Abstract
p27kip1 is a cyclin-dependent kinase inhibitor and a tumor suppressor. In some tumors, p27 suppresses tumor growth by inhibition of cell proliferation. However, this is not universally observed, implying additional mechanisms of tumor suppression by p27. p27-deficient mice are particularly susceptibility to genotoxin-induced tumors, suggesting a role for p27 in the DNA damage response. To test this hypothesis, we measured genotoxin-induced mutations and chromosome damage in p27-deficient mice. Both p27+/− and p27−/− mice displayed a higher N-ethyl-N-nitrosourea-induced mutation frequency in the colon than p27+/+ littermates. Furthermore, cells from irradiated p27-deficient mice exhibited a higher number of chromatid breaks and showed modestly increased micronucleus formation compared to cells from wild-type littermates. To determine if this mutator phenotype was related to the cell cycle-inhibitory function of p27, we measured cell cycle arrest in response to DNA damage. Both normal and tumor cells from p27-deficient mice showed impaired G2/M arrest following low doses of ionizing radiation. Thus, p27 may inhibit tumor development through two mechanisms. The first is by reducing the proliferation of cells that have already sustained an oncogenic lesion. The second is by transient inhibition of cell cycle progression following genotoxic insult, thereby minimizing chromosome damage and fixation of mutations.
Levels of p27 protein have prognostic significance in a wide variety of human cancers (reviewed in references 29 and 43). Decreased levels of p27 are associated with high tumor grade and stage in human colorectal (8, 37), gastric (48), breast (5, 15), and other cancers. Reduction of p27 protein in tumors correlates significantly with decreased survival in colorectal (18), gastric (20), breast (32), and esophageal squamous cell (38) carcinoma patients, among others. This is in contrast to other cyclin-dependent kinase (CDK) inhibitors such as p21 and p18, for which prognostic significance is limited (reviewed in reference 43), leading us to question the specific role of p27 in tumor suppression.
These observations hinted that p27 plays a fundamental role in tumor suppression, although a cause-and-effect relationship was difficult to establish because somatic mutations in the CDKN1B gene, encoding p27, are rare in tumors (16, 30, 31). Studies of the p27 knockout mouse clearly showed that p27 is a potent tumor suppressor in multiple tissues, with a particularly strong effect within the gastrointestinal (GI) tract. Although spontaneous GI tumors are rare in p27-deficient mice fed a standard diet, these mice are highly predisposed to adenomas and adenocarcinomas throughout the small intestine and colon when treated with the genotoxic carcinogens N-ethyl-N-nitrosourea (ENU), gamma irradiation, and 1,2-dimethylhydrazine (12, 28, 47).
Two primary, and sometimes overlapping, functions of tumor suppressors are inhibition of proliferation and maintenance of genetic integrity. Previously, we demonstrated that p27 deficiency resulted in an increased mitotic index (MI) in GI tumors (28), indicating that p27 functions, at least in part, to inhibit proliferation of tumor cells. However, other studies show a lack of correlation between p27 levels and proliferation, suggesting that there may be more than one mechanism of tumor suppression by p27 (24, 25, 39, 42). Here we investigated the role of p27 in maintenance of genetic integrity using the same target tissue, the GI tract, and the same agents, ENU and gamma radiation, used previously to induce tumors.
We found that p27 deficiency significantly increased both large- and small-scale genetic lesions in response to ENU and low doses of gamma irradiation using three separate genotoxic assays (the Big Blue mutagenicity assay, analysis of chromatid gaps and breaks, and the micronucleus assay). p27 deficiency also impaired G2/M arrest in the crypt progenitor cells of the GI epithelium following genotoxic exposure. Together, these results suggest that the susceptibility of p27-deficient mice to genotoxic agents can be at least partially attributed to an increase in genotoxin-induced mutation frequency (MF) due to a defect in G2/M checkpoint initiation.
MATERIALS AND METHODS
Mice.
Mixed 129/Sv × C57BL/6 p27-deficient mice were obtained from J. Roberts and genotyped as described previously (13). The p27 knockout allele was backcrossed 14 times onto the C57BL/6 strain. For analysis of the in vivo MI, mice at the age of 8 to 12 weeks were treated with a single dose of whole-body ionizing radiation (IR) (with a 137C source at 330 cGy per second). Six p27+/+, five p27+/−, and four p27−/− mice were treated with 0 Gy; four p27+/+, four p27+/−, and six p27−/− mice were treated with 0.6 Gy; eight p27+/+, five p27+/−, and five p27−/− mice were treated with 1.2 Gy; and four p27+/+, four p27+/−, and four p27−/− mice were treated with 4 Gy. For analysis of MI in tumor-bearing mice, the p27 knockout allele was crossed to C57BL/6 Apc1638N (14) or C3H Apcmin strains. When mice developed intestinal tumors, they were irradiated and sacrificed 2 to 4 h later. Eight p27+/+, eight p27+/−, and five p27−/− mice were treated with 0 Gy; two p27+/+, two p27+/−, and two p27−/− mice were treated with 1.2 Gy; and two p27+/+, two p27+/−, and one p27−/− mice were treated with 3 Gy. Five to 23 tumors per genotype per irradiation dose were examined, with the exception of p27−/− mice irradiated with 3 Gy, where only two tumors were available for examination. Mitotic figures and apoptotic bodies were counted from at least 5,000 to 25,000 tumor cells or 75 crypts in formalin-fixed, hematoxylin-eosin (H&E)-stained sections at a final magnification of ×600. All experiments were approved by the IACUC and performed in accordance with the relevant guidelines and regulations.
Somatic mutation assay.
The p27/lacI double-transgenic mice were generated by crossing Big Blue/lacI transgenic C57BL/6 mice and p27+/− C57BL/6 mice. The resulting p27+/+, p27+/−, and p27−/− lacI mice were genotyped as previously described (13, 23). Five to seven mice were assigned to each treatment group. At the age of 7 to 9 weeks, mice were treated with ENU (Sigma-Aldrich Co.) at a dose of 150 mg/kg (dissolved in 66.7 mM phosphate-buffered saline [PBS] solution) by a single intraperitoneal injection. Control mice were injected with PBS solution only. Three weeks after ENU treatment, the mice were sacrificed and colons were excised, rinsed with sterile PBS, flash frozen in liquid nitrogen, and stored at −80°C until needed. Genomic DNA was isolated as previously described (41). lacI transgenes were recovered from purified mouse chromosomal DNA by in vitro λ packaging (Stratagene, La Jolla, CA). Packaged phage were plated on an SCS-8 bacterial lawn in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Phage with a mutated lacI gene yield blue plaques, while wild-type phage form colorless plaques. lacI mutants were picked and purified by replating at low density. A 1.5-kb lacI-containing fragment was amplified by PCR (11), and the mutation specificity was determined by direct sequencing. The MF was calculated as the ratio of the number of independent mutants to the total number of plaques screened. MF data are presented with standard error of the mean (SEM) and were analyzed using COCHARM (created by Troy Johnson, Procter & Gamble, Cincinnati, OH), a computer program that executes the general Cochran-Armitage test (4). Fisher's exact test was used for the comparison of mutation changes, and the Bonferonni correction was used when comparing data from more than two groups.
Chromatid gap/break assay.
Spleens were removed from adult mice of all three p27 genotypes and placed in 1.5 ml of RPMI 1640, and cells were teased out of the spleen using sterile, bent 23-gauge needles. Spleen cell pellets were equally divided into thirds and cultured in T25 flasks with 5 ml RPMI 1640 containing 10% fetal bovine serum (FBS) and 40 μg/ml lipopolysaccharide (Sigma, L4391). Cells were cultured at 37°C for 46 h, followed by treatment with either 0, 0.6, or 1.2 Gy gamma irradiation. After irradiation, cells were incubated for an additional 3 h at 37°C, with the final hour of incubation in the presence of 0.04 μg/ml KaryoMAX Colcemid (Invitrogen). Cells were harvested for metaphase chromosome preparations using 0.075 M KCl (15 min at 37°C), followed by three changes of 3:1 methanol-acetic acid fixative. Slides were prepared and either G banded or solid stained using Wright stain. Fifty to 150 cells from each culture were counted for chromatid gaps and breaks by a blinded scorer and pooled for statistical analysis. Two or three mice per p27 genotype were analyzed in two separate experiments. Cells were not used for scoring if they had overlapping chromosomes, if debris was covering chromosomes, or if they were tetraploid.
