroacetic acid, and the extent of modification was determined with Triton-acid-urea (TAU) gels, followed by staining with Coomassie blue.
Self-association assays.
Mg2+-dependent self-association assays were performed as described previously (26, 27). The DNA concentration in the original nucleosome arrays was about 80 μg/ml. Seventy-microliter nucleosome arrays were mixed with equal volumes of salt solution containing various concentrations of MgCl2 in 10 mM Tris, pH 8.0, 0.25 mM EDTA and then incubated at room temperature for 10 min. The mixture was centrifuged at 16,000 × g for 10 min, and the DNA concentration in the supernatant was determined by measurement of absorbance at 260 nm. The final data were expressed as a percentage of the A260 value in the supernatant versus the A260 in the mixture without MgCl2. Mg50 values (the points at which 50% of the nucleosome arrays were self-associated) were taken directly from plots of the data (Fig. 1B).
FIG. 1.
Reconstitution of saturated and subsaturated nucleosome arrays. (A) 208-12 nucleosome arrays were reconstituted at histone octamer-to-DNA repeat ratios from 0.9 to 1.6, as indicated in the table (right), and then digested with EcoRI, and the reaction products were run on a 0.7% native agarose gel. The gel was stained with ethidium bromide, and the percentage of the sample migrating as naked DNA (bottom band) was determined. The corresponding level of saturation (nucleosomes per DNA repeat) was calculated (see Materials and Methods) (5). Blank entries in the table indicate oversaturation. (B) MgCl2-dependent self-association of 208-12, 207-12, and 177-12 arrays reconstituted with core histone octamer-to-DNA repeat ratios of 0.9 and 1.2. The fraction of samples remaining in solution versus the MgCl2 concentration was determined as described in Materials and Methods and plotted. The data points are based on an n of ≥3; the error bars represent ±1 standard deviation. (C) 208-12 nucleosome arrays reconstituted with 16 combinations of wild-type and mutant core histones were analyzed by SDS-PAGE (top) and EcoRI digestion analysis (bottom). Note that the K→Q substitutions within the histones alter the mobilities of the proteins. The numbers under the lanes correspond to reconstitution sets described in Table 1. The locations of free DNA repeats (D), mononucleosomes (M), and products larger than mononucleosomes (>M) are indicated.
RESULTS
We wished to determine the role of acetylation of specific histone tail domains in modulating the stability of tertiary chromatin structures by using reconstituted model nucleosome arrays. To control for the effects of the extent of array saturation, nucleosome repeat length, and DNA sequence, we assembled arrays with two different amounts of core histones and three different DNA templates: a 12-mer repeat of a 208-bp 5S rRNA gene fragment (208-12) and two templates comprised of 12 tandem repeats of 207- and 177-bp DNA fragments containing the high-affinity 601 nucleosome-positioning sequence (207-12 and 177-12) (21, 30). Levels of template saturation were determined using a restriction enzyme cleavage assay (5). Consistent with previous results (5), we found that input histone octamer-to-DNA repeat ratios of 1.2:1 and 0.9:1 corresponded to ∼100% and ∼80% saturation of 208-12 arrays, respectively (Fig. 1A). For example, the EcoRI digestion results (Fig. 1A) showed that ∼5.5% of the 208-12 DNA repeats appeared as naked DNA after digestion of arrays reconstituted at a histone octamer-to-DNA repeat ratio of 1.2:1, while digestion of arrays reconstituted at a ratio of 0.9:1 showed a percentage of naked DNA (18.7%) that corresponded to about 10 octamers per template (5).
Self-association assays with the reconstituted 208-12 arrays also indicated levels of saturation similar to that determined by EcoRI digestion. Self-association of arrays reconstituted at a ratio of 1.2:1 occurred in >2 mM MgCl2, forming large complexes that rapidly sedimented in the microcentrifuge assay with 50% of the arrays self-associated (Mg50) at 2.8 mM MgCl2, indicative of saturated arrays (27) (Fig. 1B). In contrast, 208-12 arrays reconstituted at a ratio of 0.9:1 oligomerized in >3 mM MgCl2, were completely condensed at 10 mM MgCl2, and exhibited an Mg50 value of 4.52 mM MgCl2, indicative of a subsaturated array (27). Note that although subsaturated arrays do not fold efficiently in MgCl2, such arrays do undergo self-association, albeit at higher MgCl2 concentrations (13). Substitution of the 207-12 or 177-12 template for the 208-12 template did not significantly alter the levels of nucleosome saturation, as indicated by restriction enzyme digestion analyses (results not shown). However, the 207-12 and 177-12 arrays apparently undergo self-association at slightly lower Mg2+ concentrations (Fig. 1B), perhaps due to the higher affinity of the 601 sequence for histones (21).
To examine the roles of specific histone tail domains in regulating chromatin higher-order structure, we prepared nucleosome arrays containing 16 different combinations of wild-type core histones and core histones containing specific K→Q mutations in their tail domains (Table 1). (Histones containing K→Q substitutions will hereafter be referred to as aH2A, aH2B, aH3, and aH4). Arrays were reconstituted to either 100% or 80% saturation, and the histone compositions of the saturated and subsaturated arrays were confirmed by SDS-PAGE (Fig. 1C and results not shown). Note that the K→Q substitutions within the tail domains slightly altered the mobilities of proteins compared to the wild-type histones in SDS-PAGE (Fig. 1C). In addition, the levels of saturation of all reconstituted arrays were confirmed by restriction enzyme digestion analysis (Fig. 1C, bottom, and results not shown).
