Abstract
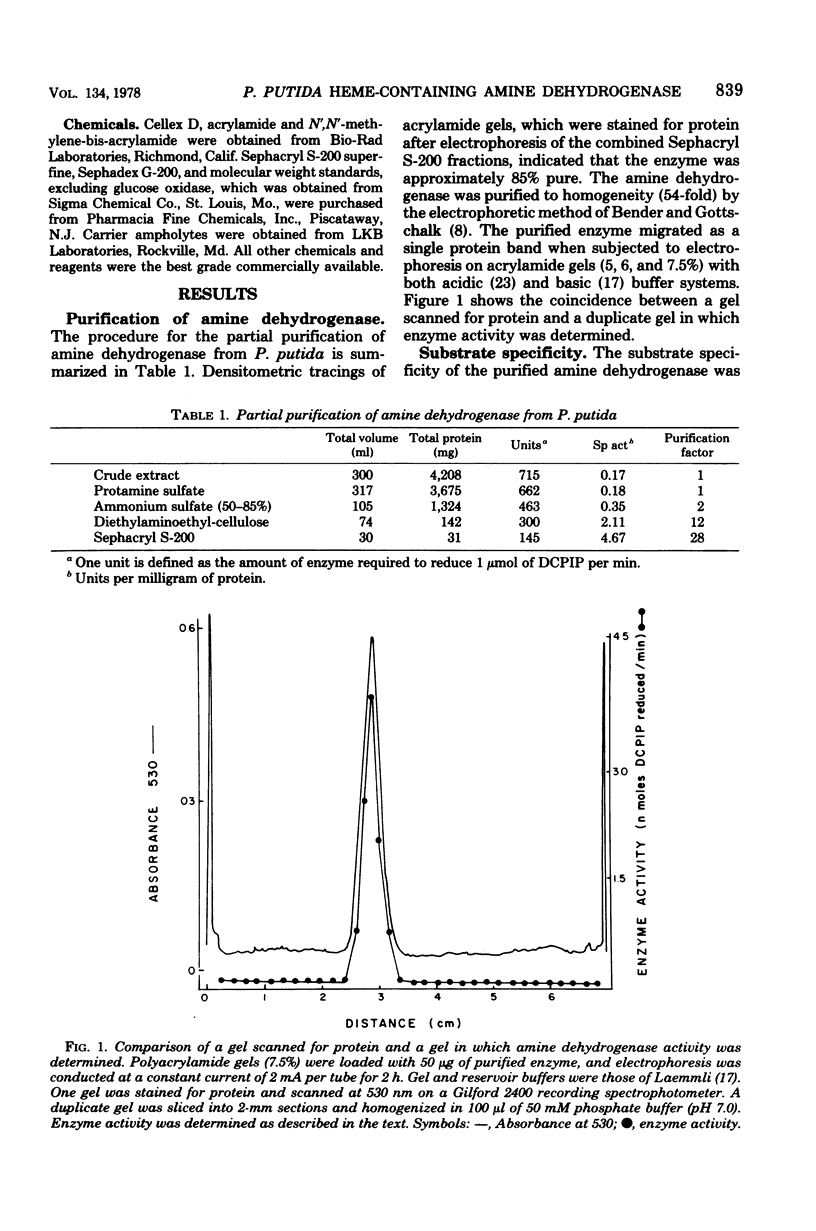
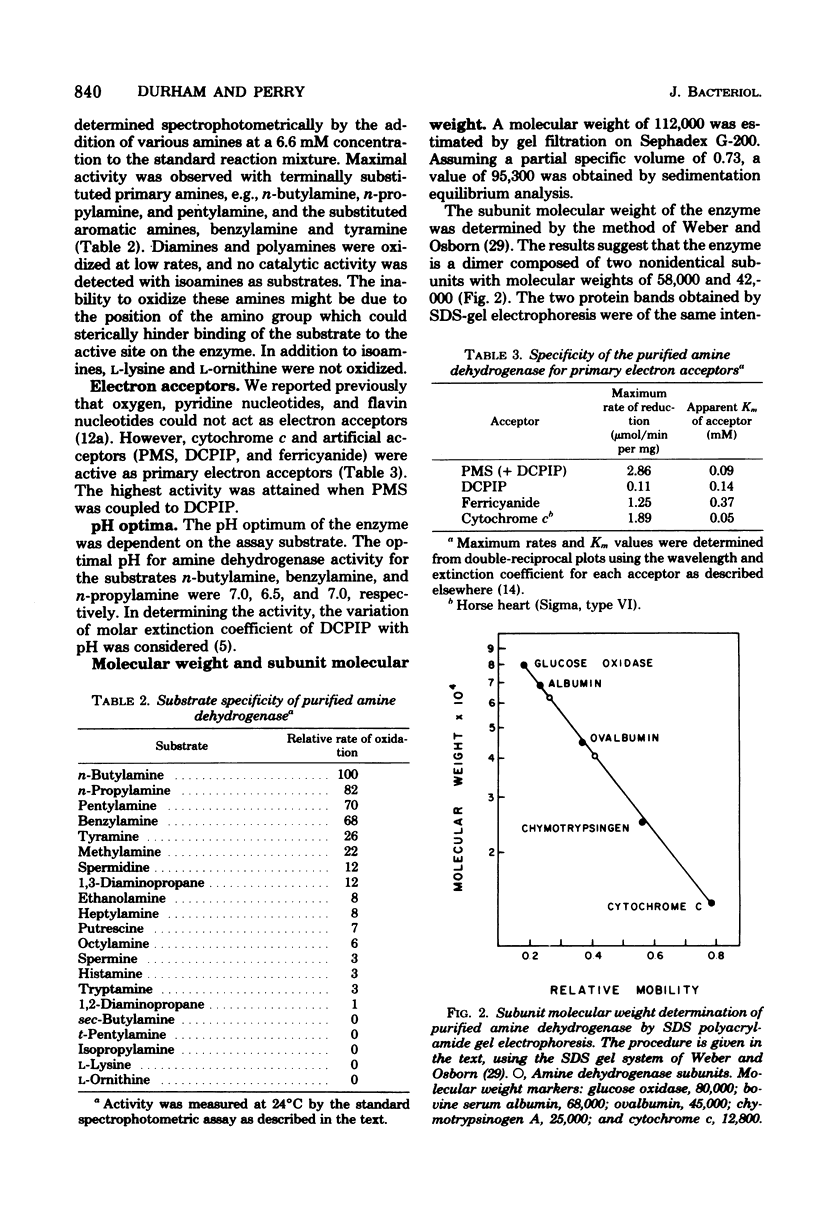
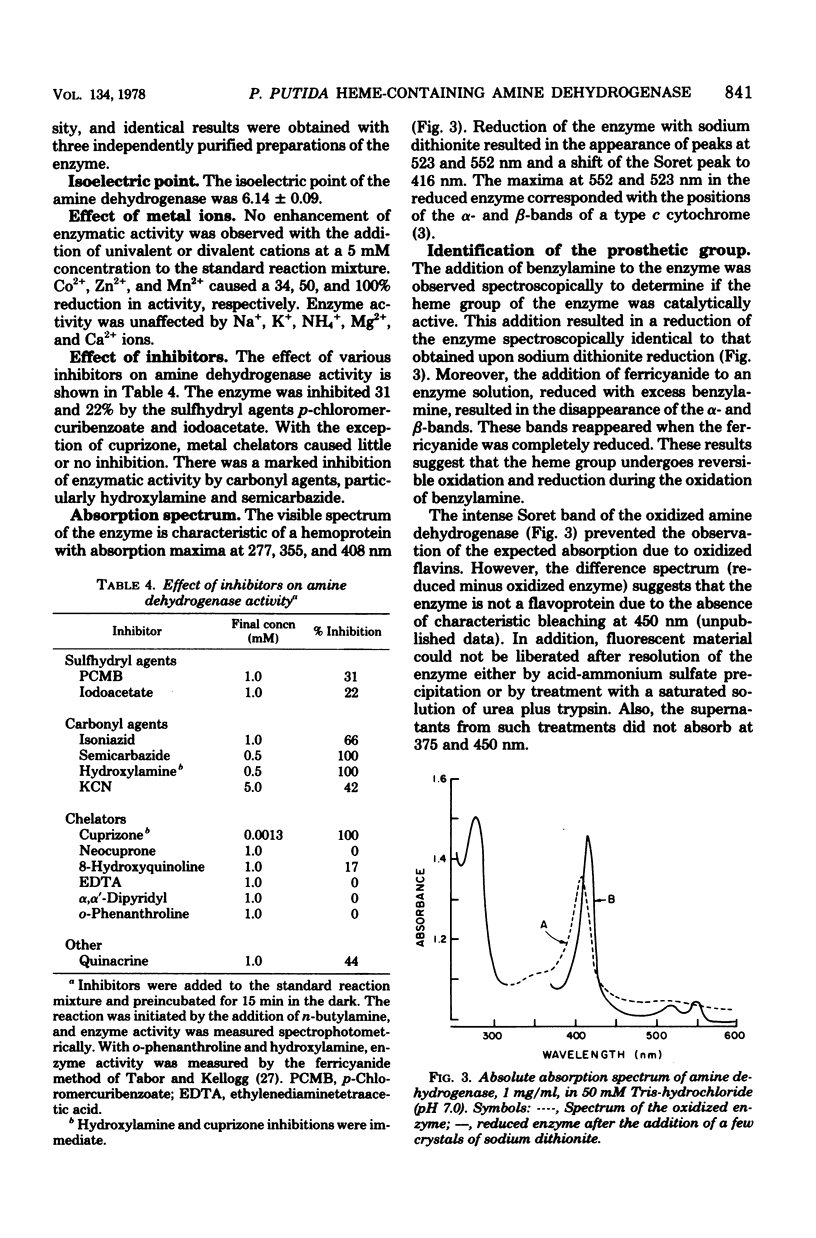
The primary amine dehydrogenase of Pseudomonas putida NP was purified to homogeneity as judged by polyacrylamide gel electrophoresis. Cytochrome c or an artificial electron acceptor was required for amine dehydrogenase activity. The enzyme was nonspecific, readily oxidizing primary monoamines, benzylamine, and tyramine; little or no measurable activity was detected with isoamines, L-ornithine, L-lysine, and certain diamines or polyamines. The pH optima for n-butylamine, benzylamine, and n-propylamine were 7.0, 6.5, and 7.0, respectively. The molecular weight of the enzyme was 112,000 as determined by gel filtration and 95,300 as analyzed by sedimentation equilibrium. Subunit analysis by sodium dodecyl sulfate gel electrophoresis suggested that the enzyme was composed of two nonidentical subunits with molecular weights of 58,000 and 42,000. The absorption spectrum of the purified enzyme was indicative of a hemoprotein, exhibiting absorption maxima at 277, 355, and 408 nm. Reduction with sodium dithionite or amine substrates resulted in absorption maxima at 523 and 552 nm and a shift in the Soret peak to 416 nm. These results suggested that the enzyme is a hemoprotein of the type c cytochrome. There was no evidence that flavins were present.