Abstract
Whether p27 is a cyclin D-cdk4/6 inhibitor or not is controversial, and how it might switch between these two modes is unknown. Arguing for a two-state mechanism, we show that p27 bound to cyclin D-cdk4 can be both inhibitory and noninhibitory, due to its differential-growth-state-dependent tyrosine phosphorylation. We found that p27 from proliferating cells was noninhibitory but that p27 from arrested cells was inhibitory, and the transition from a bound noninhibitor to a bound inhibitor was not due to an increase in p27 concentration. Rather, two tyrosine residues (Y88 and Y89) in p27's cdk interaction domain were phosphorylated preferentially in proliferating cells, which converted p27 to a noninhibitor. Concordantly, mutation of these sites rendered p27 resistant to phosphorylation and locked it into the bound-inhibitor mode in vivo and in vitro. Y88 was directly phosphorylated in vitro by the tyrosine kinase Abl, which converted p27 to a cdk4-bound noninhibitor. These data show that the growth-state-dependent tyrosine phosphorylation of p27 modulates its inhibitory activity in vivo.
Cell cycle progression through the G1 phase is regulated by the action of cyclin D-cdk4, cyclin D-cdk6, and cyclin E-cdk2 (30, 49). These serine/threonine kinases phosphorylate and inactivate substrates, such as the tumor suppressor retinoblastoma (Rb), which prevents S-phase entry (16, 54). cdk activity is tightly regulated by a combination of mechanisms, including changes in the cyclin level, mitogen-dependent assembly, the phosphorylation of positive and negative regulatory sites on the cdk partner, cellular localization, and interaction with stoichiometric cyclin kinase inhibitors (CKIs), such as p27Kip1, p21Cip1, p15Ink4b, and p16Ink4a (49).
p27 is a crucial molecule in a cell's response to extracellular mitogenic and antimitogenic signals, mediating growth arrest induced by transforming growth factor β (25), contact inhibition (26), growth in suspension (60), and cyclic AMP analogues (23). It was originally described as a “universal” cdk inhibitor, able to inhibit cdk4, cdk6, cdk2, and cdk1 in vitro (40, 50, 53). The three-dimensional structure of p27 complexed with cyclin A-cdk2 reveals that p27 has separate binding sites for the cdk and the cyclin, although it appears to interact exclusively with the cyclin-cdk dimer (46). p27 interacts with the cyclin through a conserved sequence, the “LFG” region (cyclin binding domain) (2, 11, 12). This domain is present in all Cip/Kip inhibitors, as well as in several cdk substrates, including Rb and p107 (1), and it appears to be the docking site for both inhibitors and substrates. p27 also interacts with cdk2 via the kinase binding region (KB) (45). p27 inhibition of cyclin-cdk activity is thought to occur by at least three mechanisms: (i) blocking the active site of the cdk, (ii) preventing substrate access by occlusing the substrate binding domain on the cyclin, and (iii) preventing an activating phosphorylation on the cdk by the cdk-activating kinase (24). In vitro, the N-terminal 108 amino acids of p27 exhibits full inhibitory function, and this region is sufficient to arrest cells in G1 (40).
The relationship of p27 and cyclin D-cdk4/6 in the cell is complex. Cyclin D-cdk4/6 complexes are mitogen sensors (37) and have at least two main roles in the cell: one catalytic and the other noncatalytic (28, 49, 59). The catalytic activity involves the phosphorylation of Rb and related family members, p107 and p130. The noncatalytic function of cyclin D-cdk4/6 involves its ability to act as a reservoir for p27 or p21. The majority of p27 in the proliferating cell is associated with cyclin D-cdk4, and this sequestration of p27 ensures that cdk2 will be catalytically active. This reservoir of p27 aids in the rapid inhibition of cdk2 when cells are challenged with antiproliferative signals. Antimitogenic signals, such as transforming growth factor β, induce the shuttling of p27 from cdk4 to cdk2 by the induction of another CKI, p15, which in turn reduces the available cyclin D-cdk4 complex (31, 43, 47). Other antimitogens, which perturb the integrity of the cyclin D-cdk4 sink, cause the same outcome (35, 41).
While it is generally accepted that p27 is always a potent cdk2 inhibitor in vitro and in vivo (8, 25), the effect of p27 binding to cyclin D-cdk4/6 complexes is controversial. In proliferating keratinocytes and epithelial or lymphoid cells, p27 does not inhibit cyclin D-cdk4, and p27-cyclin D-cdk4 complexes are always catalytically active (8, 13, 29, 49, 51). p27-associated complexes are able to phosphorylate exogenous Rb substrates, due to cdk4 and cdk6 association (8, 51). In vitro, recombinant p27 can bind cyclin D-cdk4 without causing inhibition (8, 27), suggesting that under certain circumstances, p27 may not be a cyclin D-cdk4 inhibitor in vitro as well. Others have extended this idea even further, suggesting that p27 may in fact be a required cyclin D-cdk4 activator (4, 14, 24, 27), serving to assemble or stabilize the cyclin D-cdk4 complex.