TABLE 1.
Mg50 values for nucleosome arrays containing all combinations of wild-type core histones and core histones bearing K→Q mutations as mimics of acetylationa
| Array set | Histone(s) used in each set
|
Total no. of charges neutralized | Mg50 value (mM)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 208-12
|
177-12
|
207-12
|
||||||||
| H2A | H2B | H3 | H4 | 0.9 | 1.2 | 0.9 | 1.2 | 0.9 | ||
| 1 | 0 | 4.52 ± 0.22 | 2.78 ± 0.10 | 3.70 ± 0.14 | 2.38 ± 0.12 | 3.28 ± 0.02 | ||||
| 2 | X | 2 | 4.63 ± 0.08 | 2.72 ± 0.05 | 3.01 ± 0.02 | 1.74 ± 0.02 | 3.38 ± 0.03 | |||
| 3 | X | 6 | 7.28 ± 0.33 | 4.53 ± 0.06 | 8.64 ± 0.07 | 4.04 ± 0.06 | 5.45 ± 0.05 | |||
| 4 | X | 6 | 5.63 ± 0.17 | 3.36 ± 0.01 | 4.41 ± 0.06 | 3.05 ± 0.05 | 4.65 ± 0.05 | |||
| 5 | X | 4 | 8.08 ± 0.32 | 4.45 ± 0.06 | 6.23 ± 0.06 | 4.14 ± 0.11 | 6.47 ± 0.06 | |||
| 6 | X | X | 8 | 8.12 ± 0.34 | 5.80 ± 0.02 | 6.56 ± 0.06 | 3.93 ± 0.04 | 5.71 ± 0.07 | ||
| 7 | X | X | 10 | 10.82 ± 0.79 | 5.16 ± 0.08 | 8.21 ± 0.14 | 4.63 ± 0.05 | 9.34 ± 0.10 | ||
| 8 | X | X | X | X | 18 | 11.50 ± 0.04 | 16.42 ± 0.72 | |||
| 9 | X | X | X | 16 | 9.58 ± 0.05 | |||||
| 10 | X | X | X | 12 | 9.45 ± 0.54 | 4.54 ± 0.10 | 6.42 ± 0.09 | 3.82 ± 0.02 | 9.36 ± 0.29 | |
| 11 | X | X | X | 12 | 19.85 ± 1.76 | 9.15 ± 0.15 | 17.04 ± 0.29 | 11.23 ± 0.40 | 16.50 ± 0.39 | |
| 12 | X | X | X | 14 | 12.93 ± 1.34 | 6.38 ± 0.04 | 9.00 ± 0.15 | 6.31 ± 0.09 | 9.65 ± 0.13 | |
| 13 | X | X | 8 | 5.71 ± 0.14 | 3.30 ± 0.05 | 3.76 ± 0.09 | 2.64 ± 0.03 | 4.87 ± 0.06 | ||
| 14 | X | X | 6 | 7.21 ± 0.30 | 3.88 ± 0.08 | 5.04 ± 0.13 | 3.15 ± 0.55 | 6.55 ± 0.10 | ||
| 15 | X | X | 12 | 10.38 ± 0.65 | 5.89 ± 0.07 | 16.20 ± 0.34 | 6.95 ± 0.13 | 8.60 ± 0.09 | ||
| 16 | X | X | 10 | 19.20 ± 0.93 | 8.93 ± 0.25 | 17.00 ± 0.28 | ||||
Numbers in the first column indicate sets of proteins used for reconstitution. The sets contain wild-type proteins (blank spaces) and mutants (X) as indicated. Lysine-to-glutamine substitutions are at lysines 5 and 9 for aH2A; 5, 12, 15, 20, 24, and 27 for aH2B; 4, 9, 14, 18, 23, and 27 for aH3; and 5, 8, 12, and 16 for aH4. Also indicated are the total numbers of charges neutralized in each set.
Mg50 values are expressed ±1 standard deviation; n ≥ 3. Empty cells indicate failure of the array to exhibit self-association.
To determine if the accessibility of nucleosome DNA was affected by the modifications within the core histone tail domains, we examined the micrococcal nuclease digestion kinetics of nucleosome arrays assembled onto the three different DNA templates under conditions (0.125 mM free CaCl2) not expected to induce folding or self-association of these subsaturated arrays (10, 13) (Fig. 2). At the initial stages of the digestion of arrays containing wild-type histones, a ladder of bands was observed, each a multiple of the expected nucleosome repeat, as the linker DNA is readily accessed by micrococcal nuclease (Fig. 2A, G, and M, lanes 1 to 3). After further digestion, a band at ∼168 bp appeared (lanes 6 to 8), indicating that ∼20 bp of linker DNA is protected, possibly because of the interactions of the N-terminal histone tails within each nucleosome (1, 36, 41). Extensive digestion (lanes 9 to 10) produced mainly a 147-bp band that corresponds to the length of DNA in nucleosome core particles (36). Smaller bands, 128 bp and 104 bp, are also present, representing previously characterized subnucleosome digestion products (10, 36, 37) (Fig. 2A). Control experiments showed that the naked DNA templates were much more rapidly digested than the nucleosome arrays, with only DNA fragments less than 100 bp in length remaining after 4 min of digestion (not shown).