Micronucleus assay.
Peripheral blood samples were obtained from seven to nine mice per p27 genotype by retroorbital bleeding immediately prior to, as well as 24 h and 48 h following, one dose of whole-body 0.6 Gy IR at the age of 8 to 12 weeks. Blood samples were fixed according to the manufacturer's specifications and processed by flow cytometry at Litron Laboratories. The micronucleus frequencies of high CD71+ micronucleated reticulocytes were assayed.
Histopathology and immunohistochemistry.
The small intestine and colon were removed, fixed in 10% neutral buffered formalin for 4 to 6 h, and embedded in paraffin. After high-temperature antigen retrieval in 10 mM citrate buffer, pH 6.0, 5-mm sections were stained for p27 (mouse monoclonal antibody; Neomarkers, Fremont, CA), phospho-histone H3 (Ser 10) (Cell Signaling Tech 9701) for 1 h. Standard avidin-biotin peroxidase complex (ABC) techniques were used for primary antibody detection (biotinylated goat anti-rabbit antibody [Vector Labs Inc., Burlingame, CA]; streptavidin ABC [DAKO Corp., Carpinteria, CA]). The slides were developed in 3,3′-diaminobenzidine-NiCl and then counterstained with methyl green. Controls included no primary antibody and/or normal rabbit serum and tissues from p27−/− mice. Bromodeoxyuridine labeling was done as described previously (28).
Cell cycle analysis.
To generate mouse embryonic fibroblasts (MEFs), p27+/− mice were crossed and embryos were dissected at 12.5 to 13.5 days after detection of vaginal plugs. The head and internal organs were removed, and the embryos were minced and incubated in 0.05% trypsin for 5 min. The cells were resuspended in Dulbecco modified Eagle medium supplemented with 10% FBS. After centrifugation, the supernatant was discarded and the cell suspension from each embryo was cultivated on a 10-cm dish in 10 ml Dulbecco modified Eagle medium with 10% FBS until confluence was reached. After this time, the cells were treated with trypsin, counted, and plated at 0.5 × 106 cells per 10-cm dish or at 0.025 × 106 cells per well in a LabtekII chamber slide (Nalge Nunc International). Immortalized MEF lines were prepared according to the NIH 3T3 protocol and were a generous gift from A. Besson and J. Roberts. Adherent primary MEFs were irradiated with 5 Gy at passages 1 to 5, with all p27 genotypes matched for passage within a given experiment. The MI of MEFs was determined using H&E staining of chamber slides in three to five independent experiments. At least three different MEF lines per p27 genotype were analyzed before and after IR. The results shown are expressed as percentages of the unirradiated MI. For synchronization experiments, cells were grown to confluence and held for 48 h before passage into 5 μg/ml aphidicolin. After 16 h, the cells were washed twice with PBS and released into fresh medium without aphidicolin. For fluorescence-activated cell sorter (FACS) analysis, 0.5 × 106 cells were fixed in 70% ethanol at −20°C for 1 h, washed twice with 2% fetal calf serum-PBS, and stained with 25 μg/ml propidium iodide and 1 mg/ml RNase A in 2% fetal calf serum-PBS overnight at 4°C. Samples were acquired on a FACScan for DNA content and analyzed using Cellquest software (Becton Dickinson, Mountain View, CA) and FlowJo software (TreeStar Inc., Ashland, OR). Fifty percent of cells entered S phase by 4 h after release (see Fig. 4). Therefore, cells were irradiated (5 Gy) at 6 h after release, when a large percentage would be in S/G2, and then collected 2 h later for mitotic and kinase assays. For analysis of phospho-H2A.X, cells were synchronized as described above and seeded in four-chamber slides at 0.025 × 106 cells per well in a LabtekII chamber slide (Nalge Nunc International). At appropriate time points, cells were fixed with 95% ethanol and 5% acetic acid for 10 min, followed by a 10-min fixation/permeation with 1% formaldehyde and 0.25% Triton X-100. Slides were blocked in 3% BSA-1× PBS for 30 min at room temperature and then incubated with anti-phospho-H2A.X (ser139)-fluorescein isothiocyanate conjugate (Upstate) according to the manufacturer's specifications overnight at 4°C. Slides were washed five times with PBS and coverslipped with mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories). Images were visualized using a Nikon E800 and acquired using Metamorph software (Universal Imaging Corp.). The experiment was run in triplicate, and all images for a given experiment were acquired using the same exposure, range, and gamma settings.
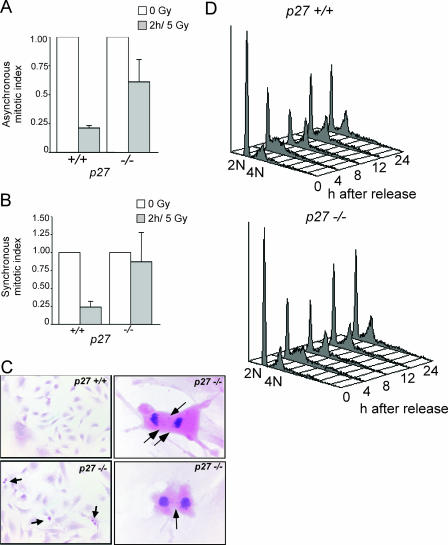
FIG. 4.
p27 deficiency impairs the G2/M checkpoint in irradiated MEFs. The MI of primary MEFs was determined using H&E-stained chamber slides. The mean MIs plus the SEMs from three to five independent experiments are shown as a percentage of the unirradiated MI. Both asynchronous (A) and synchronous (B) p27−/− MEFs have impaired G2/M arrest at early time points following low-dose gamma irradiation. (C) H&E-stained chamber slides of MEFs at 2 h following IR. The upper left panel shows a lack of p27+/+ mitotic cells due to IR-induced G2/M arrest (magnification, ×100). The lower left panel shows persistence of p27−/− mitotic cells following IR (magnification, ×100). Arrows indicate mitotic figures. The right panels show aberrant mitotic figures in p27−/− MEFs, including lagging chromosomes and anaphase bridges (arrows) (magnification, ×600). (D) FACS analysis shows that both p27+/+ and p27−/− cells were synchronized and proceed into cell cycle after release at similar rates. The data in panel B were from a similar population of cells synchronized at the G1/S boundary, released, irradiated 6 h later (in late S/G2), and collected 2 h later.
Cyclin B1-associated kinase assay.
p27+/+ and p27−/− immortalized MEFs were lysed in PBS (Sigma, P4417) (pH 7.3), 0.5 mM EDTA, 20 mM EGTA, 15 mM MgCl2, 1 mM dithiothreitol, 2 mM NaF, 1 mM NaVO4, 10% glycerol, 0.5% I-gepal, and 1× protease inhibitors (Roche Complete). Lysates were centrifuged for 45 min at 18,000 × g and 4°C, and supernatants were used for kinase assays. Protein concentrations were measured using the Bradford assay (Bio-Rad). Cyclin B1 agarose-conjugated antibody (Santa Cruz, sc-245AC) (5 μg) was incubated with lysates (500 μg) plus BSA at 0.5 mg/ml for 12 h at 4°C. Immunoprecipitated protein complexes [cyclinB1/Cdk1(Cdc2)] were washed twice in lysis buffer and twice in kinase reaction buffer (80 mM β-glycerophosphate [pH 7.3], 20 mM EGTA, 15 mM MgCl2, 10 mM dithiothreitol). For kinase assays, each sample was incubated with 20 μl of kinase reaction buffer, 20 μM ATP, 5 to 10 μCi [γ-32P]ATP (Perkin-Elmer Life Sciences, BLU-502A), and 2.0 μg histone H1 (Roche, 1004875) at 30°C for 30 min. Reactions were terminated by the addition of 10 μl of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Samples were separated on 12% polyacrylamide gels. Phosphorylation was analyzed by autoradiography and quantified by phosphorimager analysis (Molecular Probes, Typhoon Trio).