On the other hand, overexpression of p27 has been shown to inhibit cdk4/6 in some cell types (36, 56). p27-cyclin D-cdk4/6 complexes in quiescent epithelial and resting lymphoid cells are inactive (5, 35), suggesting that the controversy about the effect of p27 bound to cyclin D-cdk4 may be related to the growth state of the cell. To address this question, we analyzed cyclin-cdk complexes from proliferating and contact-arrested mink lung epithelial (Mv1Lu) cells. We found that p27-associated cyclin D-cdk4 complexes were active in cycling cells and inactive in noncycling cells. Using a p27 induction system, where the level of p27 can be increased 15-fold in an otherwise cycling cell, we were unable to inhibit cyclin D-cdk4/6, suggesting that the stoichiometry of p27 is not important. Rather, the cell has the capacity to regulate the transition between p27's bound, noninhibitory (cycling) and its bound, inhibitory (noncycling) forms in a growth-state-dependent manner. We have mapped this difference to two tyrosine residues (Y88 and Y89) in the N terminus of p27, whose phosphorylation occurs exclusively in proliferating cells. Loss of this phosphorylation in vitro and in vivo converts p27 to a bound inhibitor of cyclin D-cdk4. We suggest that this phosphorylation modulates p27's inhibitory activity and may account for p27's functional difference in cycling and noncycling cells.
MATERIALS AND METHODS
Cell culture and transfection.
Mv1Lu cells were maintained in minimal essential medium supplemented with 10% fetal bovine serum. The tetracycline (Tet)-p27 Mv1Lu line was previously described (8). All Tet lines were maintained in minimal essential medium supplemented with 10% fetal bovine serum plus 0.5 mg/ml G418, 0.3 mg/ml hygromycin, and 1 μg/ml Tet. Human p27 cDNA with a C-terminal histidine tag was cloned into the XbaI site of the pUHD10-3 hygromycin vector and transfected into Mv1Lu-tTA cells (42) by using Lipofectin (GIBCO-BRL) to generate the Tet-His-p27 cell line. Asynchronously growing (A) cells were harvested from plates no greater than 60% confluent. Contact-arrested (G0) cells were harvested 5 days after visible contact arrest. Complete medium was replaced every other day, and fluorescence-activated cell sorter analysis confirmed that >95% of cells were in G1. For fluorescence-activated cell sorter assays, cells were fixed in ethanol for 1 h at 4°C and stained with propidium iodide for 30 min at 37°C, followed by analysis on a FACScan apparatus (Becton-Dickson).
Antibodies.
Antibodies used in this study were as follows. Anti-mouse p27, anti-mink cdk4, and anti-mouse cdk2 were a generous gift from J. Massagué (8). Anti-mouse p27 (DCS-72.F6) and anti-mouse cdk4 (DCS-35) were from NeoMarkers. Phospho-S10-p27 (catalog no. 34-6300), phosphothreonine (no. 71-8200), and phosphoserine (no. 61-8100) were from Zymed Laboratories. Cyclin D1 (AHF0092) was from BioSource International, and cdk6 (K6.83) was from Cell Sciences. Cyclin D1 (sc-450, sc-718, sc-753, and sc-177), cdk6 (sc-177 and sc-7961), and cdk2 (sc-6248) were from Santa Cruz Biotechnology. Phospho-threonine-proline (P-Thr-Pro101) and phosphotyrosine (P-Tyr100) were from Cell Signaling Technology; phosphotyrosine (4G10) was from Upstate; and phosphothreonine (ab9337) and phosphoserine (ab6639) were from Abcam.
Western blot analysis, immunoprecipitation, kinase assay, and gel filtration analysis.
Cell pellets were lysed in a Tween 20 lysis buffer as described previously (8). Immunoprecipitations and Western blot analysis were performed by standard protocols. Kinase assays and gel filtration analysis were performed as described elsewhere (8, 57).
Two-dimensional isoelectric focusing analysis (2DIEF).
Ten milligrams of A or G0 cell lysate was immunoprecipitated with p27 antibodies. Immunocomplexes were boiled in 40 μl of Tris-sodium dodecyl sulfate (SDS) solution (3.5% SDS, 0.215 M Tris, pH 6.8). Buffer exchange was performed using Tris Micro Bio-Spin chromatography columns (Bio-Rad). Samples were diluted in rehydration buffer (Bio-Rad) to a final volume of 185 μl, loaded onto ReadyStrip IPG strips (pHs 5 to 8; Bio-Rad), and focused on a Protein (2DIEF) Cell (Bio-Rad). Strips were loaded onto SDS-12.5% polyacrylamide gel electrophoresis Criterion gels (Bio-Rad), transferred to polyvinylidene difluoride, and analyzed by immunoblot analysis.