FIG. 2.
Analysis of nucleosome arrays assembled with various combinations of wild-type (WT) and mutant core histones by micrococcal-nuclease digestion. Identical amounts of 177-12 (A to F), 207-12 (G to L), or 208-12 (M to R) subsaturated nucleosome arrays were digested by micrococcal nuclease in the presence of 0.125 mM CaCl2 for 0.5, 1, 2, 4, 8, 15, 30, 60, 120, and 180 min (lanes 1 to 10). The reactions were stopped by the addition of EDTA and SDS, and the products were separated on 1.8% agarose gels. The arrays contain the indicated mutant histones with otherwise-wild-type proteins; aOctamer indicates arrays containing aH2A/aH2B/aH3/aH4. M1 is a 1-kb-plus DNA ladder; M2 is MspI-digested pBR322 DNA. The sizes of selected marker bands are indicated.
Most of the nucleosome arrays containing various combinations of mutant and wild-type histone tails (sets 1 to 16 [Table 1]) exhibited digestion profiles similar to those of the wild-type nucleosome arrays throughout the time course (Fig. 2 and results not shown). However, some interesting differences were observed for all 207-12 and 177-12 arrays containing aH3 at the longest time in the digestions. Extended digestion of these arrays yielded a main band of 128 bp instead of 147 bp (Fig. 2D and J, lanes 8 to 10, and results not shown), indicating that inclusion of aH3 in these arrays resulted in a decrease in the stability of wrapping DNA near the edge of the nucleosome core region (36, 41). Moreover, inclusion of aH2A, aH2B, or aH4 did not alter the protection of the nucleosome DNA afforded by the H3 tail domain (Fig. 2B, C, E, H, I, and K), although a 104-bp product became more prominent after digestion of 207-12 and 177-12 arrays containing completely “acetylated” histones (Fig. 2F and L). In contrast, although 208-12 arrays containing completely “acetylated” histones also exhibited more internal cleavage (Fig. 2R), inclusion of aH3 alone within 208-12 arrays did not lead to more internal cleavage by micrococcal nuclease (Fig. 2P), suggesting that the H3-dependent protection can be masked by a more micrococcal-nuclease-resistant sequence at the edge of the core region within the 5S-based arrays than in the 601-based arrays. In general, our micrococcal-nuclease digestion results suggest that more intranucleosome access can be caused by H3 tail acetylation than by acetylation of other tails. These results are consistent with a recent analysis of the effects of tail deletions on DNA wrapping within nucleosomes (8) (see Discussion).
We next performed self-association assays with the arrays reconstituted with the 16 combinations of wild-type and mutant histones (Table 1). The fractions of the samples remaining unoligomerized were plotted versus MgCl2 concentrations for each array, and the Mg50 values were determined, indicating the point at which 50% of the nucleosome arrays were self-associated. These values are listed in Table 1 for saturated and subsaturated 208-12 and 177-12 arrays and subsaturated 207-12 arrays.
Figure 3 shows the self-association curves for arrays containing a single histone tail mutant (sets 2 to 5 in Table 1) for subsaturated and saturated 208-12 and 177-12 arrays and subsaturated 207-12 arrays. A comparison of the self-association curves and the Mg50 values for the 208-12 arrays (Fig. 3A and B and Table 1) indicates that arrays containing aH2B or aH4 exhibit the largest increase in Mg50 compared to all other saturated or subsaturated 208-12 arrays (Fig. 3A and B). Interestingly, self-association data for the subsaturated 177-12 arrays, which contain shorter linker DNA and a higher-affinity nucleosome-positioning sequence, indicate that arrays containing aH2B required higher MgCl2 concentrations for self-association than aH4 arrays (Fig. 3C) while saturated 177-12 arrays exhibited self-association characteristics similar to those of the 208-12 arrays. A comparison of profiles for the 207-12 arrays, based on the 601 positioning sequence, indicated again that aH2B and aH4 cause the largest abrogation in the ability of the arrays to undergo MgCl2-dependent self-association, suggesting that the greater effect of aH2B on the subsaturated 177-12 arrays is due to the shorter nucleosome repeat length. In summary, these data indicated that K→Q mutations mimicking acetylation within the H2B and H4 tails causes the most drastic reduction in the propensity of all arrays to undergo self-association.
FIG. 3.
Mutations mimicking acetylation within the H2B and H4 tail domains cause the largest reduction in propensity for array self-association. Self-association assays were performed as for Fig. 1B with nucleosome arrays containing wild-type histones (WT) and with a single K→Q substitution mutant. (A and B) Subsaturated and saturated 208-12 arrays. (C and D) Subsaturated and saturated 177-12 arrays. (E) Subsaturated 207-12 arrays. A, B, 3, and 4 indicate the presence of aH2A, aH2B, aH3, and aH4, respectively, in arrays with otherwise-wild-type histones. The data points are based on an n of ≥3; the error bars represent ±1 standard deviation.