Immunoblot analysis.
Total protein lysates were prepared from primary MEFs using a lysis buffer consisting of 150 mM NaCl, 50 mM Tris [pH 8.0], and 1% NP-40. Protein concentrations were standardized using the Bradford assay (Bio-Rad), and equal loading was confirmed by Ponceau S staining of polyvinylidene difluoride membranes after electroblotting as well as with a loading control (α-tubulin). The antibodies used for immunoblotting were to p27 (N20) and Cdc2 [P34(17)] from Santa Cruz, p21 (rabbit anti-mouse antibody) from BD Pharmingen, p53 Novocastra (CM5), phospho-Chk1 (133D3) and phospho-Cdc2 Thr161 from Cell Signaling, and α-tubulin (clone B-5-1-2) from Sigma. Blots were developed using a chemiluminescence detection kit for horseradish peroxidase (Pierce).
Statistical methods.
Two-sample unpaired t tests for samples with unequal variance were used for comparison unless otherwise stated. The 95% confidence limits and P values are two sided.
RESULTS
Mutations in genotoxin-treated p27-deficient mice.
Given that p27 functions as a tumor suppressor in multiple epithelial tissues but has no apparent effect on the steady-state proliferative index of adult epithelial tissues (21), we hypothesized that p27 may be important in the cellular response to DNA damage. We therefore investigated the role of p27 in somatic mutagenesis by crossing p27-deficient mice to lacI transgenic mice (Big Blue mice) and determining the frequency of ENU-induced mutations in the lacI gene in colonic epithelium. The use of Big Blue mice in a mammalian mutation assay offers the advantage of determining the in vivo mutagenicity of chemicals in any target organ as well as the ability to identify the specific nature of the induced genetic lesion through recovery and sequencing of the transgene (10). The spontaneous MFs (± SEM) in colons from p27+/+, p27+/−, and p27−/− mice were similar: 4.3 × 10−5 ± 0.3 × 10−5, 3.8 × 10−5 ± 0.3 × 10−5, and 5.1 × 10−5 ± 1.0 × 10−5, respectively (Table 1). Three weeks after ENU treatment, the MFs increased to 51.0 × 10−5 ± 3.3 × 10−5, 63.0 × 10−5 ± 2.0 × 10−5, and 117.3 × 10−5 ± 4.7 × 10−5 in mice with the three different p27 genotypes, respectively (Table 1). The ENU-induced MFs in both p27+/− and p27−/− mice were significantly higher than that in p27+/+ mice (Fig. 1A) (Cochran-Armitage test), with p27−/− mice demonstrating a greater-than-twofold increase in MF compared to p27+/+ mice. The number of cells bearing distinct mutations recovered in these MF assays was significantly higher in p27-deficient mice, indicating that clonal expansion after mutation fixation does not explain the increase in the frequency of recovered mutations.
TABLE 1.
MF in control and ENU-treated groups
| Genotype | Control
|
ENU treatment
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mouse | PFU (105) | No. of mutantsa | MF (10−5) | Mouse | PFU (105) | No. of mutants | MF (10−5) | |
| p27+/+ | 1 | 3.61 | 13 | 3.6 | 1 | 3.63 | 159 | 43.8 |
| 2 | 3.80 | 19 | 5.0 | 2 | 2.61 | 104 | 39.8 | |
| 3 | 2.88 | 15 | 5.2 | 3 | 3.91 | 203 | 51.9 | |
| 4 | 3.02 | 10 | 3.3 | 4 | 3.37 | 178 | 52.8 | |
| 5 | 2.88 | 11 | 3.8 | 5 | 2.62 | 167 | 63.6 | |
| 6 | 2.31 | 10 | 4.3 | 6 | 1.73 | 100 | 57.9 | |
| 7 | 3.25 | 15 | 4.6 | |||||
| Mean ± SEM | 4.3 ± 0.3 | 51.0 ± 3.3 | ||||||
| p27+/− | 1 | 5.68 | 23 | 4.1 | 1 | 4.36 | 253 | 58.1 |
| 2 | 3.42 | 14 | 4.1 | 2 | 2.88 | 180 | 62.6 | |
| 3 | 2.72 | 14 | 5.1 | 3 | 4.35 | 253 | 58.2 | |
| 4 | 2.69 | 11 | 4.1 | 4 | 3.02 | 197 | 65.2 | |
| 5 | 3.01 | 8 | 2.7 | 5 | 3.55 | 250 | 70.5 | |
| 6 | 3.05 | 11 | 3.6 | 6 | 2.62 | 176 | 67.1 | |
| 7 | 3.45 | 10 | 2.9 | |||||
| Mean ± SEM | 3.8 ± 0.3 | 63.0 ± 2.0 | ||||||
| p27−/− | 1 | 2.04 | 19 | 4.4 | 1 | 1.88 | 223 | 118.3 |
| 2 | 2.52 | 7 | 2.8 | 2 | 1.97 | 186 | 94.6 | |
| 3 | 1.21 | 3 | 2.5 | 3 | 2.13 | 255 | 119.9 | |
| 4 | 2.46 | 24 | 9.8 | 4 | 2.28 | 284 | 124.8 | |
| 5 | 2.08 | 9 | 4.3 | 5 | 2.57 | 321 | 124.9 | |
| 6 | 2.38 | 11 | 4.6 | |||||
| 7 | 2.20 | 13 | 5.9 | |||||
| Mean ± SEM | 5.1 ± 1.0 | 117.3 ± 4.7 | ||||||
The number of mutants is corrected for potential clonal expansion.
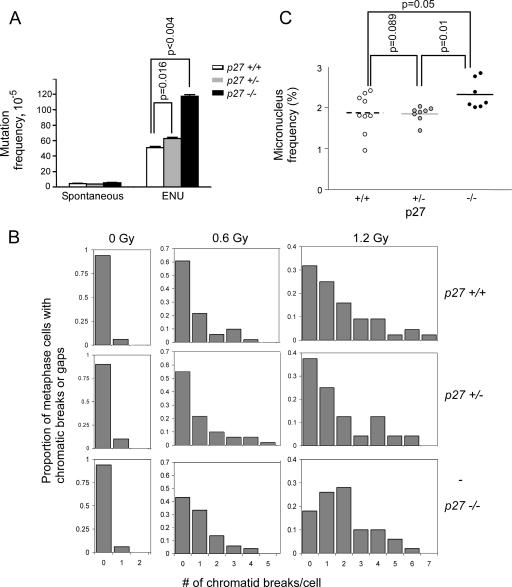
FIG. 1.
Increase in DNA damage-induced chromosome damage and mutations in p27-deficient mice. (A) Mice of all three p27 genotypes were analyzed for MF in colonic epithelium of lacI transgenic mice following treatment with the point mutagen ENU. p27+/− and p27−/− mice had significantly higher ENU-induced mutation frequencies. Vertical bars represent the SEM. (B) Spleen cultures were prepared from mice of each p27 genotype. At least 100 metaphase spreads per p27 genotype were analyzed for chromatid gaps and breaks following gamma irradiation in two independent experiments, one of which is shown. (C) Mice were subjected to retroorbital bleeds before and 48 h following 0.6 Gy gamma irradiation. p27−/− mice have a significantly higher micronucleus frequency in reticulocytes than either p27+/+ or p27+/− mice. Horizontal bars indicate the mean frequency of micronucleus formation.
