Abstract
Binding immunoglobulin protein (BiP) is an endoplasmic reticulum (ER) molecular chaperone that is central to ER function. We examined knock-in mice expressing a mutant BiP in order to elucidate physiological processes that are sensitive to BiP functions during development and adulthood. The mutant BiP lacked the retrieval sequence that normally functions to return BiP to the ER from the secretory pathway. This allowed us to examine the effects of a defect in ER function without completely eliminating BiP function. The homozygous mutant BiP neonates died after birth due to respiratory failure. Besides that, the mutant BiP mice displayed disordered layer formation in the cerebral cortex and cerebellum, a neurological phenotype of reeler mutant-like malformation. Consistent with the phenotype, Cajal-Retzius (CR) cells did not secrete reelin, and the expression of reelin was markedly reduced posttranscriptionally. Furthermore, the reduction in the size of the whole brain and the apparent scattering of CR cells throughout the cortex, which were distinct from the reeler phenotype, were also seen. These findings suggest that the maturation and secretion of reelin in CR cells and other factors related to neural migration may be sensitive to aberrant ER quality control, which may cause various neurological disorders.
Proteins destined for the secretory pathway are inserted into the endoplasmic reticulum (ER) cotranslationally and subjected to quality control (12, 25). Aberrant protein folding due to extracellular stimuli such as ischemia, hypoxia, and genetic mutations results in the accumulation of misfolded proteins in the ER, which causes ER stress and initiates the unfolded protein response (UPR) (35, 39) that enhances the capacity for ER quality control by reducing general protein synthesis (18), producing ER chaperones, and promoting ER-associated degradation (4, 6). A failure of this adaptation mechanism may cause cellular dysfunction and cell death, resulting in diverse human disorders (24, 26) such as neurodegenerative disease (21, 23), cardiomyopathy (15), and diabetes (17, 34). Furthermore, mutant mouse models have revealed that the UPR plays a vital role during normal development by increasing protein synthesis, as necessary, of dedicated secretory cells (46) such as pancreatic beta cells (38), plasma cells (37), hepatocytes (36), and alveolar type II epithelial cells (29). Inadequate adaptation to these physiological demands may lead to diverse diseases.
ER molecular chaperones and folding enzymes such as binding immunoglobulin protein (BiP), calnexin, and protein disulfide isomerase facilitate the correct folding or degradation of these newly synthesized proteins as well as of misfolded proteins. BiP, also called the 78-kDa glucose-regulated protein (GRP78), is a member of the heat shock protein 70 (HSP70) family of proteins and is one of the most abundant ER chaperones, assisting in protein translocation, folding, and degradation (31). ER chaperones localize to the ER by two mechanisms: retention and retrieval (40). BiP is retained in the ER by interacting with other ER proteins and the ER matrix. When misfolded proteins accumulate in the ER, BiP is secreted from the ER together with the misfolded proteins, where it assists with protein refolding, or it helps in the degradation of these proteins (16, 47). In post-ER compartments, the carboxyl-terminal Lys-Asp-Glu-Leu (KDEL) sequence of BiP is then recognized by the KDEL receptor, which facilitates the return of BiP to the ER (27, 30).
The complete depletion of BiP has lethal effects on mammalian early embryonic cells (28). Saccharomyces cerevisiae BiP (Kar2p) is essential for survival, while the deletion of the retrieval sequence (His-Asp-Glu-Leu [HDEL] in yeast) is dispensable because the UPR is activated, and the loss of the chaperone in the ER is compensated for (3). Therefore, to elucidate physiological processes that are sensitive to BiP functions during development and adulthood in multicellular organisms, we produced knock-in mice expressing a mutant BiP in which the retrieval sequence was deleted by homologous recombination. The mutant BiP mice died within several hours after birth due to impaired pulmonary surfactant biosynthesis and respiratory failure (29). We also found disordered layer formation in the cerebral cortex and cerebellum in the mutant BiP neonates. Although altered quality control in the ER due to mutant BiP may affect the expression of several proteins with regard to corticogenesis, we found that the expression of one such protein, reelin, secreted by Cajal-Retzius (CR) cells (9), was markedly reduced. These findings suggest that committed secretory cells, such as CR cells, have a threshold of protein-folding capacity to cope with the normal physiological protein overload in the ER during development, and BiP plays an important role.
MATERIALS AND METHODS
Reagents.
The following antibodies were used: mouse monoclonal antibody (mAb) CR50 against reelin (a gift from M. Ogawa, Brain Science Institute, RIKEN, Japan), rabbit antiserum against the hemagglutinin (HA) epitope (Zymed, San Francisco, CA), mouse mAb G10 against reelin, rabbit antiserum against Dab1 (Chemicon, Temecula, CA), rabbit antiserum against Dab1 (phospho-Y220) (Abcam, Cambridge, United Kingdom), mouse mAb EP5 against fibronectin, mouse mAb 6A6 against very-low-density lipoprotein receptor (VLDLR), rabbit antiserum against CHOP/GADD153, rabbit antiserum against ubiquitin, goat polyclonal antiserum against BiP/GRP78, mouse mAb J-3 against Cdk5 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse mAb 9E10 against the Myc epitope (ATCC, Manassas, VA), mouse mAb against γ-tubulin (Sigma Chemical, St. Louis, MO), mouse mAb SPA-827 against BiP (KDEL sequence) (Stressgen, Ann Arbor, MI), Cy2-conjugated donkey antibody against rabbit immunoglobulin G (IgG), and Cy3-conjugated donkey antibody against mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA). TO-PRO-3 and a Slow-Fade antifade kit were purchased from Molecular Probes (Invitrogen, Carlsbad, CA).
Plasmids and transfection.
A reelin cDNA (pCrl) was kindly provided by T. Curran (St. Jude Children's Research Hospital, Memphis, TN) (10). To express a Myc-tagged mutant BiP lacking the KDEL sequence, a cDNA encoding a mutant BiP with residues 1 to 650 was obtained by PCR using rat BiP cDNA (a gift from H. R. B. Pelham, MRC Laboratory of Molecular Biology, United Kingdom). The PCR product was subcloned into a pcDNA3.1 Myc-His vector (Invitrogen, Carlsbad, CA). Transfection was performed with the calcium phosphate method (20).
Mutant BiP mice.
We used homologous recombination to establish knock-in mice expressing BiP lacking the carboxyl-terminal KDEL sequence (29). The missing KDEL sequence was replaced by an HA tag. All animal experimental procedures were performed in accordance with a protocol approved by the Institutional Animal Care Committee of Chiba University, Chiba, Japan.