The effects of acetylation mimics within either the H2B or H4 tail domains can also be observed in the midst of K→Q substitutions within other tail domains. For example, aH2A/aH2B arrays exhibited higher Mg50 values than aH2A/aH3 arrays (sets 6 and 13 in Table 1), and higher Mg50 values were observed for aH2A/aH2B/aH4 arrays than for aH2A/aH2B/aH3 arrays (sets 11 and 12 in Table 1). Moreover, the presence of both aH2B and aH4 in the same array (sets 8, 9, 11, and 16 in Table 1) resulted in either the largest observed Mg50 values or a loss of the ability of the arrays to undergo oligomerization altogether. For example, arrays containing both of these mutants and wild-type H2A and H3 (set 16 in Table 1) exhibited Mg50 values of 8.9 mM, 19.2 mM, and 17.0 mM for saturated and subsaturated 208-12 arrays and subsaturated 207-12 arrays, respectively, the highest Mg50 values for arrays including any two mutant proteins. Moreover, both subsaturated and saturated 177-12 arrays containing these proteins were unable to oligomerize at any MgCl2 concentration tested (Fig. 4A). Addition of aH3 to the aH2B/aH4 arrays (set 9 in Table 1) resulted in a loss of observable oligomerization for subsaturated 208-12 and 207-12 arrays (Fig. 4A and results not shown) and an increase of ∼0.7 mM in the Mg50 for saturated 208-12 arrays (Table 1). Addition of aH2A to the aH2B/aH3/aH4 arrays to generate completely “acetylated” saturated 208-12 arrays (set 8 in Table 1) resulted in an increase of ∼1.9 mM in the Mg50 value to 11.5 mM (Table 1). In only a few cases did we find that the effect of multiple tails bearing acetylation mimics on array self-association was nucleosome saturation dependent. For example, subsaturated 208-12 aH3/aH4 arrays (set 7 in Table 1) oligomerized at a higher MgCl2 concentration than aH2A/aH2B arrays, while saturated 208-12 arrays containing these histone sets exhibited similar self-association curves (Fig. 4B). However, in most cases, the relative ability to undergo self-association was not dependent upon nucleosome saturation.
FIG. 4.
MgCl2-dependent self-association profiles for selected nucleosome arrays containing combinations of histone tail mutants mimicking acetylation. Self-association assays were performed as for Fig. 1B. (A) Self-association curves for subsaturated 208-12 or 177-12 arrays containing all wild-type histones (WT) or various combinations of aH2A, aH2B, aH3, and aH4 (A, B, 3, and 4, respectively) mutants, as indicated. (B) Self-association curves for arrays containing aH2A/aH2B dimers or aH3/aH4 tetramers (AB and 34, respectively) within 208-12 arrays, as indicated. The data for saturated 208-12 arrays are depicted by blue lines; data for subsaturated arrays are in red. The data points are based on an n of ≥3; the error bars represent ±1 standard deviation.
Interestingly, a comparison of the self-association of arrays containing and lacking aH2A suggests that acetylation of the H2A tail causes context-dependent changes in the propensity for self-association, in contrast to the other tail domains (Fig. 5 and data not shown). For example, unlike aH2B, aH3, and aH4, the presence of aH2A within a 208-12 array containing otherwise-wild-type histones had the same Mg50 value as that of completely native arrays (Fig. 3A and B and 5A). However, the same substitution within either subsaturated or saturated 208-12 arrays containing aH2B resulted in an increase in the Mg50 value of 0.84 mM or 1.3 mM, respectively (Fig. 5A and Table 1). Conversely, the addition of aH2A to either subsaturated or saturated 208-12 aH4 arrays resulted in a drop in the Mg50 value of 0.87 mM or 0.57 mM, respectively (Fig. 5A and Table 1). The presence of aH2A did not affect the Mg50 values of the aH3 arrays (Fig. 5A). Finally, the opposing effects of addition of aH2A to either the aH2B- or aH4-containing 208-12 arrays apparently canceled out when aH2A was added to the aH2B/aH4 arrays (compare sets 11 and 16 in Table 1). Similar results were obtained with the 207-12 arrays (Fig. 5C and Table 1).
FIG. 5.
The effects of aH2A on the propensity for array oligomerization are context dependent. The change in Mg50 values after virtual addition of aH2A (A, C, and E) or aH2B (B, D, and F) to arrays containing the mutant histones listed at the top of each graph (80% saturation) was calculated and plotted. Bars with an up or down arrow indicate that the resultant or original array, respectively, does not oligomerize. (A and B) 208-12 arrays. (C and D) 207-12 arrays. (E and F) 177-12 arrays. The error bars represent one standard deviation.
In contrast to the 208-12 and 207-12 arrays, addition of aH2A to 177-12 arrays containing any combinations of mutant or wild-type histones resulted in a reduction in the Mg50 value and thus a greater propensity for self-association, regardless of the level of nucleosome saturation (Fig. 5E and Table 1). For example, substitution of aH2A for wild-type H2A in subsaturated 177-12 arrays containing aH2B/aH3 caused a marked reduction in the Mg50 value from 16.2 mM to 9.0 mM (sets 12 and 15 in Table 1 and Fig. 5E). Likewise, saturated 177-12 arrays containing aH2B, aH3, and aH4 were unable to oligomerize; however, the addition of aH2A to these arrays generated a completely “acetylated” array that oligomerized at 16.4 mM MgCl2 (Table 1, sets 8 and 9). On the other hand, substitution of aH2B, aH3, or aH4 for wild-type H2B, H3, or H4, respectively, increased the Mg50 value for all nucleosome arrays we examined (Table 1 and Fig. 5B, D, and F).