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast. Enzymic and chemical properties of the crystalline enzyme. Biochem J. 1959 Nov;73:539–550. doi: 10.1042/bj0730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- Anthony C. The microbial metabolism of C1 compounds. The cytochromes of Pseudomaonas AM1. Biochem J. 1975 Feb;146(2):289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Bamforth C. W., Large P. J. Solubilization, partial purification and properties of N-methylglutamate dehydrogenase from Pseudomonas aminovorans. Biochem J. 1977 Feb 1;161(2):357–370. doi: 10.1042/bj1610357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R., Gottschalk G. Purification and properties of D-gluconate dehydratase from Clostridium pasteurianum. Eur J Biochem. 1973 Dec 3;40(1):309–321. doi: 10.1111/j.1432-1033.1973.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Blevins W. T., Perry J. J. Metabolism of Propane, n-Propylamine, and Propionate by Hydrocarbon-Utilizing Bacteria. J Bacteriol. 1972 Oct;112(1):513–518. doi: 10.1128/jb.112.1.513-518.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Perry J. J. Metabolism of n-propylamine, isopropylamine, and 1,3-propane diamine by Mycobacterium convolutum. J Bacteriol. 1975 Oct;124(1):285–289. doi: 10.1128/jb.124.1.285-289.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Mehta R. J., Hoare D. S. New obligate methylotroph. J Bacteriol. 1972 Feb;109(2):916–921. doi: 10.1128/jb.109.2.916-921.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGE J. G. Purification and properties of glucose dehydrogenase and cytochrome b from Bacterium anitratum. Biochim Biophys Acta. 1960 Dec 4;45:250–262. doi: 10.1016/0006-3002(60)91449-9. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindström A., Pettersson G. The mechanism of inhibition of pig-plasma benzylamine oxidase by the copper-chelating reagent cuprizone. Eur J Biochem. 1974 Oct 1;48(1):229–236. doi: 10.1111/j.1432-1033.1974.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Mehta R. J. Methylamine dehydrogenase from the obligate methylotroph Methylomonas methylovora. Can J Microbiol. 1977 Apr;23(4):402–406. doi: 10.1139/m77-059. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA T. Studies on acetic acid bacteria. IV. Purification and properties of a new type of alcohol dehydrogenase, alcohol-cytochrome-553 reductase. J Biochem. 1961 Mar;49:240–251. doi: 10.1093/oxfordjournals.jbchem.a127289. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. Identification of flavin adenine dinucleotide and heme in a homogeneous spermidine dehydrogenase from Serratia marcescens. J Biol Chem. 1970 Oct 25;245(20):5424–5433. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