Western blot.
The brains removed from the mice and cells were homogenized in a buffer containing 0.4% Nonidet P-40, 0.2% N-lauroylsarcosine, 30 mM Tris-HCl (pH 8.0), 1 mM EDTA, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, and 30 μg ml−1 N-acetyl-l-leucinal-l-lecinal-l-norleucinal (ALLN; Sigma Chemical). The lysates were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Gels were transferred onto polyvinylidene fluoride membranes (Immobilon-P; Millipore Corp., Billerica, MA), blocked with 5% nonfat dry milk in the buffer described above, incubated with a primary antibody followed by peroxidase-conjugated donkey anti-goat, anti-mouse, or anti-rabbit IgG, and developed by chemiluminescence (ECL; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Imaging was obtained by using LAS1000 and Image Gauge software (Fuji Photo Film Co. Ltd., Tokyo, Japan).
Primary neuronal culture.
Cortical neurons of mouse embryos were derived from embryos at day 17.5 to 18.5 according to standard procedures (1). After removing the meninges, cortical lobes were isolated in phosphate-buffered saline (PBS), dissected into small pieces, and digested with 0.25% trypsin and 0.02% DNase I in PBS with 5% glucose at 37°C for 20 min. Trypsin was then neutralized with a half volume of horse serum, and the solution was centrifuged at 440 × g for 5 min at 4°C. The resultant cells were triturated in Dulbecco's modified Eagle's medium (DMEM)-F-12 medium containing 10% fetal bovine serum using a siliconized Pasteur pipette and scattered at about 2 × 105 to 4 × 105 cells/cm2 on plates coated with poly-l-lysine (Sigma). Cells were maintained in DMEM-F-12 medium containing 10% fetal bovine serum-1% penicillin-streptomycin for 3 days at 37°C and then replaced with opti-MEM (Invitrogen) containing 1% of an insulin-transferrin-selenium A mixture (ITS; Invitrogen) and antibiotics. On the following day, the supernatants were collected, centrifuged using a table-top machine at 440 × g for 5 min at 4°C, and concentrated by centrifugation (YM50; Millipore). The neurons were treated with control or reelin-containing medium (prepared as previously described) (8) at 37°C for 20 min and collected for Western blotting. 293T cells were transfected with full-length mouse reelin expression construct pCrl. The following day, the cells were washed with serum-free DMEM and maintained in opti-MEM containing 1% ITS and antibiotics. After three more days, the conditioned medium was collected, centrifuged at 440 × g for 5 min at 4°C, and used as the reelin-containing medium.
Confocal and immunofluorescence microscopy of primary neurons.
Cells on coverslips were fixed in cold methanol for 10 min at −20°C and then processed as previously described (20). The stained cells were examined by either confocal laser scanning microscopy (LSM510 fitted with krypton and argon lasers; Carl Zeiss, Oberkochen, Germany) or fluorescence microscopy (Axiovert 200 M; Carl Zeiss).
Northern blot.
Northern blot analysis was done as previously described (15). The expression level of the reelin and BiP mRNAs was assessed relative to that of β-actin mRNA using densitometry by Image Gauge software (Fuji Photo Film).
In situ hybridization histochemistry.
reelin cDNA extending from nucleotides 4716 to 5476 (GenBank accession number U24703) (9) was cloned into the pGEM-T Easy vector (Promega, Madison, WI). In vitro transcription from reelin cDNA was performed using a digoxigenin-UTP RNA labeling kit (Roche Applied Science, Mannheim, Germany) to prepare the antisense and sense cRNA probes according to the manufacturer's instructions.
The brains of mouse embryos at embryonic day 15.5 (E15.5) were fixed with 4% paraformaldehyde dissolved in 0.1 M sodium phosphate buffer (pH 7.4), and postfixed overnight at 4°C with the same fixative. The brains were embedded in 2% agar in PBS and sliced coronally into 150-μm sections with a Microslicer (DTK-3000; Dosaka EM, Kyoto, Japan). Hybridization and detection procedures were performed as described below. The free-floating sections were incubated with proteinase K (20 μg/ml) in 0.1% Tween 20 in PBS (PBST) for 10 min at room temperature. Sections were rinsed with PBST, refixed in 4% paraformaldehyde for 20 min, and again rinsed with PBST three times each for 20 min. Sections were prehybridized in hybridization buffer (50% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 50 μg/ml heparin, 0.1% Tween 20, 5 mg/ml torula RNA) for 30 min at 65°C. Subsequently, sections were hybridized overnight at 65°C with digoxigenin-UTP-labeled antisense or sense riboprobes (0.2 μg/ml) in the hybridization buffer. Sections were then sequentially rinsed in 2× SSCT (0.1% Tween 20 in SSC)-50% formamide twice each for 30 min at 65°C, 2× SSCT for 15 min at 65°C, and 0.2× SSCT twice each for 30 min at 65°C and then incubated overnight with alkaline phosphatase-coupled anti-digoxigenin antibody (1:4,000 dilution; Roche Applied Science, Mannheim, Germany). After washing with PBST three times and then once with 0.1 M Tris-HCl (pH 8.2), sections were stained by use of a solution prepared from FastRed tablets (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instruction and then washed with PBST three times. Sections were then coverslipped with 80% glycerol, and fluorescence images were obtained directly with a confocal laser scanning microscope (LSM5 Pa; Zeiss, Oberkochen, Germany). No labeling was detectable in the control sections that were hybridized with the sense riboprobe (data not shown).
Immunohistochemistry.
Pregnant mice were deeply anesthetized by Nembutal, and embryos were removed by cesarean section. The embryos were fixed by transcardiac perfusion with 4% paraformaldehyde in PBS, and the heads were further immersion fixed for 12 h at 4°C. Embryonic brains were then embedded in 3% agar in PBS, and sections at a thickness of 200 μm were prepared on a Microslicer (Dosaka EM, Kyoto, Japan). The sections were incubated with 10% normal goat serum in PBS for 30 min to block nonspecific antibody binding and then incubated with a mixture of CR50 mouse monoclonal antibody (1:200 dilution) (32) and rabbit anti-calretinin (1:2,000; Swant, Switzerland) in PBS for 12 h at 4°C. The sections were rinsed with PBS and then incubated with a mixture of Cy2-conjugated anti-rabbit IgG (1:100; Jackson Immunoresearch) and Cy3-conjugated anti-mouse IgG (1:200; Chemicon) in PBS for 2 h at 4°C. For calbindin immunohistochemistry of the cerebellum, rabbit anti-calbindin (1:2,000; Swant) was used as the primary antibody, and Cy2-conjugated anti-rabbit IgG was the secondary antibody. The sections were then rinsed with PBS and mounted onto glass slides with 80% glycerol. For counterstaining with a DNA dye, the sections immunostained with anti-calbindin antibody were stained with TO-PRO-3 (Invitrogen) and mounted with SlowFade (Molecular Probes). The sections were observed under a confocal laser scanning microscope (LSM5 Pa; Carl Zeiss).