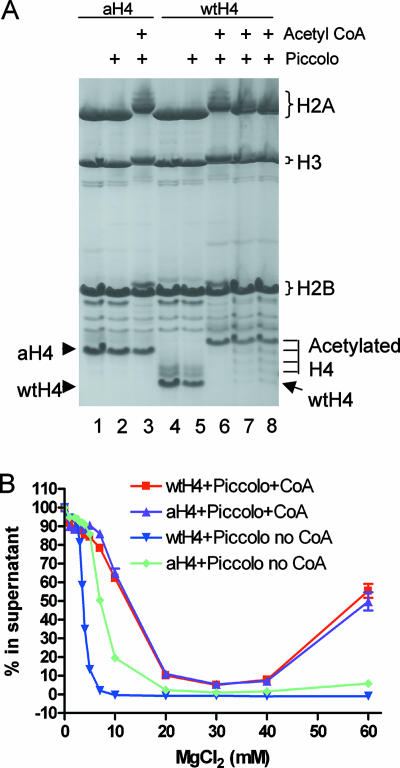
The effects of the K→Q mutations modeling acetylation on nucleosome array self-association observed here raise the question of whether these mutations accurately mimic actual lysine acetylation with regard to self-association. To address this issue, we prepared the Piccolo v55 HAT complex, which can tetra-acetylate the H4 tail domain at positions 5, 8, 12, and 16 in nucleosomes in an acetyl-CoA-dependent fashion (4, 28). We thus compared the effects of four K→Q substitutions within the H4 tail and H4 tetra-acetylated by the Piccolo v55 HAT complex on nucleosome array self-association. Nucleosome arrays containing wild-type H4 or aH4 were incubated with the Piccolo v55 HAT complex in the presence or absence of acetyl-CoA. Histone acetylation was monitored by running samples on TAU gels (Fig. 6A). Wild-type H4 was completely tetra-acetylated within the arrays, while a single acetylation event occurred on H2A and H3. A fraction of H2B (less than 10%) was also monoacetylated. Importantly, treatment of arrays containing aH4 resulted in no change in the acetylation status of the protein while identical extents of acetylation were observed for the other three core histones (Fig. 6A). The arrays containing all wild-type histones or aH4 were treated with the Piccolo v55 HAT complex and then purified by salt precipitation, resuspended in TE buffer, and subjected to self-association assays. The analysis shows that both arrays exhibited identical self-association curves (Fig. 6B), indicating that the H4 K→Q mutations modeling acetylation had exactly the same effect on nucleosome array self-association as tetra-acetylated H4. Interestingly, the Mg50 values for both acetylated arrays were increased compared to the self-association curves of unacetylated nucleosome arrays containing aH4, likely due to the partial acetylation of the other three core histones.
FIG. 6.
Glutamine is an effective mimic of acetylated lysine with regard to array self-association. Nucleosome arrays reconstituted with 208-12 DNA and wild-type (wt) or aH4 at a histone octamer-to-DNA repeat ratio of 0.9 were acetylated with the Piccolo v55 HAT complex for 30 min. (A) A TAU gel shows the extent of core histone acetylation in nucleosome arrays. Lanes 1 to 3 and 4 to 6 show arrays containing aH4 or wild-type H4, respectively, lanes 1 and 3 show arrays only, lanes 2 and 4 show arrays plus Piccolo complex, and lanes 3 and 6 show arrays plus Piccolo complex plus acetyl-CoA. Lanes 7 and 8 are identical to lane 6, except that the reaction time was 20 or 10 min, respectively. (B) Self-association assays with wild-type or aH4 arrays treated with Piccolo in the presence or absence of acetyl-CoA, as indicated. The data points are based on an n of ≥3; the error bars represent ±1 standard deviation.
DISCUSSION
Posttranslational acetylation in the core histone tail domains has multiple functions within eukaryotic chromatin. However, the mechanisms by which acetylation directly alters higher-order chromatin structure remain to be elucidated. Here, we determined the effects of mutations mimicking acetylation within individual core histone tail domains on the self-association of nucleosome arrays, a process that recapitulates essential interactions involved in the formation of higher-order tertiary chromatin structures (13). Glutamine resembles acetylated lysine in chemical structure and charge and can functionally replace this modification in vivo. For example, glutamine substitutions within the H4 tail function as acetylated H4 to prevent Sir3p binding and spreading to form heterochromatin (17). Moreover, in Saccharomyces cerevisiae, an H3-K56Q mutation partially suppresses the loss of acetylation of K56 in asf1 cells (23). Importantly, we provide evidence that the effect of acetylation within the histone H4 tail domain on array oligomerization can be effectively recapitulated by lysine-to-glutamine substitutions (Fig. 6B). These results support the idea that glutamine substitutions can provide insights into the role of acetylation in regulating higher-order chromatin structures.