Phosphatase treatments.
Immunoprecipitated complexes were washed with 50 mM imidazole and 100 μl of tyrosine phosphatase buffer (25 mM imidazole-HCl, pH 7.2, 1 mg/ml bovine serum albumin, 0.1% [vol/vol] β-mercaptoethanol) or 1× protein tyrosine phosphatase (PTP) buffer (NEB). Twenty units of recombinant human T-cell PTP, generated in Escherichia coli (NEB, Calbiochem), was added and incubated at 30°C for 30 min. Alternatively, complexes were washed with 50 mM PIPES [piperazine N,N′-bis(2-ethanesulfonic acid), pH 6.0], followed by incubation in 100 μl of 50 mM PIPES-1 mM dithiothreitol for 10 min at 30°C. Ten to 80 U of potato acid phosphatase (Roche) was added, and the mixture was incubated at 30°C for 15 to 30 min.
Purification of A- and G0His-p27 from Tet cells.
His-tagged-p27-inducible (Tet-His-p27) cell lines were grown under A or G0 conditions and then were cultured in the absence of Tet for 20 h (AHis-p27 and G0His-p27, respectively). Cells were lysed in 8 M urea, sonicated, and centrifuged at 50,000 rpm for 30 min. Extracts were then loaded on HisTrap chelating columns (GE Healthcare) equilibrated with binding buffer (6 M urea, 0.5 M NaCl, 50 mM Tris, and 20% glycerol). Urea was removed by a gradual buffer exchange into buffer B (0.5 M NaCl, 50 mM Tris-HCl, pH 7.5, 10% glycerol) to refold the His-p27. His-p27 was eluted from the column (200 mM imidazole, 20 mM HEPES, pH 7.4, 1 M KCl, 0.1 M EDTA). Fractions were dialyzed overnight in 25 mM HEPES, pH 7.7, 150 mM NaCl, 5 mM MgCl2, and 0.05% NP-40.
Purification of recombinant cyclin D1-cdk4.
Recombinant His-cyclin D1-cdk4 was harvested from coinfected High5 cells as described previously (8). Cell extracts were lysed in buffer containing 10 mM NaPO4, 10 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, and protease and phosphatase inhibitors for 2 h on ice. Extracts were then homogenized in a Dounce homogenizer several times, and NaPO4 was adjusted to 50 mM and NaCl to 200 mM; the extracts were homogenized in a Dounce homogenizer again and clarified by centrifugation at 45,000 rpm for 45 min. This material was used as the High5 cell lysate expressing cyclin D1-cdk4. Alternatively, this material was loaded on a metal agarose HiTrap column (GE Healthcare). His-cyclin D1-cdk4 was eluted with 400 mM imidazole, 50 mM NaPO4, 200 mM NaCl, 10% glycerol. Fractions were subjected to SDS-polyacrylamide gel analysis, Coomassie blue staining, and immunoblot analysis to confirm the presence of cyclin D1-cdk4. In vitro kinase assay was done to determine the active fractions. The High5 cell lysate expressing cyclin D1-cdk4 and the pure cyclin D1-cdk4 was standardized by comparison of cdk4-associated cyclin D1 immunoblot analysis. Recombinant cyclin A-cdk2 was harvested and purified from coinfected High5 cells as described previously (8).
Construction of YF mutant p27s.
Purified, histidine-tagged p27s were generated from E. coli as described elsewhere (8). Human p27 cDNA with a C-terminal histidine tag was used as a template in PCR mutagenesis with oligonucleotides carrying the Y74F, Y88F, Y89F, YY88 89FF, and YY88 89EE point mutations. The PCR fragments were ligated to the T7pGEMEX human His-p27 plasmid for expression in E. coli (8) or to pUHD10-3 for transfection into Mv1Lu cells and selection of clones expressing the Y-to-F (YF) mutant p27s.
Preincubation experiment.
Ten micrograms of His-p27 was incubated in 1 mg of uninfected High5 cell extract or mock incubated in Tween 20 lysis buffer for 1 h at 37°C in the presence of an ATP-regenerating system (400 mM creatine phosphate [Roche], 2 mg/ml creatine kinase [Roche], 0.3 mM ATP). Samples were desalted using Bio-Gel P-6 DG desalting gel (Bio-Rad) by buffer exchange (buffer A contained 6 M urea, 0.5 M NaCl, 50 mM Tris, 20% glycerol). His-p27 was recovered by metal agarose chromatography using Talon metal affinity resin (BD Biosciences). Urea was removed by a gradual buffer exchange into buffer B (0.5 M NaCl, 50 mM Tris, 10% glycerol) to refold the His-p27. His-p27s were eluted from the Talon beads with 70 μl of 0.5 M EDTA.