BrdU labeling.
Bromodeoxyuridine (BrdU) (80 mg · kg−1) was administered intraperitoneally to pregnant mice at the E13 or E15 three times a day (at 10:00, 16:00, and 22:00). At E18, the embryos were removed by cesarean section and perfusion fixed as mentioned above. The brains were embedded in agar, and sections with a thickness of 200 μm were prepared and incubated with 2 N HCl for 1 h at room temperature. The sections were then rinsed with PBS, treated with 10% normal goat serum, and then incubated with a mouse mAb against BrdU (1:50; Becton Dickinson, San Jose, CA) for 12 h. After rinsing with PBS, the sections were incubated with Alexa 488-conjugated goat anti-mouse IgG (1:500 dilution; Invitrogen) in PBS for 2 h at 4°C. The sections were then rinsed again with PBS and mounted onto glass slides with glycerol. Immunolocalization was observed under a confocal laser scanning microscope. The primordium of the somatosensory cortex was divided into 10 layers from the ventricular surface to the pial surface. The densities of BrdU-labeled cells in each layer were determined in four serial sections from a representative brain of each genotype. BrdU-labeled cells in the defined area of each section of the neocortical primordium were counted from the ventricular surface to the pial surface to obtain the total numbers of labeled cells, and the average percentages of the labeled cells in each layer were plotted on a histogram.
RESULTS
Defective neocortical layer formation in mutant BiP mice.
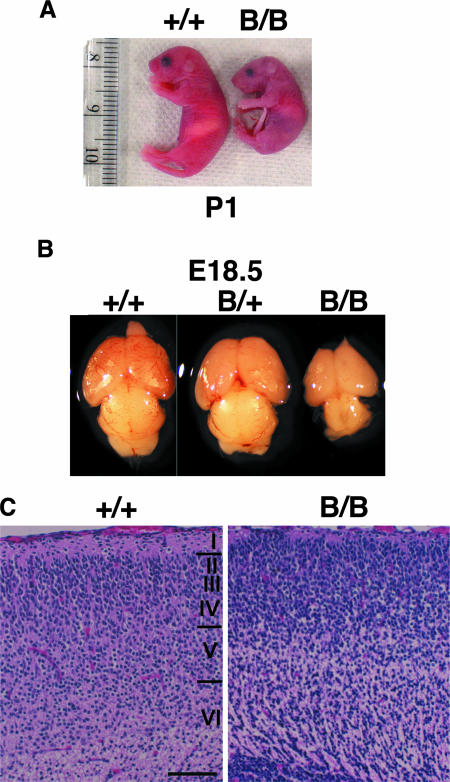
The homozygous mutant BiP mice were born at the expected Mendelian ratio, and they died within 1 day after birth due to respiratory failure (29). They moved and responded to painful stimuli but appeared pale and were significantly smaller than wild-type mice (Fig. 1A). Among the various organs, the mutant brain, including the cerebral cortex and cerebellum, was substantially smaller than those of wild-type mice (Fig. 1B), suggesting that the brain was particularly affected by the BiP mutation. In fact, the neocortical stratification at E18, as observed with hematoxylin-eosin staining, was defective in the mutant BiP mice. The mutant brain had a relatively high density of neurons in neocortical layer I (Fig. 1C, right), in contrast to a low density of neuronal arrangement in the control (Fig. 1C, left).
FIG. 1.
Absence of the KDEL retrieval sequence from BiP impairs brain development. (A) Newborns at P1. (B) Brains from E18.5 embryos. (C) At E18, large numbers of neurons are distributed in the superficial layer of the mutant (right), in contrast to the cell-sparse layer I of the control (left). (C) Hematoxylin-eosin staining. Scale bar, 100 μm. B/B, homozygous; B/+, heterozygous; +/+, wild type.
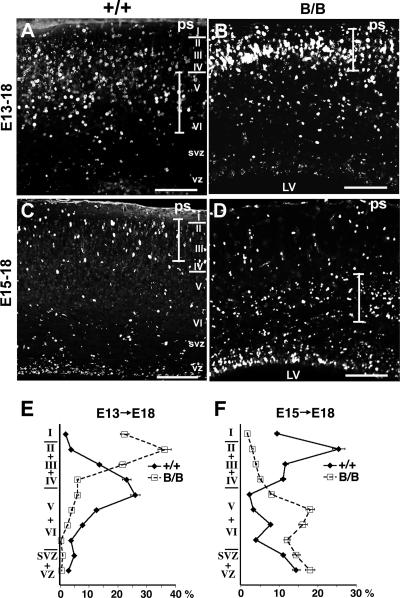
Cortical neurogenesis occurs in the ventricular zone, and the new neurons migrate through other new neurons to the marginal zone and then move to their final destination during embryogenesis. To further investigate the defect in layer formation during neocortical development, birth date analysis of the neocortical neurons was carried out by BrdU labeling (Fig. 2A to D). Neuronal precursors in the ventricular zone became labeled with BrdU during proliferation and migrated after the final mitosis through earlier-born neurons to the cortical plate in normal corticogenesis. When BrdU was administered at E13, heavily labeled cells were distributed in forming layers V and VI, and lightly labeled cells that repeated mitosis after BrdU incorporation were distributed in the upper layers at E18 in the control (Fig. 2A and E), as reported previously by Caviness (7). In the mutant, however, heavily labeled cells were distributed in the upper layer up to superficial layer I, and few labeled cells were observed in the lower layer at E18 (Fig. 2B and E). When BrdU was administered at E15, in the control, the heavily labeled cells reached upper layers II and III at E18 (Fig. 2C and F), as reported previously by Caviness (7), but in the mutant, only a small number of heavily labeled cells reached these upper layers, and most of the labeled cells were distributed in the lower layer (Fig. 2D and F). These findings indicate that, in the mutant brain, the earlier-born neurons reached the superficial layer and remained there and that the later-born neurons did not reach the upper layer, remaining in the lower layer. The mutant BiP mice exhibited an outside-in pattern of neocortical layer formation, in contrast to the inside-out pattern in the control (7), indicating that neocortical layer formation was impaired.