Our results in general confirm and extend previous experiments examining the effects of deletion of specific tail domains on array self-association (11, 33) and show that acetylation of most of the histone tails decreases the propensity of arrays to undergo divalent-cation-dependent oligomerization in a manner similar to tail deletion. However, we observed several interesting differences between the effects of tail deletion and acetylation. Whereas tail deletion indicates that the H3 and H4 tail domains make the largest contributions to array oligomerization, our data indicate that acetylation of the H2B and H4 tails causes the greatest abrogation of nucleosome array self-association. Thus, the contribution of the H3 tail domain does not appear to be particularly sensitive to acetylation while acetylation of the H2B tail has a much larger negative impact on array self-association than would be expected based on deletion of this tail domain (11). Indeed, in general, compared to tail deletion (11), we found that nucleosome arrays containing the same DNA sequence (208-12 DNA), the same recombinant core histones, and the same ratio of histone octamer to 208 DNA repeat (0.9) have higher Mg50 values, with the exception of aH2A/aH4 arrays. As discussed below, this reduction is likely due to the negative effect of the aH2A tail on self-association. For example, the Mg50 value is about 7.7 mM for 208-12 arrays lacking the H2B/H4 tails (11), while the Mg50 value is 19.2 mM for aH2B/aH4 208-12 arrays (set 16 in Table 1), suggesting that tail deletions mimic some but not all properties of histone tail acetylation. One possibility is that acetylation alters the conformation of the histone tail domains in the nucleosome arrays, as indicated by spectroscopic investigations (39), so that acetylated tails may have a distinct negative effect on nucleosome array oligomerization.
Likewise, while in general we found that the more positive charges that were neutralized by K→Q mutations modeling acetylation the higher the Mg50 value that was required for array self-association, our data indicate that the effect of acetylation of individual tails on nucleosome array self-association is not simply charge dependent. For example, arrays corresponding to sets 10, 11, and 15 in Table 1 have the same extent of positive charge neutralization (12 charges neutralized); however, the Mg50 values of these three arrays exhibited wide variation, with aH2A/aH2B/aH4 arrays having Mg50 values two- to threefold higher than those of aH2A/aH3/aH4 arrays (sets 10 and 11 in Table 1). Furthermore, the Mg50 value is 8.12 mM for aH2A/aH2B arrays with eight combined K→Q mutations in subsaturated 208-12 arrays. However, adding six or four K→Q substitutions by inclusion of aH3 or aH4 increased the Mg50 value to 12.93 mM or 19.85 mM (sets 6, 11, and 12 in Table 1). Thus, the additional six or four neutralized positive charges increased the Mg50 value by 4.8 mM or 11.7 mM, respectively. Indeed, in all cases, addition of aH3 (six charges neutralized) did not increase the Mg50 value as much as addition of aH2B (six charges neutralized) or aH4 (four charges neutralized). Moreover, deletion of the H3 and H4 tails resulted in the loss of the positive charges of lysines, and also arginines. However, 208-12 arrays lacking H3 and H4 tails exhibited lower Mg50 values (∼9 mM) for self-association (11) despite the greater loss of positive charge resulting from tail deletion compared to arrays containing aH3/aH4 (10.82 mM). These data indicate that charge neutralization is not the only effect of core histone tail acetylation and that an additional effect(s) must be operative within arrays containing acetylated tail domains. As suggested above, acetylation may alter tail conformation or otherwise compel the tail to serve as an obstacle to nucleosome array oligomerization.
Our results also suggest that the stability of DNA association at the edge of the nucleosome core region is weakened by acetylation of the H3 tail domain and that this effect is distinct from acetylation within the other three histone tail domains. Combined with the observation that acetylation of H3 does not have a relatively large effect on self-association, our data suggest that acetylation of this domain may primarily regulate DNA accessibility at the nucleosome level. These data correlate with a previous micrococcal-nuclease digestion study of nucleosome arrays showing that removal of the tail domains by trypsin proteolysis allowed more trimming into nucleosome core DNA (10). Our results also agree with a recent analysis by Ferreira and colleagues (8) of the effect of the deletion of specific tail domains on the stability of DNA wrapping near the edge of the nucleosome core region. Indeed, these authors found that nucleosomes specifically lacking the H3 tail domain exhibited a significant reduction in the stability of DNA association at the edge of the core, perhaps due to perturbation of interactions with the adjacent N-terminal α-helix within H3, which makes critical contacts with DNA. These data are also consistent with earlier observations that deletion of the H3 tail increases binding of a transcription factor to nucleosome DNA (38). Thus, acetylation of the H3 tail domain may regulate access to DNA within nucleosomes in a manner similar to tail removal.