In vitro Abl kinase assay.
Recombinant wild-type (Wt) His-p27 or His-YF mutant p27s were incubated with Abl kinase buffer (60 mM HEPES, pH 7.5, 5 mM MgCl2, 5 mM MnCl2, 3 mM Na3VO4, 1.25 mM dithiothreitol, 20 μM ATP, 0.066 μM [γ-32P]ATP) and 40 units of Abl kinase (Cell Signaling and NEB) for 1 h at room temperature. p27 was recovered by immunoprecipitation with Talon metal affinity resin (BD Biosciences) and used in immunoblot analysis and in vitro kinase assays.
RESULTS
Growth-state-dependent effects on p27-associated kinase activity.
When grown to confluence, Mv1Lu cells exit the cell cycle and enter a G0 state, while replating of these cells at low density induces reentry into the cell cycle. In our experiments, Mv1Lu cells were assayed from A cell populations (with a G1 content between 60 and 65% [hereinafter called A cells]) or following growth to confluence (with a G1 content between 90 and 95% [hereinafter called G0 cells]) (Fig. 1A). Unlike the situation in serum-starved cells, where cyclin D levels decrease due to the lack of mitogenic stimulation, in confluent Mv1Lu cells in the presence of high levels of serum, cyclin D1, cdk6, and cdk4 levels remained high and were no different than the amounts in A cell lysates (Fig. 1B). A decrease in the faster-migrating, phosphorylated cdk2 form was detected in G0 cells and corresponds to the loss of catalytically active cdk2. Total p27 levels increase approximately 10-fold when cells are grown to confluence (Fig. 1B) (8). The cdk inhibitors p21 and p15 are not detected in A or G0 Mv1Lu cells (data not shown), and p27 is the only CKI expressed.
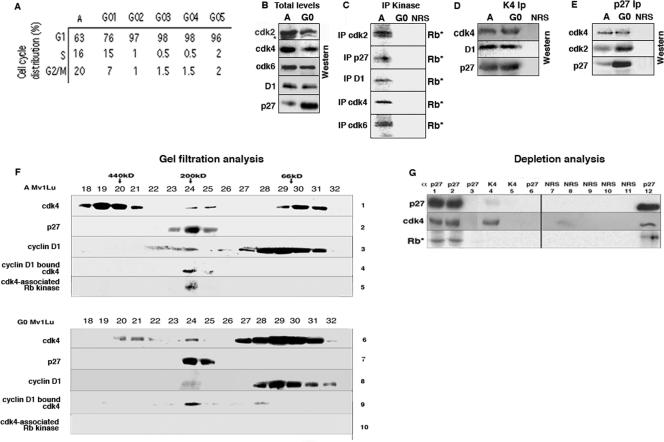
FIG. 1.
Analysis of cell cycle progression and G1 regulators from A and G0 cells. (A) A Mv1lu cells or Mv1lu cells arrested by contact inhibition (G0 cells) were harvested for flow cytometric analysis of DNA content. G01 to G05 indicate the numbers of days after visible contact arrest that the cells were harvested. (B) A and G0 cell extracts were analyzed directly by immunoblotting with the antibodies indicated on the left. D1, anti-cyclin D1. (C) Cdk2, p27, cyclin D1, cdk4, and cdk6 were immunoprecipitated (IP) from A and G0 cell lysates, and the ability of the complexes to phosphorylate exogenous Rb substrates was determined by kinase assays (Rb*). In all cases, immunoprecipitation with NRS was used as an immunoprecipitation control. (D and E) Extracts were immunoprecipitated with cdk4 (D) or p27 (E) antibodies, followed by immunoblot analysis with the antibodies indicated on the left. (F) Fractions from Superdex 200 gel filtration of lysates from A or G0 Mv1lu cells were analyzed by immunoblotting and immunoprecipitation to determine the compositions of endogenous complexes. The positions of protein molecular mass markers are indicated at the top. Fractions were subjected directly to anti-cdk4 (lanes 1 and 6), anti-p27 (lanes 2 and 7), and anti-cyclin D1 (lanes 3 and 8) immunoblotting. Immunoprecipitations with cyclin D1 antibodies (lanes 4 and 9) were subjected to anti-cdk4 immunoblotting. Fractions were immunoprecipitated with anti-cdk4 antibodies, followed by kinase assays (lanes 5 and 10). (G) A cell extracts were subjected to three cycles of immunoprecipitation with p27 antibodies (lanes 1 to 3), two cycles with cdk4 (lanes 4 and 5), and a final cycle with p27 antibodies (lanes 6 and 12). Lanes 7 to 11 were mock depleted. All immunoprecipitations were immunoblotted with p27 (top panel) and cdk4 antibodies (middle panel). The kinase activity associated with each immunoprecipitation was determined by Rb kinase assay (Rb*) (bottom panel).