FIG. 2.
Mutant BiP mice exhibit an outside-in pattern of neocortical layer formation. Shown is birth date analysis of the neocortical neurons. (A, B, and E) BrdU was administered at E13, and the distribution of the labeled cells was examined at E18. In control mice, heavily labeled cells were stratified in the lower layers (layers V and VI) (A), in contrast to the significant reduction of labeled cells in the lower layers and their significant increase in the superficial layer in the mutant (B). A quantitative analysis is represented in E. (C, D, and F) BrdU was administered at E15, and the distribution of the labeled cells was examined at E18. In the control, heavily labeled cells reached the upper layers (layers II and III), and the still-migrating cells were also found in the lower layer (C). In contrast, only small numbers of the labeled cells reached the upper layers (layers II and III), and a large proportion of them were distributed in the lower layer in the mutant (D). A quantitative analysis is represented in F. The graphs in E and F represent the averages ± standard errors of the means of BrdU-labeled cells in each layer of four serial sections from a representative brain of each genotype. The extent of the layers in that the cortical neurons were intensely labeled with BrdU is indicated by a vertical bar in each picture. B/B, homozygous mutant; +/+, wild-type mice; LV, lateral ventricle; ps, pial surface; svz, subventricular zone; vz, ventricular zone; I to VI, neocortical layers. Scale bars, 100 μm.
Mutant BiP mice have reduced expression of reelin.
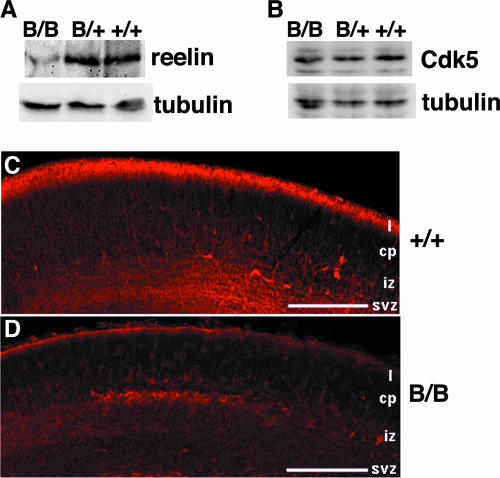
The above-described findings suggested that aberrant neocortical formation is due to the defects in layer formation, like a deficiency in reelin signaling in a reeler mutant malformation (9, 13) or a deficiency in Cdk5 signaling (33). Indeed, analysis of the embryonic cerebral neocortex revealed significantly reduced reelin expression by Western blotting and immunoreactivity in superficial layer I of the mutant BiP mice (Fig. 3A, C, and D), while the expression of Cdk5 was preserved (Fig. 3B). These results are consistent with the fact that reelin is a secretory protein that may interact with BiP in the ER, whereas Cdk5 is a cytosolic protein that is apart from BiP.
FIG. 3.
Significant downregulation of reelin expression in superficial layer I of the mutant BiP neocortical primordium. (A and B) The levels of expression of reelin, Cdk5, and γ-tubulin in brains of E18.5 embryos were evaluated by Western blotting. (C and D) At E16, intense reelin immunoreactivity was found in the superficial layer of the control (C), but immunoreactivity was significantly reduced in the mutant brain (D). Scale bars, 200 μm. cp, cortical plate; iz, intermediate zone; svz, subventricular zone; I, layer I; B/B, homozygous mice; B/+, heterozygous mutant mice; +/+, wild-type mice.
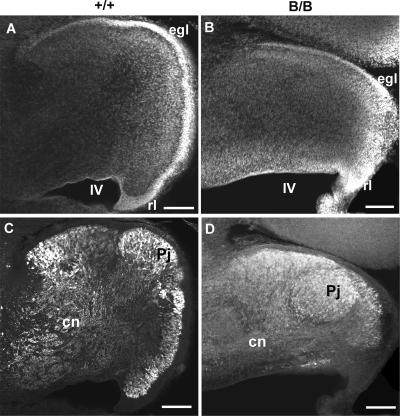
Because reeler malformation is also well documented in the cerebellum with regard to the migration defect of Purkinje cells (48), the structure of the cerebellum was examined at E18. The growth of the mutant cerebellum was significantly retarded as shown by staining with DNA dye (Fig. 4A and B). Although the external granular layer (EGL) was formed in both genotypes, the development of an EGL migrating tangentially from the rhombic lip was significantly retarded in the mutant BiP mice (Fig. 4A and B). A large number of Purkinje cells remained in the subcortical region, in contrast to the cortical arrangement of Purkinje cells in the control (Fig. 4C and D). Hippocampal layer formation showed little defect in the mutant BiP mice based on hematoxylin-eosin-stained sections (data not shown).
FIG. 4.
Mutant BiP mice exhibit defective cerebellar development. (A and B) Staining of the cerebellar primordium at E18 with a DNA dye, TO-PRO-3. The cerebellum of the homozygote (B) is much smaller than that of the wild type (A). An EGL is formed in both genotypes, but the progression of EGL formation in the caudorostral direction is retarded in the homozygote (B). (C and D) Purkinje cell distribution in the cerebellum at E18. Calbindin-immunoreactive Purkinje cells are distributed in the cortical layer in the cerebellum of the control (C). In contrast, large numbers of immunoreactive cells stay in the subcortical region of the mutant cerebellum (D). Scale bars, 100 μm. B/B, homozygous mice; +/+, wild-type mice; cn, cerebellar nucleus; egl, EGL; Pj, Purkinje cell layer; rl, rhombic lip; IV, the fourth ventricle.