The predominant spontaneous mutations were G·C→A·T transitions and G·C→T·A transversions, regardless of p27 genotype (data not shown), which is consistent with previous studies (9). Spontaneous −1 frameshifts were relatively minor in p27+/+ mice (3.5%) but accounted for 8.4% and 9.4% in the p27+/− and p27−/− mice, respectively (P = 0.036, Fisher's exact test), suggesting that repair in the absence of p27 may rely more heavily on the activity of DNA polymerases that are prone to introducing −1 frameshifts, such as Pol κ, a member of the Y family of translesional DNA polymerases (34). Treatment with ENU altered the mutation spectrum of the colonic epithelium in all p27 genotypes. ENU induced an increase in the frequency of nearly all types of base substitutions except G·C→C·G transversions, with A·T→T·A substitutions accounting for 30.8% of the spectrum. The frequencies of G·C→A·T, G·C→T·A, and A·T→C·G substitutions in p27−/− mice was significantly higher than that found in wild-type mice (Table 2). p27+/− mice displayed an intermediate phenotype, indicating that p27 is haploinsufficient for the ability to guard against ENU-induced mutation. The frequency of ENU-induced −1 frameshifts remained low in the p27+/+ and p27+/− mice (0.6 × 10−5 ± 0.3 × 10−5 and 0.8 ± 0.4 × 10−5, respectively) but was increased to 8.2 × 10−5 ± 0.4 × 10−5 in p27−/− mice (Table 2), providing further evidence that a distinct repair process favoring −1 frameshifts may occur in p27−/− mice.
TABLE 2.
ENU-induced mutation specificity
| Mutation type |
p27+/+
|
p27+/−
|
p27−/−
|
|||
|---|---|---|---|---|---|---|
| Mutation % (no./total)a | Mean MF ± SEM (10−5)b | Mutation % (no./total) | Mean MF ± SEM (10−5) | Mutation % (no./total) | Mean MF ± SEM (10−5) | |
| Transition | ||||||
| G·C>A·T | 30.8 (36/117) | 17.0 ± 4.5 | 33.1 (43/130) | 21.2 ± 2.3 | 26.8 (19/71) | 30.5 ± 4.0c |
| A·T>G·C | 18.8 (22/117) | 7.1 ± 2.5 | 18.5 (24/130) | 10.0 ± 3.0 | 12.7 (9/71) | 16.0 ± 8.0 |
| Transversion | ||||||
| G·C>T·A | 8.5 (10/117) | 3.9 ± 1.8 | 12.3 (16/130) | 7.3 ± 2.0 | 16.9 (12/71) | 19.6 ± 2.0d |
| G·C>C·G | 0 | 0 | 2.3 (3/130) | 1.3 ± 0.6 | 1.4 (1/71) | 1.8 ± 0.9 |
| A·T>T·A | 30.8 (36/117) | 16.2 ± 3.0 | 23.8 (31/130) | 16.0 ± 3.5 | 26.8 (19/71) | 30.6 ± 7.0 |
| A·T>C·G | 7.7 (9/117) | 2.8 ± 1.4 | 8.5 (11/130) | 5.2 ± 1.4 | 7.0 (5/71) | 8.9 ± 0.4e |
| Other | ||||||
| +1 frameshifts | 0 | 0 | 0 | 0 | 0 | 0 |
| −1 frameshifts | 1.7 (2/117) | 0.6 ± 0.3 | 0.8 (1/130) | 0.8 ± 0.4 | 7.0 (5/71) | 8.2 ± 0.4 |
| Deletion | 0 | 0 | 0.8 (1/130) | 0.8 ± 0.4 | 0 | 0 |
| Insertion | 0 | 0 | 0 | 0 | 0 | 0 |
| Complex changes | 1.8 (2/117) | 1.2 ± 0.6 | 0 | 0 | 1.4 (1/71) | 1.8 ± 0.8 |
The number of mutants is corrected for potential clonal expansion.
Mutation frequency in each class of mutation.
P = 0.01, Fisher's exact test.
P = 0.001, Fisher's exact test.
P = 0.004, Fisher's exact test.
Frequency of chromatid gaps and breaks.
Chromatid gaps and breaks represent dangerous DNA double-strand breaks that when unrepaired lead to unjoined chromosome elements and if misrepaired may promote breakage-bridge-fusion cycles and continued genetic instability. The frequency of chromatid gaps and breaks is often used as a measure of the cell's ability to repair its DNA or of its ability to arrest prior to entering mitosis. The spontaneous frequencies of chromatid gaps and breaks in lipopolysaccharide-stimulated splenocytes were similar among the p27 genotypes (Fig. 1B). However, following 0.6 Gy and especially 1.2 Gy gamma irradiation, the frequency of cells containing breaks and the number of breaks per cell were increased in splenocytes from p27−/− mice compared to those from p27+/+ mice. p27+/− mice displayed an intermediate response (P = 0.03, test for an ordered effect across genotypes).
Frequency of micronucleus formation.
The micronucleus assay is a standard test used for the detection of chromosomal damage. It measures the ability of cells to repair DNA damage by detecting the formation of micronuclei (acentric fragments or complete chromosomes that failed to segregate properly and were excluded from the nucleus). Whereas the spleen culture assay involves a short period of in vitro culture prior to assessment of DNA damage, the micronucleus assay directly measures the in vivo response of peripheral blood cells without intermediate culture and thus provides a direct picture of the DNA damage response of cells within the context of the organism. We analyzed micronucleus formation in p27+/+, p27+/−, and p27−/− mice following low-dose gamma irradiation (high-dose irradiation arrests the majority of reticulocytes and thereby prevents the formation and subsequent recovery of micronuclei). While the spontaneous micronucleus frequencies (mean ± SEM) were similar in the p27+/+, p27+/−, and p27−/− genotypes (0.30% ± 0.02%, 0.26% ± 0.02%, and 0.29% ± 0.02%, respectively), by 48 h following 0.6 Gy gamma irradiation, it had increased to 1.9% ± 0.16%, 1.8% ± 0.07%, and 2.3% ± 0.13% (Fig. 1C). The micronucleus frequency from p27−/− mice was significantly higher than that from either p27+/− or p27+/+ mice.
p27 is expressed in the proliferative compartment of the intestinal crypt.
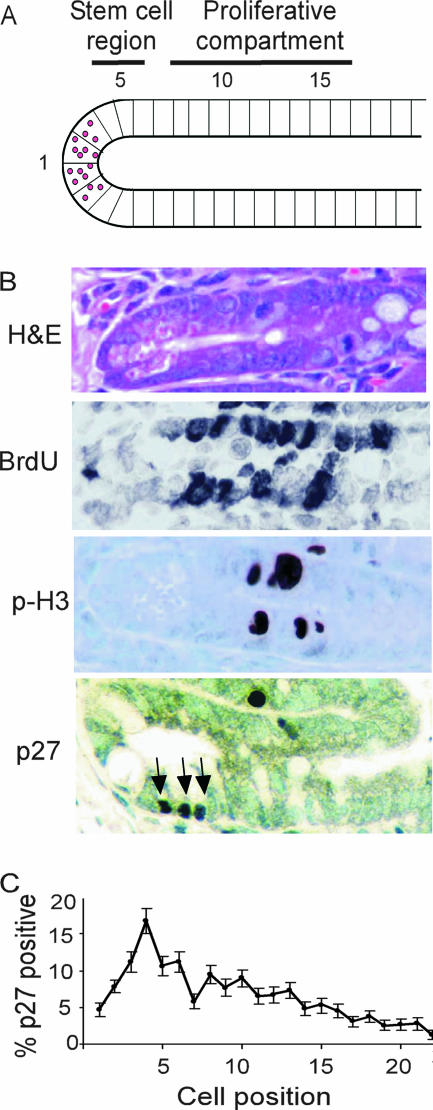
p27 is a known inhibitor of GI tumorigenesis, and p27-deficient colon cells show an increase in MF following genotoxic insult. In order to evaluate the potential target cells of p27 deficiency in mutagenesis and intestinal tumorigenesis, we assessed the spatial distribution of p27 expression within the intestinal crypts from normal mice. p27-positive cells were found throughout the intestinal crypt; however, the majority were within the bottom 10 cell positions, peaking at cell position four (Fig. 2). Extensive kinetic analysis by Potten and colleagues has shown that the presumptive stem cell of the intestinal crypt resides at or near cell position 4 and that cell positions 5 to 15 contain the transit-amplifying cells, with proliferation peaking at cell position 10 (reviewed in reference 1) (Fig. 2B). Thus, p27 expression occurs within the proliferative compartment of the intestinal epithelium.