To examine the catalytic activity of the G1 kinases, cdk2-, cdk4-, cdk6-, and cyclin D1-associated kinase complexes were immunoprecipitated from A and G0 cell lysates and in vitro Rb kinase assays were performed (Fig. 1C). While cdk2, cdk4, cdk6, and cyclin D1-associated kinase activities were robust in A cell lysates, all of these kinases were catalytically inactive in G0 cell lysates (Fig. 1C). By using contact inhibition rather than serum starvation to induce cell cycle arrest, the inactivity of the cdk's in G0 is not due to the loss of the cyclin component because cdk4 remains associated with cyclin D1 (Fig. 1D and E). p27 immunoprecipitations from A cell lysates also contained robust Rb kinase activity, and this p27-associated kinase activity has been shown to be due specifically to cdk4 and cdk6 rather than cdk2 (8, 51). p27-associated kinase activity was not detected in G0 cell lysates, even though the amount of p27-associated cdk4 was unchanged (Fig. 1D and E). Thus, p27 is associated with cyclin D1 complexes from both A and G0 cells, suggesting the existence of two p27 states: a bound-inhibitor (G0 cell) form and a bound-noninhibitor (A cell) form.
We next examined cdk4 from A and G0 cells by gel filtration chromatography, analyzing the resulting fractions by immunoprecipitation-immunoblot analysis or direct immunoblot analysis (Fig. 1F). As previously described, cdk4 was present in three pools in epithelial cells: pools with molecular masses of >450 kDa, 200 kDa, and 66 kDa (Fig. 1F) (29, 32, 38, 52, 57). As determined by cyclin D1 immunoprecipitation (lane 4) and cdk4-associated kinase assays (lane 5), neither the >450-kDa (fractions 18 to 21) nor the 66-kDa (fractions 29 to 31) pool had associated cyclin D1 (lane 4), p27 (lane 2), or kinase (lane 5) activity. A majority of cdk4 in A cells is found in these two inactive complexes (∼90% of cdk4 was found in the >450-kDa pool [60%] and the 66-kDa pool [30%]). The 66-kDa pool is due to monomeric cdk4, as Inks are not expressed in these cells under proliferating or contact-arrested conditions (43). Only the third, minor cdk4 pool (∼10% of the total cdk4; 200 kDa, fractions 23 to 25) in the A cells had kinase activity, as determined by cdk4-associated kinase assays (lane 5).
Cyclin D1 is detected in the 200-kDa complex, but more is at 70 kDa (Fig. 1F, lane 3). cdk4 does not coprecipitate with cyclin D1 from this 70-kDa pool (lane 4), suggesting that this pool of cyclin D1 is cdk free. p27-free, cyclin D1-cdk4 dimers are not detected in these cells, consistent with the idea that p27 might be required for cyclin D1-cdk4 complex formation and activity. All of the p27 in the cell migrates at 200 kDa, suggesting that monomeric p27 is also not present.
All three cdk4 pools were still present in gel filtration chromatography of the G0 cell lysates (Fig. 1F, lane 6), although their relative distributions were different. Confluence-induced arrest resulted in the loss of the 450-kDa pool and an increase in the 66-kDa cdk4 monomeric pool (lane 6). The ratio of the 200-kDa pool relative to the total amount of cdk4 in the cell remained constant, at approximately 10%. The large 70-kDa pool of cyclin D1 was present and was still cdk4 free, as seen in the cyclin D1 immunoprecipitation (lane 9). p27 was detected only at 200 kDa (lane 7), suggesting that it is entirely occupied in complex.
However, cdk4-associated kinase assays (Fig. 1F, lane 10) demonstrated that while the 200-kDa cyclin D1-cdk4-p27 complex was intact in G0, it was catalytically inactive. In both A and G0 cells, p27 associated with the cyclin D1-cdk4 complex, but the outcomes of this association were different.