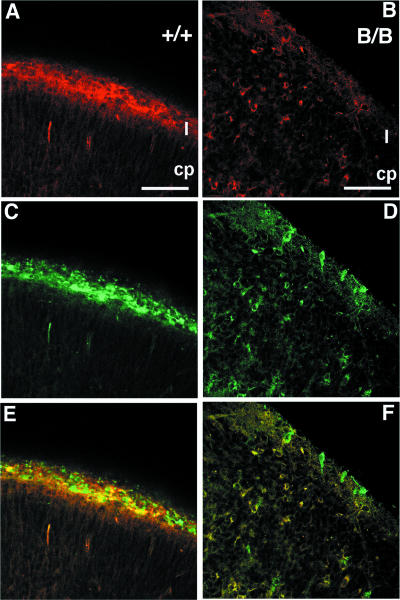
The structure of the superficial layer of the neocortical primordium was further examined by double immunohistochemical labeling for both reelin and calretinin. Calretinin-immunopositive neurons, corresponding to CR cells in the neocortical primordium, were found in the superficial layer of the mutant BiP mice, but their numbers were significantly reduced, and reelin immunoreactivity was barely detected (Fig. 5B, D, and F), in contrast to the localization of reelin immunoreactivity in the calretinin-positive neurons in superficial layer I of the wild-type mice (Fig. 5A, C, and E). Some of calretinin-immunopositive CR cells of the mutant neocortex appeared in a disorganized, scattered pattern other than the marginal zone (Fig. 5F). This finding was confirmed by in situ hybridization histochemistry of the neocortical primordium by using reelin cRNA probe as the marker for CR cells. The cells positive for reelin mRNA formed a thin superficial layer in wild-type mice (Fig. 6A and B). In contrast, the cells positive for reelin mRNA were scattered in the upper layer of the neocortical primordium of the mutant BiP mice (Fig. 6C and D). These findings of in situ hybridization histochemistry correspond well with those of calretinin-immunoreactive cells. Furthermore, the present findings indicate that the transcription of the reelin gene takes place to a similar degree in both mutant and wild-type mice, but the reelin protein is significantly reduced in the CR cells of the mutant.
FIG. 5.
Distribution of CR cells in the neocortical primordium. At E16, reelin immunoreactivity (red) in the superficial layer (I) of control (A) and mutant (B) brains was present. Calretinin-immunopositive CR cells (green) were found in the superficial layers of both control (C) and mutant (D) mice. Reelin immunoreactivity colocalizes with calretinin in CR cells in the control (E) (A and C were merged), but reelin immunoreactivity is hardly detectable in the calretinin-positive cells in superficial layer I of the mutant (F) (B and D were merged). Furthermore, the cells that were double labeled with reelin and calretinin were found in the layers below the superficial layer in the mutant (F), although such cells were not detected in the wild type (E). Scale bars, 50 μm.
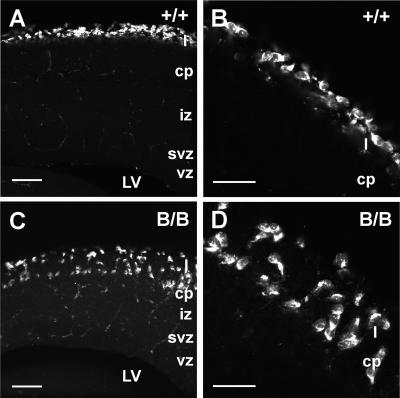
FIG. 6.
The cells positive for reelin mRNA were scattered in the neocortical primordium of mutant BiP mice. The distribution of CR cells was further confirmed by in situ hybridization for reelin mRNA as the marker of CR cells in the neocortical primordium at E15.5. In the control, reelin mRNA-positive cells were situated in the superficial layer (layer I) as shown in A. The higher magnification of the upper cortical area represents the characteristic horizontal arrangement of CR cells (B). In contrast, reelin mRNA-positive cells were distributed from the superficial layer into the cortical plate in the homozygote (C). (D) Higher magnification of the upper cortical area in C. The random orientation of reelin-positive cells is evident. Scale bars, 100 μm in A and C and 50 μm in B and D.
While this mouse does have features of a reeler mutant phenotype, such as an outside-in pattern of neocortical layer formation and the migration defect of Purkinje cells in the cerebellum, it also has other phenotypes in the brain that are distinct from the reeler phenotype. These include the reduction in the size of the whole brain and the apparent scattering of reelin- and calretinin-positive neurons throughout the cortex. This is not surprising since BiP likely has a multitude of substrates that are significant for brain development. Among them, we decided to focus on reelin since at least one of the reeler mutants has a defect in intracellular transport of reelin (11) rather than the production of reelin, and the transport mutant BiP may affect the folding and secretion of reelin.
Reelin secretion is impaired in the mutant BiP brain.
Reelin is a large secreted glycoprotein (9) produced by some cortical neurons such as CR cells in the marginal zone during development. Reelin mediates cortical laminar formation through binding to VLDLR and apolipoprotein E receptor type 2 (ApoER2) on cortical neurons (8, 43). In reeler mice deficient in the reelin gene (9), the cortical neurons lack the ability to localize properly and settle inside the earlier-migrating neurons (7).
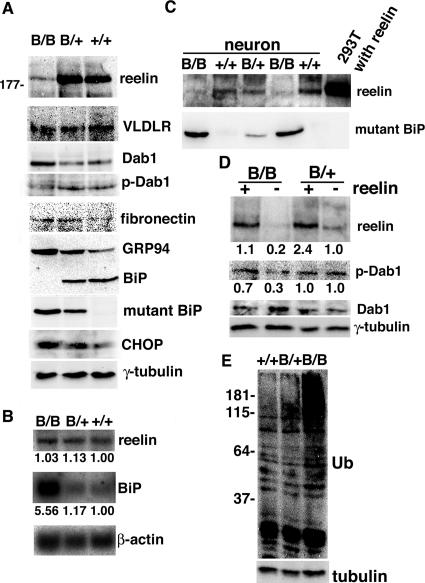
In E18.5 mice, we used an antibody directed against the amino terminus of reelin to detect a fragment of reelin (∼180 kDa) in the wild-type cerebral cortex; however, these fragments were much less intense in the homozygous mutant BiP cortex (Fig. 7A), consistent with histological observations. Although VLDLR expression was equivalent between wild-type and mutant BiP cortices, dephosphorylated Dab1 accumulated in the mutant BiP brain, indicating that the reelin signaling pathway was inactivated there. The expression of another secreted glycoprotein, fibronectin, was preserved in the mutant brain. The reelin deficiency was not a consequence of reduced transcription, because reelin mRNA expression did not differ in control and mutant brains (Fig. 7B), consistent with the in situ hybridization experiment (Fig. 6). The expression of BiP mRNA as well as CHOP protein (a cell death-related transcriptional factor of the UPR) (50) was enhanced in the mutant brain (Fig. 7A and B), suggesting that the mutant brain suffered from ER stress.
FIG. 7.