We also found that acetylation within the histone H2A tail exhibited a complex, context-dependent effect on nucleosome array oligomerization. The presence of aH2A either reduced or increased the propensity of arrays to undergo self-association, depending upon the acetylation states of the other tail domains. A possible explanation for this phenomenon is that histone H2A tail acetylation may exert a cooperative effect with the other core histone tail domains, perhaps involving acetylation-dependent histone-histone interactions. Indeed, careful analysis of divalent-cation-mediated array condensation and surface charge density led Fletcher and Hansen to conclude that tail-tail interactions occur during the folding of nucleosome arrays (9). Perhaps acetylation increases tail-tail interactions important for intra-array folding at the expense of interarray interactions important for self-association. Indeed, we previously showed that the H3 tail domain participates in both short- and long-range internucleosome interactions that are likely important for secondary- and tertiary-structure formation, respectively (18, 43). Moreover, our results indicate that in some cases the effects of H2A histone tail acetylation can be dependent on the nucleosome repeat length. As mentioned above, addition of aH2A to 177-12 arrays containing any combination of the other core histones resulted in a reduction in the Mg50 value, quite distinct from the 208-12 arrays (Table 2). Substitution of 177-12 DNA for the 208-12 DNA in the aH2B/aH4 arrays decreases interarray fiber-fiber interactions, to the extent that aH2B/aH4 arrays containing 177-12 DNA cannot oligomerize (set 16 in Table 1). However, the 207-12 arrays, which are based on the same nucleosome-positioning sequence as the 177-12 arrays, behave in general like the 208-12 arrays, indicating that the effect is due to the shorter repeat length in the 177-12 arrays. These observations were not dependent on the level of array saturation. It will be interesting to further investigate the mechanism of interaction between the H2A tail and other tails with respect to self-association and whether specific combinations of acetylated tail domains have special roles in chromatin decondensation.
Acknowledgments
We thank Song Tan for the Piccolo expression construct, Craig Peterson for the 177-12 array plasmid, and Jeff Hansen for the 207-12 array plasmid.
This work was supported by NIH grant GM52426.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Ausio, J., F. Dong, and K. E. van Holde. 1989. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone “tails” in the stabilization of the nucleosome. J. Mol. Biol. 206451-463. [DOI] [PubMed] [Google Scholar]
- 2.Belmont, A. S., and K. Bruce. 1994. Visualization of G1 chromosomes: a folded, twisted, supercoiled chromonema model of interphase chromatid structure. J. Cell Biol. 127287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, S. L. 2007. The complex language of chromatin regulation during transcription. Nature 447407-412. [DOI] [PubMed] [Google Scholar]
- 4.Berndsen, C. E., W. Selleck, S. J. McBryant, J. C. Hansen, S. Tan, and J. M. Denu. 2007. Nucleosome recognition by the Piccolo NuA4 histone acetyltransferase complex. Biochemistry 462091-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers, L. M., C. Tse, K. P. Walker III, and J. C. Hansen. 1999. Assembly of defined nucleosomal and chromatin arrays from pure components. Methods Enzymol. 30419-35. [DOI] [PubMed] [Google Scholar]
- 6.Chung, S. Y., W. E. Hill, and P. Doty. 1978. Characterization of the histone core complex. Proc. Natl. Acad. Sci. USA 751680-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Nadal, E., M. Zapater, P. M. Alepuz, L. Sumoy, G. Mas, and F. Posas. 2004. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427370-374. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira, H., J. Somers, R. Webster, A. Flaus, and T. Owen-Hughes. 2007. Histone tails and the H3 αN helix regulate nucleosome mobility and stability. Mol. Cell. Biol. 274037-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher, T. M., and J. C. Hansen. 1996. The nucleosomal array: structure/function relationships. Crit. Rev. Eukaryot. Gene Expr. 6149-188. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Ramirez, M., F. Dong, and J. Ausio. 1992. Role of the histone “tails” in the folding of oligonucleosomes depleted of histone H1. J. Biol. Chem. 26719587-19595. [PubMed] [Google Scholar]
- 11.Gordon, F., K. Luger, and J. C. Hansen. 2005. The core histone N-terminal tail domains function independently and additively during salt-dependent oligomerization of nucleosomal arrays. J. Biol. Chem. 28033701-33706. [DOI] [PubMed] [Google Scholar]
- 12.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 111640-1650. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, J. C. 2002. Conformational dynamics of the chromatin fiber in solution: determinants, mechanisms, and functions. Annu. Rev. Biophys. Biomol. Struct. 31361-392. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, J. C., J. Ausio, V. H. Stanik, and K. E. van Holde. 1989. Homogeneous reconstituted oligonucleosomes, evidence for salt-dependent folding in the absence of histone H1. Biochemistry 289129-9136. [DOI] [PubMed] [Google Scholar]
- 15.Hansen, J. C., and D. Lohr. 1993. Assembly and structural properties of subsaturated chromatin arrays. J. Biol. Chem. 2685840-5848. [PubMed] [Google Scholar]