Depletion of lysates by multiple immunoprecipitations with p27 antibodies demonstrated that only p27-associated cdk4 complexes were catalytically active (Fig. 1G). After two rounds of immunoprecipitation, p27 was depleted and associated cdk4 was detected (Fig. 1G, lanes 1 and 2). The depleted lysates were then immunoprecipitated with cdk4 antibodies (lanes 4 and 5), and some p27-free cdk4 was detected (lane 4). All of these immunoprecipitates were used in parallel kinase assays (those from lanes 1, 2, 3, 6, and 12 in the p27-associated kinase assay; those from lanes 4 and 5 in the cdk4-associated kinase assay; and those from lanes 7 to 11 in the normal rabbit serum [NRS] assay). Kinase activity was detected in the p27-associated immunoprecipitates (lanes 1 to 3), but the p27-free cdk4 (lane 4) was inactive. Thus, while pools of p27-free cdk4 exist in A cells (the 450- and 66-kDa pools), only the p27-associated cyclin D1-cdk4 complex has kinase activity, suggesting that p27 associates with cdk4 complexes in a noninhibitory manner.
The stoichiometry of p27 cannot explain why it is a cyclin D-cdk4 inhibitor specifically in G0 cells.
A stoichiometric model has been suggested to explain how p27's association with cyclin D-cdk4 could be noninhibitory in A cells but inhibitory in G0 cells, where the level of p27 increases 10-fold. However, we did not detect more p27 associated with the inactive cyclin D1-cdk4 complex in G0 (Fig. 1D and E). To ascertain whether the concentration of p27 could be increased in vivo to a level where p27-dependent inhibition of cdk4 would be detected in an otherwise cycling cell, we used a previously described Tet-repressible-p27 induction system in Mv1Lu cells (Tet-p27) (8). In this system, Mv1Lu derivatives express a p27 cDNA under the negative control of the Tet transactivator (18). In the absence of Tet, p27 was expressed to levels roughly 15-fold above basal levels, and the cells arrested in G1 due to p27-mediated inhibition of cdk2 (8). We compared Tet-p27 cells in the absence of Tet (exogenous p27 was expressed) to A and G0 cells to determine whether this p27-induced arrest was similar to that seen in G0 cells (Fig. 2). cdk4 and cdk6 levels were unchanged under the three conditions, and cdk2 was detected predominantly in its slower-migrating, nonphosphorylated form in G0 and Tet-p27 cells (Fig. 2A). Cyclin D1 levels were slightly increased in the Tet-p27 cells. p27 levels in Tet-p27 cells were actually greater than those detected in G0 cells. It is difficult to visualize p27 in A cells without overexposing the amount of p27 in the Tet-p27 cells, but a direct comparison between A and G0 cells is made in Fig. 1.
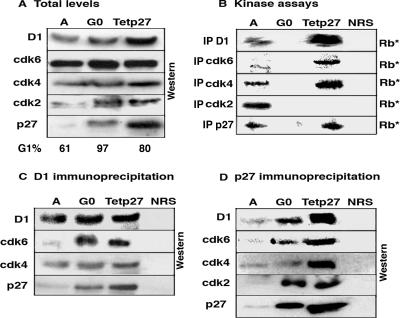
FIG. 2.
Comparison of cell cycle proteins in A, G0, and Tet-p27 cells. Equal amounts of extract from these three conditions were analyzed to determine the effect of overexpression of p27 in Tet-p27 cells. The Tet-p27 cells were harvested 24 h after Tet removal. (A) Extracts were analyzed directly by immunoblotting with the antibodies indicated on the left. The percent G1 content is listed at the bottom of the panel. D1, cyclin D1. (B) Cyclin D1, cdk6, cdk4, cdk2, and p27 were immunoprecipitated (IP) and used in kinase assays with exogenous Rb as a substrate (Rb*). In all cases, immunoprecipitation with NRS was used as a control. (C and D) Extracts were immunoprecipitated with cyclin D1 (C) or p27 (D) antibodies, followed by immunoblot analysis with the antibodies indicated on the left.
We assayed for cyclin D1-, cdk2-, cdk4-, cdk6-, and p27-associated kinase activity under all three conditions (Fig. 2B). As expected, all of the kinases were active in A cells but were inactive in G0 cells. In Tet-p27 cells, only cdk2 was inactive; cyclin D1-, cdk4-, cdk6-, and p27-associated complexes were still active. This suggested not only that arrest in the Tet-p27 cells was due to cdk2 inhibition but also that p27 could not inhibit cdk4 in an otherwise cycling cell. As the level of p27 in Tet-p27 cells, where cdk4 is not inhibited, is in fact greater than that seen in G0 cells, where cdk4 is inhibited, the concentration of p27 must not be the only determinant of cdk4 activity.