Reelin expression is reduced posttranscriptionally in the mutant BiP brain. (A) The levels of expression of reelin, VLDLR, Dab1, tyrosine-phosphorylated Dab1 (p-Dab1), fibronectin, GRP94, BiP, mutant BiP, CHOP, and γ-tubulin in brains of E18.5 embryos were evaluated by Western blotting. In mutant BiP, the carboxyl-terminal KDEL sequence was replaced by an HA tag. (B) Northern blotting using probes for reelin, BiP, and β-actin mRNAs in brains at E18.5. The expression levels of reelin and BiP mRNAs were assessed by the relative ratio to β-actin mRNA. (C) Secretion of reelin and mutant BiP in the culture medium from the primary neurons and 293T cells transfected with reelin cDNA, as evaluated by Western blotting. (D) Primary neurons were treated with control (−) or reelin-containing medium (+) at 37°C for 20 min, collected, and subjected to Western blotting with antibodies against reelin, Dab1, tyrosine-phosphorylated Dab1, and γ-tubulin. The expression levels of reelin and tyrosine-phosphorylated Dab1 were assessed by the relative ratios to γ-tubulin. (E) Expression of ubiquitinated (Ub) proteins in the cerebrum at E18.5 embryos, as evaluated by Western blotting. B/B, homozygous mice; B/+, heterozygous mutant mice; +/+, wild-type mice.
Mutant BiP might impair the folding of reelin, leading to its degradation by the ER-associated degradation pathway or to its secretion as an immature form from the CR cells due to an escape from ER quality control. To test this possibility, we used primary neurons derived from embryonic brains and found a significant decrease in reelin secretion by the homozygous mutant BiP neurons compared with that of wild-type or heterozygous neurons (Fig. 7C). To investigate whether the homozygous mutant BiP neurons maintained their responsiveness to reelin stimulation, we incubated primary neurons with conditioned culture medium containing a severalfold physiological level of reelin secreted by 293T cells transiently transfected with reelin cDNA (Fig. 7D). Exogenous reelin seemed to be active on the homozygous neurons, leading to the activation of the reelin signaling pathway, as demonstrated by a reduced amount of Dab1 expression and an increased amount of phospho-Dab1 expression. On the other hand, the reelin signaling pathway in the heterozygous mutant cortical neurons seems to be constitutively active with endogenous reelin even without exogenous reelin stimulation. Thus, Dab1 expression and phosphorylation (Fig. 7D) are rather unchanged in the heterozygous mutant. These results suggest that the impaired secretion of reelin by the CR cells rather than defective responsiveness in the cortical neurons may be responsible for the neurological phenotype of reeler mutant-like malformations in mutant BiP mice. Thus, the impaired retrieval of BiP may promote the degradation of misfolded proteins by the ubiquitin/proteasome pathway. In fact, ubiquitinated proteins accumulated in the mutant cerebrum (Fig. 7E).
BiP may enhance the folding of reelin.
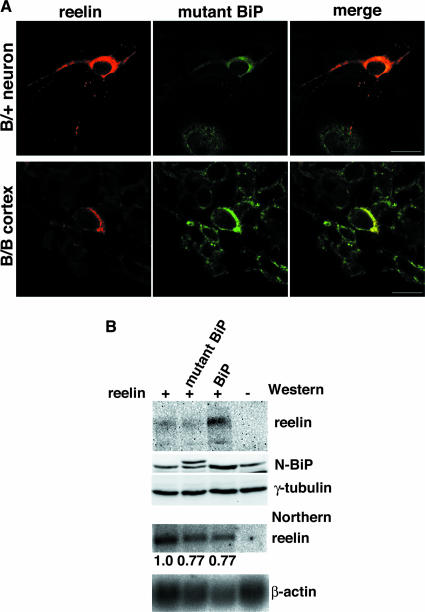
Mutant BiP was detected in the ER (29), but a significant fraction was also secreted from cells because of the lack of the retrieval motif (KDEL) (Fig. 7C). We examined the subcellular localization of reelin to establish its relationship with mutant and wild-type BiP. Reelin colocalized with mutant BiP in the ER in primary neurons derived from heterozygous mutant BiP embryos; this was also the case in cortical neurons in the homozygous mutant postnatal brain, where the expression of reelin was reduced (Fig. 8A). To obtain further insight into the interaction of BiP and reelin, we performed cotransfection experiments in HeLa cells. Coexpression of reelin and wild-type BiP, but not the mutant BiP lacking the KDEL sequence, greatly enhanced the expression of reelin protein (reelin mRNA levels were equivalent in the two transfections) (Fig. 8B). These results suggest that BiP promotes the folding of reelin.
FIG. 8.
BiP may enhance the maturation of reelin. (A) Subcellular localization of endogenous reelin and mutant BiP in primary neurons from the heterozygous (B/+) mutant BiP embryo and the cortex of the homozygous (B/B) mutant BiP embryo (E18.5), as evaluated by confocal laser scanning microscopy, with double labeling using a mouse mAb for reelin and a rabbit antiserum for HA. Scale bars, 10 μm. (B) HeLa cells were transiently transfected with reelin alone or cotransfected with either mutant BiP in which the KDEL sequence was replaced by a Myc tag or wild-type BiP (the Myc-tagged mutant BiP has a higher molecular weight than wild-type BiP). The levels of expression of reelin, BiP, and γ-tubulin were evaluated by Western blotting, and the levels of expression of reelin and β-actin mRNA were evaluated by Northern blotting. The expression level of reelin mRNA was assessed relative to that of β-actin mRNA.
DISCUSSION
We produced knock-in mice expressing a mutant BiP with the retrieval sequence deleted, which allowed us to examine the effects of a defect in the response to secretory pathway stress without completely eliminating BiP function, as would be the case with BiP knockout mice (28). The loss of BiP function was compensated for by the UPR in embryonic fibroblasts. However, neonates expressing mutant BiP suffered respiratory failure caused by the impaired secretion of pulmonary surfactant in alveolar type II epithelial cells (29). Furthermore, we observed abnormal corticogenesis in mutant BiP mice. Mutant BiP may predominantly affect dedicated secretory cells, such as alveolar type II cells and CR cells, in which active secretion is particularly important; thus, protein folding was probably affected in these cells. Indeed, we found an impaired secretion of reelin in CR cells, which may account for one aspect of cortical malformation in the cerebrum and cerebellum of mutant BiP mice. We also demonstrated increased Dab1 protein levels and a reduction in Dab1 tyrosine phosphorylation, which are consistent with a reeler-like phenotype. On the other hand, this mouse has other phenotypes in the brain that are distinct from the reeler phenotype. These include the reduction in the size of the whole brain and the apparent scattering of CR cells throughout the cortex, suggesting that mutant BiP may likely interfere with other substrates in addition to the reelin required for brain development.