FIG. 2.
p27 is expressed in the proliferative compartment of the intestinal crypt (A) Graphic representation of crypt cell position in the small intestine. (B) Representative section of small intestinal crypt stained with H&E and antibodies to bromodeoxyuridine, phospho-histone H3, and p27 (magnification, ×600). Arrows point to p27-positive crypt cells. (C) Distribution of p27-positive staining in the crypt. Error bars indicate SEMs.
p27 participates in the G2/M checkpoint in intestinal crypt cells in response to low-dose gamma irradiation.
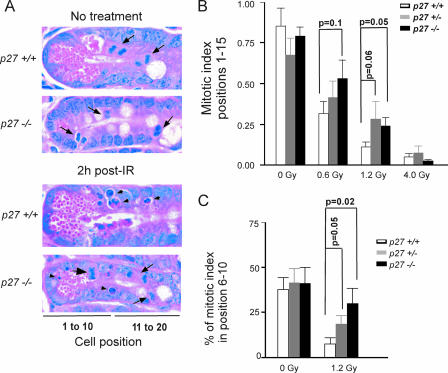
As p27 is expressed in the GI crypt cells and p27 deficiency increases MF in the GI, we measured the effect of p27 on cell cycle kinetics in response to a specific genotoxic agent, gamma irradiation. Radiation has been used extensively to study cell kinetics with the intestinal crypt (33). Nakayama et al. demonstrated previously that p27 deficiency does not impair arrest at the G1/S checkpoint in response to gamma irradiation (21), and our unpublished results were consistent with this finding. We therefore investigated the role of p27 at the radiation-induced G2/M checkpoint. p27+/+, p27+/−, and p27−/− mice were exposed to various doses of gamma irradiation from 0.6 to 4.0 Gy, and the MI of GI crypt cells was assessed.
The MIs within the intestinal crypts from unirradiated mice were similar between p27 genotypes (Fig. 3B). The p27 genotype did not alter the apoptotic index at any dose of radiation (data not shown). Radiation-induced G2/M arrest, however, was impaired in p27-deficient mice at low doses (0.6 and 1.2 Gy), although only 1.2 Gy reached statistical significance (Fig. 3). At higher doses (4 Gy), mitosis was equally inhibited in both p27+/+ and p27−/− intestinal epithelia, indicating that the effect of p27 is only seen at low doses of irradiation. Radiation-induced cell cycle arrest was most pronounced in cell positions 6 to 10 within the crypt, with an 80% reduction in mitotic figures in wild-type mice (P = 0.001) compared to only a 27% reduction in p27−/− mice (P = 0.39). The MI in position 6 to 10 from irradiated p27+/+ mice was reduced significantly compared to that in p27−/− mice (P = 0.02). p27+/− animals again displayed an intermediate phenotype, indicating that even partial loss of p27 impairs G2/M arrest. Thus, the impaired radiation-induced G2/M arrest conferred by p27 deficiency was most pronounced in cell positions 6 to 10, near the peak of p27 expression (Fig. 2).
FIG. 3.
p27 deficiency impairs G2/M arrest in irradiated GI crypt cells. (A) Mitotic cells (arrows) are seen in both p27+/+ and p27−/− mice before treatment. Note the reduction of mitotic cells in irradiated p27+/+ but not p27−/− crypts. Increased apoptosis is seen in both p27+/+ and p27−/− crypts after irradiation (arrowheads) (magnification, ×1,000). (B) p27−/− mice have a significantly higher MI in cell positions 1 to 15 of the SI crypt than p27+/+ mice following 1.2 Gy gamma irradiation. p27+/− mice display an intermediate phenotype. (C) p27−/− mice have a significantly higher frequency of mitotic figures in positions 6 to 10 of the SI crypt than p27+/+ mice following 1.2 Gy gamma irradiation. Mean mitotic indices plus or minus the SEM are shown.
p27 regulates the G2/M checkpoint at early time points following gamma irradiation.
We further analyzed the impact of p27 deficiency on the response to gamma irradiation by using primary MEFs. Dose-response studies showed that a dose of 5 Gy resulted in a similar percentage of G2/M arrest in MEFs as a dose of 1.2 Gy in GI epithelium (data not shown). Whereas mitotic activity in asynchronously growing p27+/+ MEFs was reduced by an average of 79% ± 2% at 2 h following 5 Gy, mitotic activity was reduced by only 39% ± 19% in p27−/− cells (Fig. 4A). In order to analyze the role of p27 specifically at the G2/M boundary, we irradiated synchronized MEFs. Following release from synchronization, p27+/+ and p27−/− cells proceed through the cell cycle with similar kinetics (Fig. 4D). p27+/+ cells exhibited a pronounced G2/M arrest as indicated by a reduced MI at 2 h following irradiation (mean of 76% ± 8% reduction in MI), while p27−/− cells exhibited a strongly attenuated G2/M arrest (mean of 13% ± 41% reduction in MI) (Fig. 4B).
Both p27+/+ and p27−/− MEFs displayed a similar increase in the relative number of aberrant mitotic figures per total mitotic cells following gamma irradiation (data not shown). However, due to the higher MI of p27−/− MEFs, the absolute number of aberrant mitotic figures was higher in p27−/− MEFs (53 ± 10 per 106 cells) than in p27+/+ cells (21 ± 11 per 106 cells) (Fig. 4C). Together, these results indicate that a lack of G2/M arrest directly leads to an increased frequency of aberrant chromosomal segregation, and this is exacerbated in the absence of p27.
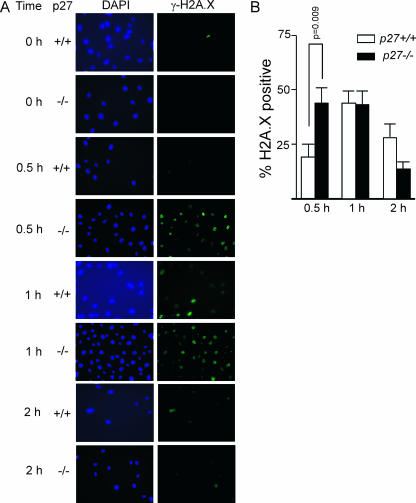
As another measure of chromosome damage at early time points following genotoxic damage, we analyzed the kinetics of DNA double-strand break formation in the absence of p27. Phosphorylation of the variant histone H2A.X (also known as gamma-H2A.X) is an early response to DNA double-strand break formation and can be visualized as foci by immunofluorescence (35, 36). Low-dose irradiation of late S/G2 phase-synchronized MEFs led to a statistically significant increase in phospho-H2A.X staining in p27−/− cells compared to p27+/+ cells (P = 0.009) by 30 min following exposure, but by 1 h and 2 h following irradiation, the frequencies of phospho-H2A.X positive cells were similar in both p27+/+ and p27−/− cells (Fig. 5).
FIG. 5.
p27 deficiency leads to an increase in DNA double-strand breaks at early time points following gamma irradiation. (A) Fluorescence images (magnification, ×200) of representative fields in synchronized primary MEFs harvested at 0 h and at 0.5 h and 1 h following 5 Gy irradiation. All cells are positive for phospho-H2A.X staining by 0.5 h following irradiation; however, the detection threshold was standardized and set to illustrate the difference in signal intensity between p27+/+ and p27−/− MEFs. (B) p27−/− MEFs have a higher frequency of phospho-H2A.X positive cells by 30 min following irradiation than p27+/+ MEFs (P = 0.009). Error bars indicate SEMs.