In fact, more cyclin D1, cdk4, cdk6, and p27 kinase activities were detected in the Tet-p27 lysates (Fig. 2B), consistent with the suggestion that p27 may help to assemble the cdk4/cdk6 complexes. More cdk6 and slightly more cdk4 were associated with cyclin D1 in Tet-p27 and/or G0 cells (Fig. 2C). More p27 was associated with cyclin D1, but rather than suggesting an increased stoichiometric association, this was consistent with the presence of more of the cyclin D1-cdk4/6 complex. Immunoprecipitation with p27 antibodies (Fig. 2D) showed an increased association of cdk2 with p27 in G0 and Tet-p27 cells, consistent with the inactivity of this kinase. By p27 immunoprecipitation, more p27-cyclin D1-cdk4 and p27-cyclin D1-cdk6 were detected in Tet-p27 cells (Fig. 2D). However, the newly assembled cyclin D1-cdk6 complex in G0 cells was inactive, while the newly assembled cdk4/6 complex in Tet-p27 cells was catalytically active. Thus, while p27 may help to assemble these complexes, this function must be separate from its ability to inhibit them. In Tet-p27 cells, the level of p27 is clearly saturating but not sufficient to inhibit cyclin D1-cdk4/6 activity, suggesting that some additional G0 cell-dependent signal must affect the activities of p27 and the cdk's.
Modification of p27 alters its ability to inhibit in vitro.
The ability of p27 to be either a bound inhibitor or a bound noninhibitor in vivo was reminiscent of the situation in vitro, in which it has been suggested that the purity of the cyclin-cdk and p27 proteins might dictate whether p27 is an inhibitor or not (8, 27). Recombinant cyclin D1-cdk4 complexes are generated by coinfection of baculoviral High5 cells, and catalytically active cyclin D1-cdk4 can be assayed from unpurified High5 cell lysates. We additionally purified cyclin D1-cdk4 from the High5 cell lysate via a histidine tag on cyclin D1 by metal agarose chromatography to generate two sources of cyclin D1-cdk4 (pure cyclin D1-cdk4 and High5 cell lysate expressing cyclin D1-cdk4). When we assayed recombinant, bacterially purified p27's ability to inhibit equal amounts of these two source materials, we found that while p27 was a potent inhibitor of pure cyclin D1-cdk4, it was a poor inhibitor of unpurified cyclin D1-cdk4 (Fig. 3A, lane 1). This was not due to the lack of an association with the cyclin D1-cdk4 complex, as cdk4-associated p27 was detected in the unpurified reaction mixture at concentrations where p27 was not inhibitory (Fig. 3A, lane 3).
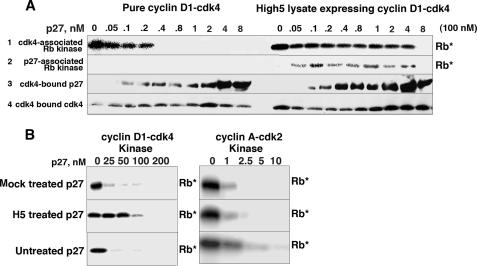
FIG. 3.
Modification of p27 in vitro affects its ability to inhibit cyclin D1-cdk4. (A) Equal amounts of recombinant cyclin D1-cdk4 were assayed from an unpurified High5 baculoviral lysate (High5 cell lysate expressing cyclin D1-cdk4) or following purification via a histidine tag on cyclin D1 (pure cyclin D1-cdk4). These were mixed with increasing amounts of recombinant p27. These mixtures were used directly (lane 1) in in vitro kinase assays or following immunoprecipitation with p27 antibodies (lane 2) to analyze only p27-associated kinase activity. Pure cyclin D1-cdk4 and the High5 cell lysate expressing cyclin D1-cdk4 were immunoprecipitated with cdk4 antibodies, followed by p27 (lane 3) and cdk4 (lane 4) immunoblot analyses. (B) Recombinant p27 was preincubated in High5 cell extract (H5 treated) or mock incubated (mock treated) in the presence of an ATP-regenerating system. This histidine-tagged p27 was recovered by metal agarose chromatography in the presence of urea and used in in vitro kinase assays with pure cyclin D1-cdk4 and cyclin A-cdk2. Untreated, recombinant p27 was used as a control. Due to the lower specific activity and inherent instability of the cyclin D1-cdk4 complex, more cyclin D1-cdk4 is used than cyclin A-cdk2, and the results can be compared only down each column. Rb*, phosphorylated Rb.
To ensure that we were observing differences in the inhibitory activities of bound p27, we assayed for kinase activity from p27 immunoprecipitations (Fig. 3A, lane 2). p27-associated kinase activity was detected from the unpurified cyclin D1-cdk4 material, but p27-associated complexes were catalytically inactive when they were formed using the pure material. Thus, the interaction of p27 with these two cyclin D1-cdk4 source materials was fundamentally different and argued for a two-state mechanism; p27 bound without causing inhibition to the cyclin D1-cdk4 complex expressed in the High5 cell lysate but bound causing the inhibition of the pure cyclin D1-cdk4 material.