The deletion of the retrieval sequence from BiP could have two possible effects. First, the lack of recycling of mutant BiP to the ER could impair the folding environment in the ER. This effect may be limited because constitutively active UPR compensates for it, and a sufficient amount of the functional mutant BiP, as long as it stays in the ER, may be produced for cell survival. Second, the impaired retrieval of mutant BiP may affect quality control in post-ER compartments. In addition to the ER itself, several studies have revealed that proper ER-to-Golgi apparatus transport and the subsequent retrieval/return of proteins and lipids to the ER may contribute to quality control (16, 19, 42, 45, 47). In this regard, the folding (and therefore function) of reelin may be dependent on the proper retrieval of BiP to the ER via interactions with the KDEL receptor.
Reelin is a large 3,461-residue secreted glycoprotein that has eight reelin repeats of ∼350 residues each that contain an epidermal growth factor motif followed by a carboxyl-terminal 33 residues rich in basic amino acids (43). During embryogenesis, CR cells secrete reelin as homo-oligomers that function in cortical layer formation through binding to lipoprotein receptors on cortical neurons (44). Although the folding, intracellular transport, and oligomerization of reelin have not been characterized in detail, we found that reelin protein expression was impaired in mutant BiP mice, indicating that BiP may play a role in the maturation of reelin. Furthermore, we found that the expression levels of BiP mRNA and the CHOP protein were enhanced in the mutant brain, suggesting that the mutant brain might have suffered from ER stress. We speculate that the folding of the reelin protein may be vulnerable to impaired quality control in the ER and the post-ER compartments of mutant CR cells. If true, this assumption suggests that environmental stresses that perturb ER quality control may also impair the reelin signaling pathway and other factors, which may cause neuronal migration defects.
In addition to brain development, several studies suggested the possible role of reelin in the pathogenesis of human mental disorders such as schizophrenia, autism, bipolar disorder, and Alzheimer's disease (5, 14, 43). Because reelin signaling through ApoER2 in adult brains modulates synaptic plasticity and memory formation (2), the defective reelin signaling pathway may contribute to the pathogenesis of adult mental disorders. Reelin and ApoE share ApoER2 on cortical neurons (8), and ApoE inhibits reelin signaling by competing for binding to ApoER2. Interestingly, the E4 allele of ApoE increases the risk of developing sporadic forms of Alzheimer's disease.
In the meantime, the persistent accumulation of misfolded proteins beyond the capacity of ER quality control causes ER stress, leading to cellular dysfunction and cell death (24, 26). This process is thought to cause human mental disorders such as neurodegenerative diseases including Alzheimer's disease (23) and Parkinson's disease (21), bipolar disorders (22), and ischemic neuronal injury (41). The involvement of impaired BiP function in neurodegenerative diseases has been reported in a mouse model where the disruption of SIL1, a cochaperone of BiP, caused protein accumulation and neurodegeneration (49). Thus, reelin signaling and ER quality control may be related to the pathogenesis of adult mental disorders, as seen in reeler mutant-like cerebral malformation in mutant BiP neonates.
The UPR is a ubiquitous mechanism in all cells to adapt to ER stress in pathological conditions, and BiP is an essential component of this system. Our results suggest that a physiological increase in the production of reelin and other factors in dedicated secretory cells like CR cells during neonatal periods may require the UPR and a proper folding capacity in the ER. Neuronal migration and stratification may be sensitive to environmental insults such as viral infection, hypoxia, and ischemia that perturb ER functions.
Acknowledgments
We thank T. Nishino, K. Toshimori, and T. Yamashita for critical comments. We also thank M. Kashio for excellent technical assistance.
This work was supported by grants-in-aid for science research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T.A. and grant 05-32 from NIBIO to S.Y.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Banker, G., and K. Goslin. 1988. Developments in neuronal cell culture. Nature 336185-186. [DOI] [PubMed] [Google Scholar]
- 2.Beffert, U., E. J. Weeber, A. Durudas, S. Qiu, I. Masiulis, J. D. Sweatt, W. P. Li, G. Adelmann, M. Frotscher, R. E. Hammer, and J. Herz. 2005. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47567-579. [DOI] [PubMed] [Google Scholar]
- 3.Beh, C. T., and M. D. Rose. 1995. Two redundant systems maintain levels of resident proteins within the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 929820-9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacino, J. S., and A. M. Weissman. 1998. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 1419-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothwell, M., and E. Giniger. 2000. Alzheimer's disease: neurodevelopment converges with neurodegeneration. Cell 102271-273. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky, J. L., and A. A. McCracken. 1999. ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 10507-513. [DOI] [PubMed] [Google Scholar]
- 7.Caviness, V. S., Jr. 1982. Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 256293-302. [DOI] [PubMed] [Google Scholar]
- 8.D'Arcangelo, G., R. Homayouni, L. Keshvara, D. S. Rice, M. Sheldon, and T. Curran. 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24471-479. [DOI] [PubMed] [Google Scholar]
- 9.D'Arcangelo, G., G. G. Miao, S. C. Chen, H. D. Soares, J. I. Morgan, and T. Curran. 1995. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374719-723. [DOI] [PubMed] [Google Scholar]
- 10.D'Arcangelo, G., K. Nakajima, T. Miyata, M. Ogawa, K. Mikoshiba, and T. Curran. 1997. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 1723-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bergeyck, V., K. Nakajima, C. Lambert de Rouvroit, B. Naerhuyzen, A. M. Goffinet, T. Miyata, M. Ogawa, and K. Mikoshiba. 1997. A truncated Reelin protein is produced but not secreted in the ‘Orleans’ reeler mutation (Reln[rl-Orl]). Brain Res. Mol. Brain Res. 5085-90. [DOI] [PubMed] [Google Scholar]
- 12.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4181-191. [DOI] [PubMed] [Google Scholar]
- 13.Falconer, D. S. 1951. Two new mutant, ‘trembler’ and ‘reeler,’ with neurological actions in the house mouse (Mus muscululus L.). J. Genet. 50192-201. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi, S. H. 2005. Reelin glycoprotein: structure, biology and roles in health and disease. Mol. Psych. 10251-257. [DOI] [PubMed] [Google Scholar]