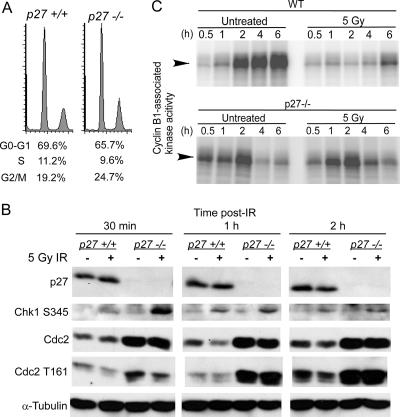
Mechanism of impaired G2/M arrest in p27-deficient cells.
Entry into and progression through mitosis are highly regulated by activation of Cdc2/cyclin B kinase via phosphorylation of Cdc2. Following DNA damage, a signal is transduced via the Chk1 kinase to inhibit Cdc2, leading to G2/M arrest. Phosphorylation of Chk1 on S345 was seen in both p27+/+ and p27−/− cells at 30 min, 1 h, and 2 h after irradiation of synchronized cultures in late S/G2 (Fig. 6B). p27 itself was not induced at early time points following irradiation (Fig. 6B), while p53 and the Cdk inhibitor p21 were expressed in both p27+/+ and p27−/− cells and induced by irradiation by 24 h (data not shown). As checkpoint signaling was intact in the absence of p27, we next examined Cdc2 levels and activity. Synchronized p27−/− cells in late S/G2 showed higher levels of Cdc2 compared to p27+/+ cells both before and after irradiation (Fig. 6B). This increase in Cdc2 was observed only in synchronized MEFs (data not shown). Accordingly, the p27−/− cells also contained higher levels of activated Cdc2 as indicated by phosphorylation on T161 (Fig. 6B). Synchronized wild-type MEFs irradiated in S/G2 showed the expected inhibition of Cdc2-associated kinase activity especially at 2 to 4 h after IR (Fig. 6C, compare untreated versus 5 Gy panels). In contrast, p27 null cells showed less inhibition of Cdc2 activity after IR, notably at the 2-h time point. As inhibition of Cdc2 activity is necessary for G2/M arrest, this explains the impairment of G2/M arrest in p27 null cells at early time points following irradiation. This may also explain why impaired arrest in p27-deficient cells is apparent only at low doses of IR and at early time points; higher doses or longer times may activate a sufficiently strong response to inhibit the higher levels of Cdc2 seen in p27−/− cells.
FIG. 6.
p27 deficiency leads to impaired inhibition of Cdc2 in irradiated G2/M cells. (A) FACS analysis demonstrates efficient synchronization of both p27+/+ and p27−/− primary MEFs used for panels B and C. (B) Whole-cell lysates were prepared from synchronized primary MEFs irradiated in late S/G2, harvested at various times following 5 Gy gamma irradiation, and immunoblotted for the indicated proteins. (C) p27+/+ and p27−/− immortalized MEFs were synchronized and then irradiated with 5 Gy in late S/G2 phase of the cell cycle. At the indicated times, cyclin B1 complexes were immunoprecipitated from cell lysates, and kinase activity toward histone H1 was measured. WT, wild type.
p27 deficiency impairs gamma irradiation-induced G2/M arrest in autochthonous tumor cells.
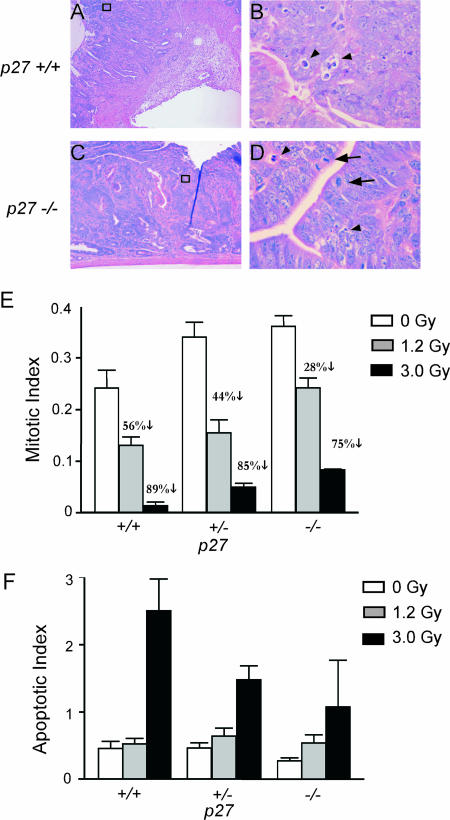
As a preliminary investigation into whether the ability of p27 to regulate the cell cycle response to genotoxic damage is related to the prognostic role of p27 in cancer, we analyzed the response of GI adenomas and adenocarcinomas from Apc mutant mice to whole-body irradiation based on p27 genotype. Although radiotherapy is rarely used in human GI cancer, assessment of MI in irradiated tumors is a direct, in vivo measure of the response of these cells to a clinically relevant genotoxic stress. The majority of human colon cancers bear mutations in APC, and thus GI tumors from Apc mutant mice serve as a relevant preclinical model for therapy response. The MI was significantly greater in GI tumor cells from p27-deficient mice, both before (P = 0.04) and after whole-body irradiation (1.2 Gy, P < 0.001; 3 Gy, P = 0.04) (Fig. 7). The MI was reduced by 56% in tumors from irradiated p27+/+ mice (1.2 Gy) but by only 44% and 28% in tumors from p27+/− and p27−/− mice, respectively. Thus, p27 deficiency impaired G2/M arrest in tumor cells from irradiated mice. This impairment was p27 gene dosage dependent, indicating that the efficiency of the G2/M checkpoint in autochthonous tumor cells is markedly sensitive to p27 levels. p27-deficient tumor cells also showed impaired apoptotic response to 3 Gy, although this did not reach statistical significance (Fig. 7F).
FIG. 7.
p27 deficiency impairs G2/M arrest in autochthonous tumor cells. Apc mutant, p27+/+, p27+/−, and p27−/− mice bearing intestinal adenomas and adenocarcinomas were irradiated and tumors removed 2 h later. (A to D) H&E-stained sections of p27+/+ and p27−/− adenocarcinomas at 2 h following 1.2 Gy (magnifications, ×100 [A and C] and ×1,000 [B and D]). Note persistent mitotic cells (black arrows) in p27−/− compared to p27+/+ adenocarcinomas. Arrowheads indicate apoptotic bodies. (E) p27+/+ GI tumors have a larger radiation-induced reduction in MI than p27−/− GI tumors. p27−/− GI tumors have a significantly higher MI than p27+/+ GI tumors, regardless of irradiation dose (unirradiated, P = 0.04; 1.2 Gy, P = 0.00001; or 3 Gy, P = 0.04). (F) p27+/+ GI tumors have a larger, although not statistically significant, radiation-induced increase in apoptotic index than p27−/− GI tumors. Mean indices plus the SEM are shown.
DISCUSSION
Two mechanisms of tumor suppression by p27.
In two models of GI tumorigenesis (chemical induction and germ line Apc mutation), we found that p27 functions as a tumor suppressor via inhibition of tumor cell proliferation (28). Here we show that p27 also functions to reduce mutation induction and chromosome damage generated by DNA-damaging agents. p27 deficiency led to an increased accumulation of point mutations as well as a modest increase in large-scale chromosomal aberrations such as chromatid breaks and micronucleus formation in response to genotoxic stress. The increase in MF in p27−/− mice in the Big Blue mouse assay was comparable to that in other mice with deficiencies of DNA damage response genes, such as Blm and Brca2 (44, 45). Oncogenes are frequently activated by point mutations, and tumor suppressors are frequently inactivated by a variety of mechanisms, from point mutation to large genomic deletions. Indeed, accumulation of mutations is generally believed to be rate limiting for cancer (19). Reduction of p27 thereby enhances both mitogenesis and mutagenesis on the incipient cancer cell. The observation that loss of p27 increases the frequency of mutations and chromosomal aberrations following genotoxic insult provides an explanation for the sensitivity of p27-deficient mice to carcinogens and lends further support for the use of p27-deficient mice as a bioassay for carcinogenicity (27). It also suggests that p27 may play a previously unrecognized role in tumor response to genotoxic therapy and subsequent patient survival.