- 16.Hayes, J. J., and K. M. Lee. 1997. In vitro reconstitution and analysis of mononucleosomes containing defined DNAs and proteins. Methods 122-9. [DOI] [PubMed] [Google Scholar]
- 17.Hecht, A., T. Laroche, S. Strahl-Bolsinger, S. M. Gasser, and M. Grunstein. 1995. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80583-592. [DOI] [PubMed] [Google Scholar]
- 18.Kan, P. Y., X. Lu, J. C. Hansen, and J. J. Hayes. 2007. The H3 tail domain participates in multiple interactions during folding and self-association of nucleosome arrays. Mol. Cell. Biol. 272084-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, K. K., and J. L. Workman. 2007. Histone acetyltransferase complexes: one size doesn't fit all. Nat. Rev. Mol. Cell Biol. 8284-295. [DOI] [PubMed] [Google Scholar]
- 20.Li, M., J. Luo, C. L. Brooks, and W. Gu. 2002. Acetylation of p53 inhibits its ubiquitination by Mdm2. J. Biol. Chem.y 27750607-50611. [DOI] [PubMed] [Google Scholar]
- 21.Lowary, P. T., and J. Widom. 1998. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 27619-42. [DOI] [PubMed] [Google Scholar]
- 22.Memedula, S., and A. S. Belmont. 2003. Sequential recruitment of HAT and SWI/SNF components to condensed chromatin by VP16. Curr. Biol. 13241-246. [DOI] [PubMed] [Google Scholar]
- 23.Recht, J., T. Tsubota, J. C. Tanny, R. L. Diaz, J. M. Berger, X. Zhang, B. A. Garcia, J. Shabanowitz, A. L. Burlingame, D. F. Hunt, P. D. Kaufman, and C. D. Allis. 2006. Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc. Natl. Acad. Sci. USA 1036988-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridsdale, J. A., M. J. Hendzel, G. P. Delcuve, and J. R. Davie. 1990. Histone acetylation alters the capacity of the H1 histones to condense transcriptionally active/competent chromatin. J. Biol. Chem. 2655150-5156. [PubMed] [Google Scholar]
- 25.Sabet, N., S. Volo, C. Yu, J. P. Madigan, and R. H. Morse. 2004. Genome-wide analysis of the relationship between transcriptional regulation by Rpd3p and the histone H3 and H4 amino termini in budding yeast. Mol. Cell. Biol. 248823-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz, P. M., A. Felthauser, T. M. Fletcher, and J. C. Hansen. 1996. Reversible oligonucleosome self-association: dependence on divalent cations and core histone tail domains. Biochemistry 354009-4015. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz, P. M., and J. C. Hansen. 1994. Formation and stability of higher order chromatin structures. Contributions of the histone octamer. J. Biol. Chem. 26916284-16289. [PubMed] [Google Scholar]
- 28.Selleck, W., I. Fortin, D. Sermwittayawong, J. Cote, and S. Tan. 2005. The Saccharomyces cerevisiae Piccolo NuA4 histone acetyltransferase complex requires the Enhancer of Polycomb A domain and chromodomain to acetylate nucleosomes. Mol. Cell. Biol. 255535-5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahbazian, M. D., and M. Grunstein. 2007. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 7675-100. [DOI] [PubMed] [Google Scholar]
- 30.Shogren-Knaak, M., H. Ishii, J. M. Sun, M. J. Pazin, J. R. Davie, and C. L. Peterson. 2006. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311844-847. [DOI] [PubMed] [Google Scholar]
- 31.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 40341-45. [DOI] [PubMed] [Google Scholar]
- 32.Thiriet, C. 2004. Analysis of chromatin assembled in vivo using exogenous histones in Physarum polycephalum. Methods 3386-92. [DOI] [PubMed] [Google Scholar]
- 33.Tse, C., and J. C. Hansen. 1997. Hybrid trypsinized nucleosomal arrays: identification of multiple functional roles of the H2A/H2B and H3/H4 N-termini in chromatin fiber compaction. Biochemistry 3611381-11388. [DOI] [PubMed] [Google Scholar]
- 34.Tse, C., T. Sera, A. P. Wolffe, and J. C. Hansen. 1998. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol. Cell. Biol. 184629-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumbar, T., G. Sudlow, and A. S. Belmont. 1999. Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J. Cell Biol. 1451341-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Holde, K. E. 1989. Chromatin. Springer Verlag, New York, NY.
- 37.Van Holde, K. E., J. R. Allen, K. Tatchell, W. O. Weischet, and D. Lohr. 1980. DNA-histone interactions in nucleosomes. Biophys. J. 32271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitolo, J. M., C. Thiriet, and J. J. Hayes. 2000. The H3-H4 N-terminal tail domains are the primary mediators of transcription factor IIIA access to 5S DNA within a nucleosome. Mol. Cell. Biol. 202167-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, X., S. C. Moore, M. Laszckzak, and J. Ausio. 2000. Acetylation increases the alpha-helical content of the histone tails of the nucleosome. J. Biol. Chem. 27535013-35020. [DOI] [PubMed] [Google Scholar]
- 40.Wegel, E., and P. Shaw. 2005. Gene activation and deactivation related changes in the three-dimensional structure of chromatin. Chromosoma 114331-337. [DOI] [PubMed] [Google Scholar]
- 41.Weischet, W. O., J. R. Allen, G. Riedel, and K. E. Van Holde. 1979. The effects of salt concentration and H-1 depletion on the digestion of calf thymus chromatin by micrococcal nuclease. Nucleic Acids Res. 61843-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng, C., and J. J. Hayes. 2003. Structures and interactions of the core histone tail domains. Biopolymers 68539-546. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, C., X. Lu, J. C. Hansen, and J. J. Hayes. 2005. Salt-dependent intra- and internucleosomal interactions of the H3 tail domain in a model oligonucleosomal array. J. Biol. Chem. 28033552-33557. [DOI] [PubMed] [Google Scholar]