- 15.Hamada, H., M. Suzuki, S. Yuasa, N. Mimura, N. Shinozuka, Y. Takada, T. Nishino, H. Nakaya, H. Koseki, and T. Aoe. 2004. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol. Cell. Biol. 248007-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond, C., and A. Helenius. 1994. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J. Cell Biol. 12641-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding, H. P., and D. Ron. 2002. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51(Suppl. 3)S455-S461. [DOI] [PubMed] [Google Scholar]
- 18.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397271-274. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, C. M., S. Caldwell, and A. A. Cooper. 2002. An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER-Golgi transport. J. Cell Biol. 15891-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, V. W., N. Shah, and R. D. Klausner. 1992. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell 69625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai, Y., M. Soda, H. Inoue, N. Hattori, Y. Mizuno, and R. Takahashi. 2001. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105891-902. [DOI] [PubMed] [Google Scholar]
- 22.Kakiuchi, C., K. Iwamoto, M. Ishiwata, M. Bundo, T. Kasahara, I. Kusumi, T. Tsujita, Y. Okazaki, S. Nanko, H. Kunugi, T. Sasaki, and T. Kato. 2003. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat. Genet. 35171-175. [DOI] [PubMed] [Google Scholar]
- 23.Katayama, T., K. Imaizumi, N. Sato, K. Miyoshi, T. Kudo, J. Hitomi, T. Morihara, T. Yoneda, F. Gomi, Y. Mori, Y. Nakano, J. Takeda, T. Tsuda, Y. Itoyama, O. Murayama, A. Takashima, P. St. George-Hyslop, M. Takeda, and M. Tohyama. 1999. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1479-485. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman, R. J. 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 1101389-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleizen, B., and I. Braakman. 2004. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 16343-349. [DOI] [PubMed] [Google Scholar]
- 26.Kopito, R. R., and D. Ron. 2000. Conformational disease. Nat. Cell Biol. 2E207-E209. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, M. J., and H. R. Pelham. 1990. A human homologue of the yeast HDEL receptor. Nature 348162-163. [DOI] [PubMed] [Google Scholar]
- 28.Luo, S., C. Mao, B. Lee, and A. S. Lee. 2006. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 265688-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimura, N., H. Hamada, M. Kashio, H. Jin, Y. Toyama, K. Kimura, M. Iida, S. Goto, H. Saisho, K. Toshimori, H. Koseki, and T. Aoe. 2007. Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ. 141475-1485. [DOI] [PubMed] [Google Scholar]
- 30.Munro, S., and H. R. Pelham. 1987. A C-terminal signal prevents secretion of luminal ER proteins. Cell 48899-907. [DOI] [PubMed] [Google Scholar]
- 31.Munro, S., and H. R. Pelham. 1986. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46291-300. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa, M., T. Miyata, K. Nakajima, K. Yagyu, M. Seike, K. Ikenaka, H. Yamamoto, and K. Mikoshiba. 1995. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14899-912. [DOI] [PubMed] [Google Scholar]
- 33.Ohshima, T., J. M. Ward, C. G. Huh, G. Longenecker, Veeranna, H. C. Pant, R. O. Brady, L. J. Martin, and A. B. Kulkarni. 1996. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 9311173-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyadomari, S., A. Koizumi, K. Takeda, T. Gotoh, S. Akira, E. Araki, and M. Mori. 2002. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Investig. 109525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil, C., and P. Walter. 2001. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13349-355. [DOI] [PubMed] [Google Scholar]
- 36.Reimold, A. M., A. Etkin, I. Clauss, A. Perkins, D. S. Friend, J. Zhang, H. F. Horton, A. Scott, S. H. Orkin, M. C. Byrne, M. J. Grusby, and L. H. Glimcher. 2000. An essential role in liver development for transcription factor XBP-1. Genes Dev. 14152-157. [PMC free article] [PubMed] [Google Scholar]
- 37.Reimold, A. M., N. N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E. M. Gravallese, D. Friend, M. J. Grusby, F. Alt, and L. H. Glimcher. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412300-307. [DOI] [PubMed] [Google Scholar]
- 38.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 71165-1176. [DOI] [PubMed] [Google Scholar]
- 39.Schroder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74739-789. [DOI] [PubMed] [Google Scholar]
- 40.Sonnichsen, B., J. Fullekrug, P. N. Van, W. Diekmann, D. G. Robinson, and G. Mieskes. 1994. Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J. Cell Sci. 1072705-2717. [DOI] [PubMed] [Google Scholar]
- 41.Tajiri, S., S. Oyadomari, S. Yano, M. Morioka, T. Gotoh, J. I. Hamada, Y. Ushio, and M. Mori. 2004. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 11403-415. [DOI] [PubMed] [Google Scholar]
- 42.Taxis, C., F. Vogel, and D. H. Wolf. 2002. ER-Golgi traffic is a prerequisite for efficient ER degradation. Mol. Biol. Cell 131806-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tissir, F., and A. M. Goffinet. 2003. Reelin and brain development. Nat. Rev. Neurosci. 4496-505. [DOI] [PubMed] [Google Scholar]
- 44.Utsunomiya-Tate, N., K. Kubo, S. Tate, M. Kainosho, E. Katayama, K. Nakajima, and K. Mikoshiba. 2000. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc. Natl. Acad. Sci. USA 979729-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vashist, S., and D. T. Ng. 2004. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J. Cell Biol. 16541-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, J., and R. J. Kaufman. 2006. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13374-384. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto, K., R. Fujii, Y. Toyofuku, T. Saito, H. Koseki, V. W. Hsu, and T. Aoe. 2001. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J. 203082-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuasa, S., J. Kitoh, S. Oda, and K. Kawamura. 1993. Obstructed migration of Purkinje cells in the developing cerebellum of the reeler mutant mouse. Anat. Embryol. (Berlin) 188317-329. [DOI] [PubMed] [Google Scholar]
- 49.Zhao, L., C. Longo-Guess, B. S. Harris, J. W. Lee, and S. L. Ackerman. 2005. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat. Genet. 37974-979. [DOI] [PubMed] [Google Scholar]
- 50.Zinszner, H., M. Kuroda, X. Wang, N. Batchvarova, R. T. Lightfoot, H. Remotti, J. L. Stevens, and D. Ron. 1998. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12982-995. [DOI] [PMC free article] [PubMed] [Google Scholar]