Role of p27 in response to genotoxic damage.
Bartek and Lukas (2) have suggested that the cell cycle checkpoint response to DNA damage may occur in two waves, with an initial, transient response occurring within 20 to 30 min and lasting only a few hours, while a second delayed, but more sustained response is mediated by the p53/p21 cascade. In normal, unperturbed intestinal epithelium, p27 is expressed in the proliferative compartment. In contrast, the expression of the related CDK inhibitor p21, while low in unirradiated murine intestinal crypts, is strongly upregulated in a p53-dependent manner by 4 h following gamma irradiation (46). p27 deficiency affected radiation-induced G2/M arrest at early (<4 h) but not later time points, suggesting a physiologic role for p27 protein in the immediate response to genotoxic insult, prior to p53-dependent upregulation of p21. The presence of p27 in the GI crypt progenitor cells may function as an immediate-early attenuator for halting cell cycle progression following genotoxic exposure during the time required for p53-dependent transcription and translation of p21 to bring about a sustained cell cycle arrest.
Although other reports have hinted that p27 functions at the G2/M transition, the physiologic relevance of this role remains unknown (7, 22). Chibazakura et al. found that p27 acts in concert with p21 and p107 to inhibit cyclin A-CDK activity following metaphase (7). Nakayama et al. showed that Skp2−/− cells undergo endoreduplication as a result of p27 accumulation in the absence of Skp2-directed proteolysis, indicating that degradation of p27 is required for entry into mitosis (22). Sugihara et al. (40) demonstrated that accumulation of p27 protein at 24 to 48 h following high-dose gamma irradiation is required for suppression of centrosome amplification and prevention of chromosomal instability; however, they did not evaluate either low-dose irradiation or time points earlier than 10 h postirradiation. We observed that p27 protein levels are not altered at early time points following irradiation. Cdc2 levels, however, are higher in G2/M phase p27−/− cells, even in the absence of irradiation, than in p27+/+ cells. Cdc2 is known to exhibit variation in both RNA and protein levels in addition to the well-characterized posttranslational regulation via inhibitory and activating phosphorylation (17). The mechanism by which p27 might affect Cdc2 protein levels, however, is unclear.
Cdc2 physically interacts with p27 and may be a target of inhibition by p27 (22). We demonstrated that inhibition of Cdc2-associated kinase activity at early time points (<4 h) following IR is impaired in the absence of p27. This agrees with the observation that p27 deficiency impairs radiation-induced G2/M arrest at early time points, and taken together, these results provide a plausible mechanism for the increase in the frequency of mutations and chromosomal aberrations following genotoxic insult in p27-deficient mice. The delay in G2/M arrest seen in p27−/− cells may be a result of higher levels of Cdc2, which would titrate inhibitory signals, thus necessitating stronger or more sustained DNA damage signaling in order to inhibit its activity and bring about cell cycle arrest. Higher genotoxin doses might elicit stronger cell cycle arrest signals, thus more efficiently inactivating CDKs even in the absence of p27. Thus, p27 functions in the immediate-early DNA damage response to low, more physiologic levels of DNA damage.
Clinical relevance.
Radiotherapy for cancer has conventionally been administered as a fractionated dose, i.e., multiple administrations of a low dose of irradiation, in order to minimize the radiosensitivity of proliferating host tissues such as bone marrow and GI epithelium. The relatively recent advent of hyperfractionated radiotherapy attempts to avoid the dose-limiting effects of host radiosensitivity by further reducing the single dose of irradiation while increasing the number of doses administered (reviewed in reference 3). It is clear that intestinal epithelium responds differently depending on the radiation dose and on the molecular background of the target cells (Fig. 3B) (6). In the case of p27-deficient cells, they are more likely to continue progression through the cell cycle with concomitant fixation of mutations specifically at the low doses of irradiation that are more likely to be used in cancer therapy. In one of the few studies to examine the response of human tumors of known p27 status to radiotherapy, Oka et al. found that a high p27 labeling index prior to radiotherapy was associated with improved disease-free survival in cervical squamous cell carcinoma patients following a total dose of 27 Gy given in four or five fractionated doses (26), indicating a role for p27 in the response of tumors to fractionated radiotherapy. Further analysis of patient response to radio- or chemotherapy dose based on the p27 status of the tumor warrants investigation.
Acknowledgments
We thank M. Fero, J. Roberts, and A. Besson for providing p27 knockout mice and immortalized MEFs and for constructive comments on the manuscript.
This work was supported by the American Cancer Society, the Life Possibilities Fund, and Public Health Service grant CA099517 from the National Cancer Institute. S.R.P. was supported by an NIH training grant in the Molecular Training Program in Cancer Research through the University of Washington.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Bach, S. P., A. G. Renehan, and C. S. Potten. 2000. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21469-476. [DOI] [PubMed] [Google Scholar]
- 2.Bartek, J., and J. Lukas. 2001. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490117-122. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, M., and H. P. Beck-Bornholdt. 1999. Hyperfractionated radiotherapy: tops or flops? Med. Pediatr. Oncol. 33399-402. [DOI] [PubMed] [Google Scholar]
- 4.Cariello, N. F., and N. J. Gorelick. 1996. Database and software for the analysis of mutations at the lacI gene in both transgenic rodents and bacteria. Environ. Mol. Mutagen. 28397-404. [DOI] [PubMed] [Google Scholar]
- 5.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3227-230. [DOI] [PubMed] [Google Scholar]
- 6.Ch'ang, H. J., J. G. Maj, F. Paris, H. R. Xing, J. Zhang, J. P. Truman, C. Cardon-Cardo, A. Haimovitz-Friedman, R. Kolesnick, and Z. Fuks. 2005. ATM regulates target switching to escalating doses of radiation in the intestines. Nat. Med. 11484-490. [DOI] [PubMed] [Google Scholar]
- 7.Chibazakura, T., S. G. McGrew, J. A. Cooper, H. Yoshikawa, and J. M. Roberts. 2004. Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107. Proc. Natl. Acad. Sci. USA 1014465-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciaparrone, M., H. Yamamoto, Y. Yao, A. Sgambato, G. Cattoretti, N. Tomita, T. Monden, H. Rotterdam, and I. B. Weinstein. 1998. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 58114-122. [PubMed] [Google Scholar]
- 9.de Boer, J. G., S. Provost, N. Gorelick, K. Tindall, and B. W. Glickman. 1998. Spontaneous mutation in lacI transgenic mice: a comparison of tissues. Mutagenesis 13109-114. [DOI] [PubMed] [Google Scholar]
- 10.Dycaico, M. J., G. S. Provost, P. L. Kretz, S. L. Ransom, J. C. Moores, and J. M. Short. 1994. The use of shuttle vectors for mutation analysis in transgenic mice and rats. Mutat. Res. 307461-478. [DOI] [PubMed] [Google Scholar]
- 11.Erfle, H. L., D. F. Walsh, J. Holcroft, N. Hague, J. G. de Boer, and B. W. Glickman. 1996. An efficient laboratory protocol for the sequencing of large numbers of lacI mutants recovered from Big Blue transgenic animals. Environ. Mol. Mutagen. 28393-396. [DOI] [PubMed] [Google Scholar]
- 12.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27-deficient mice. Cell 85733-744. [DOI] [PubMed] [Google Scholar]
- 14.Fodde, R., W. Edelmann, K. Yang, C. van Leeuwen, C. Carlson, B. Renault, C. Breukel, E. Alt, M. Lipkin, and P. M. Khan. 1994. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl. Acad. Sci. USA 918969-8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredersdorf, S., J. Burns, A. M. Milne, G. Packham, L. Fallis, C. E. Gillett, J. A. Royds, D. Peston, P. A. Hall, A. M. Hanby, D. M. Barnes, S. Shousha, M. J. O'Hare, and X. Lu. 1997. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc. Natl. Acad. Sci. USA 946380-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamata, N., R. Morosetti, C. W. Miller, D. Park, K. S. Spirin, T. Nakamaki, S. Takeuchi, Y. Hatta, J. Simpson, and S. Wilcyznski. 1995. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 552266-2269. [PubMed] [Google Scholar]
- 17.Lee, M. G., C. J. Norbury, N. K. Spurr, P. Nurse, M. G. Lee, C. J. Norbury, N. K. Spurr, and P. Nurse. 1988. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature 333676-679. [DOI] [PubMed] [Google Scholar]
- 18.Loda, M., B. Cukor, S. W. Tam, P. Lavin, M. Fiorentino, G. F. Draetta, J. M. Jessup, and M. Pagano. 1997. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3231-234. [DOI] [PubMed] [Google Scholar]
- 19.Loeb, L. A. 1991. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 513075-3079. [PubMed] [Google Scholar]
- 20.Mori, M., K. Mimori, T. Shiraishi, S. Tanaka, H. Ueo, K. Sugimachi, and T. Akiyoshi. 1997. p27 expression and gastric carcinoma. Nat. Med. 3593. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, and D. Loh. 1996. Mice lacking p27 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85707-720. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama, K., H. Nagahama, Y. A. Minamishima, S. Miyake, N. Ishida, S. Hatakeyama, M. Kitagawa, S. Iemura, T. Natsume, and K. I. Nakayama. 2004. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6661-672. [DOI] [PubMed] [Google Scholar]
- 23.Nishino, H., A. Knoll, V. L. Buettner, C. S. Frisk, Y. Maruta, J. Haavik, and S. S. Sommer. 1995. p53 wild-type and p53 nullizygous Big Blue transgenic mice have similar frequencies and patterns of observed mutation in liver, spleen and brain. Oncogene 11263-270. [PubMed] [Google Scholar]
- 24.Noguchi, T., R. Kikuchi, K. Ono, S. Takeno, H. Moriyama, and Y. Uchida. 2003. Prognostic significance of p27/kip1 and apoptosis in patients with colorectal carcinoma. Oncol. Rep. 10827-831. [PubMed] [Google Scholar]
- 25.Ohtani, M., H. Isozaki, K. Fujii, E. Nomura, M. Niki, H. Mabuchi, K. Nishiguchi, M. Toyoda, T. Ishibashi, and N. Tanigawa. 1999. Impact of the expression of cyclin-dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer 851711-1718. [PubMed] [Google Scholar]
- 26.Oka, K., Y. Suzuki, and T. Nakano. 2000. Expression of p27 and p53 in cervical squamous cell carcinoma patients treated with radiotherapy alone: radiotherapeutic effect and prognosis. Cancer 882766-2773. [DOI] [PubMed] [Google Scholar]
- 27.Payne, S. R., and C. J. Kemp. 2003. p27(Kip1) (Cdkn1b)-deficient mice are susceptible to chemical carcinogenesis and may be a useful model for carcinogen screening. Toxicol. Pathol. 31355-363. [DOI] [PubMed] [Google Scholar]
- 28.Philipp-Staheli, J., K. H. Kim, S. R. Payne, K. E. Gurley, D. Liggitt, G. Longton, and C. J. Kemp. 2002. Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell 1355-368. [DOI] [PubMed] [Google Scholar]
- 29.Philipp-Staheli, J., S. R. Payne, and C. J. Kemp. 2001. p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 264148-168. [DOI] [PubMed] [Google Scholar]
- 30.Pietenpol, J. A., S. K. Bohlander, Y. Sato, N. Papadopoulos, B. Liu, C. Friedman, B. J. Trask, J. M. Roberts, K. W. Kinzler, J. D. Rowley, et al. 1995. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 551206-1210. [PubMed] [Google Scholar]
- 31.Ponce-Castaneda, M. V., M. H. Lee, E. Latres, K. Polyak, L. Lacombe, K. Montgomery, S. Mathew, K. Krauter, J. Sheinfeld, J. Massague, et al. 1995. p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 551211-1214. [PubMed] [Google Scholar]
- 32.Porter, P. L., K. E. Malone, P. J. Heagerty, G. M. Alexander, L. A. Gatti, E. J. Firpo, J. R. Daling, and J. M. Roberts. 1997. Expression of cell-cycle regulators p27 Kip-1 and cyclin E alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 3222-225. [DOI] [PubMed] [Google Scholar]
- 33.Potten, C. S. 2004. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat. Res. 161123-136. [DOI] [PubMed] [Google Scholar]
- 34.Prakash, S., R. E. Johnson, L. Prakash, S. Prakash, R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74317-353. [DOI] [PubMed] [Google Scholar]
- 35.Rogakou, E. P., C. Boon, C. Redon, and W. M. Bonner. 1999. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogakou, E. P., D. R. Pilch, A. H. Orr, V. S. Ivanova, and W. M. Bonner. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 2735858-5868. [DOI] [PubMed] [Google Scholar]
- 37.Sgambato, A., C. Ratto, B. Faraglia, M. Merico, R. Ardito, G. Schinzari, G. Romano, and A. R. Cittadini. 1999. Reduced expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27(Kip1) in human colon cancer. Mol. Carcinog. 26172-179. [DOI] [PubMed] [Google Scholar]
- 38.Shamma, A., Y. Doki, T. Tsujinaka, H. Shiozaki, M. Inoue, M. Yano, K. Kawanishi, and M. Monden. 2000. Loss of p27(KIP1) expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Oncology 58152-158. [DOI] [PubMed] [Google Scholar]
- 39.Singh, S. P., J. Lipman, H. Goldman, F. H. Ellis, Jr., L. Aizenman, M. G. Cangi, S. Signoretti, D. S. Chiaur, M. Pagano, and M. Loda. 1998. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 581730-1735. [PubMed] [Google Scholar]
- 40.Sugihara, E., M. Kanai, S. Saito, T. Nitta, H. Toyoshima, K. Nakayama, K. I. Nakayama, K. Fukasawa, M. Schwab, H. Saya, and M. Miwa. 2006. Suppression of centrosome amplification after DNA damage depends on p27 accumulation. Cancer Res. 664020-4029. [DOI] [PubMed] [Google Scholar]
- 41.Suri, A., J. deBoer, W. Kusser, and B. W. Glickman. 1996. A 3 milliTesla 60 Hz magnetic field is neither mutagenic nor co-mutagenic in the presence of menadione and MNU in a transgenic rat cell line. Mutat. Res. 37223-31. [DOI] [PubMed] [Google Scholar]
- 42.Thomas, G. V., K. Szigeti, M. Murphy, G. Draetta, M. Pagano, and M. Loda. 1998. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am. J. Pathol. 153681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsihlias, J., L. Kapusta, and J. Slingerland. 1999. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu. Rev. Med. 50401-423. [DOI] [PubMed] [Google Scholar]
- 44.Tutt, A. N., C. T. van Oostrom, G. M. Ross, H. van Steeg, and A. Ashworth. 2002. Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation. EMBO Rep. 3255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y., and J. A. Heddle. 2004. Spontaneous and induced chromosomal damage and mutations in Bloom syndrome mice. Mutat. Res. 554131-137. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, J. W., D. M. Pritchard, J. A. Hickman, and C. S. Potten. 1998. Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am. J. Pathol. 153899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, W., L. Bancroft, J. Liang, M. Zhuang, and L. H. Augenlicht. 2005. p27kip1 in intestinal tumorigenesis and chemoprevention in the mouse. Cancer Res. 659363-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasui, W., Y. Kudo, S. Semba, H. Yokozaki, and E. Tahara. 1997. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn. J. Cancer Res. 88625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]