We hypothesized that a factor in the High5 cell lysate might modify p27 to prevent its inhibitory activity in the unpurified reaction mixture. To try to convert p27 from a bound inhibitor to a bound noninhibitor, we incubated recombinant p27 in High5 cell extract in the presence of an ATP-regenerating system (Fig. 3B). This p27 was repurified from the extract via its histidine tag, restandardized by Coomassie blue staining and immunoblot analysis (data not shown), and tested for its ability to inhibit pure cyclin D1-cdk4 and cyclin A-cdk2. Both mock- and High5 cell-incubated p27s efficiently inhibited cyclin A-cdk2. However, while mock-incubated p27 was able to inhibit cyclin D1-cdk4, High5 cell-incubated p27 was now a poor inhibitor of pure cyclin D1-cdk4. As these p27s were purified in the presence of urea, associating proteins were not copurified (data not shown), suggesting that a urea-stable modification of p27 had occurred during the preincubation, which now prevented p27's ability to inhibit pure cyclin D1-cdk4. Thus, we had an assay system where we could easily analyze p27 under two different conditions: modification free (using purified cyclin D1-cdk4) and modification inducing (using the High5 cell lysates expressing cyclin D1-cdk4).
p27 isolated from G0 cells is a better cdk4 inhibitor than p27 isolated from A cells.
The data are consistent with a model where p27 could interact with cyclin D-cdk4/6 in two alternate modes: bound inhibitory and bound noninhibitory. We generated a Tet-His-p27 cell line in Mv1Lu cells. This allowed us to purify AHis-p27 and G0His-p27 by metal agarose chromatography and to test their ability to inhibit purified recombinant cdk4's and cdk2's phosphorylation of exogenous Rb substrates in vitro (Fig. 4A and B). We purified these p27s by a standard denaturation/renaturation protocol in the presence of urea, and thus associating proteins did not copurify with the p27s (data not shown). Purified AHis-p27 and G0His-p27 were standardized by quantitative immunoblot analysis. Both AHis-p27 and G0His-p27 were able to inhibit cyclin A-cdk2 kinase equally, demonstrating that the p27s had refolded correctly (Fig. 4A). However, while G0His-p27 was a potent cyclin D1-cdk4 inhibitor similar to recombinant bacterially purified p27, an equal amount of AHis-p27 was unable to inhibit cyclin D1-cdk4, despite equal association with cdk4 (Fig. 4B). This suggested that p27 isolated from G0 cells was intrinsically different than p27 isolated from A cells, and the ability to inhibit or not to inhibit was due to a modification of p27 itself.
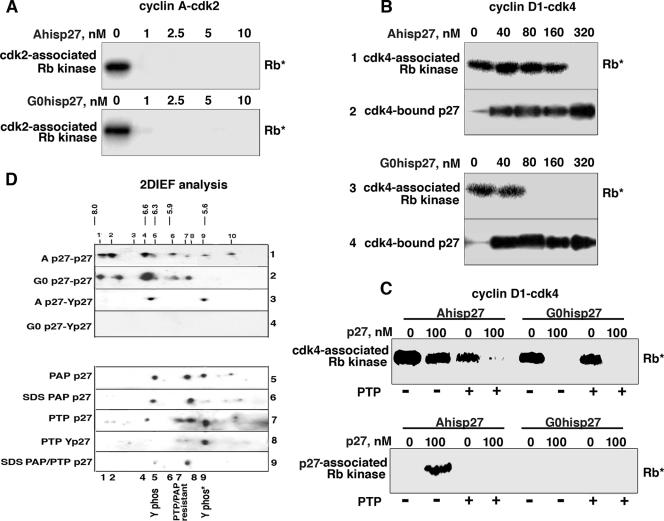
FIG. 4.
Phosphorylation of p27 in vivo affects its ability to inhibit cyclin D1-cdk4. A or G0 Tet-His-p27 cells were cultured in the absence of Tet for 20 h. His-p27 was purified by metal agarose chromatography to produce G0His-p27 and AHis-p27. (A) Increasing amounts of AHis-p27 and G0His-p27 were mixed with pure cyclin A-cdk2 and used in Rb kinase assays (Rb*) in vitro. (B) Increasing amounts of these two types of p27 were mixed with pure cyclin D1-cdk4 and used in Rb kinase assays in vitro (lanes 1 and 3) or immunoprecipitated with cdk4 antibodies, followed by p27 immunoblot analysis (lanes 2 and 4). (C) Ternary complexes with A- or G0His-p27 and pure cyclin D1-cdk4 were generated in vitro and immunoprecipitated with cdk4 (top panel) or p27 (bottom panel) antibodies. These complexes were treated (+) or not treated (−) with PTP, washed to remove PTP, and then used in in vitro Rb kinase assays. (D) Equal amounts of p27 protein (as determined by one-dimensional Western blot analysis) were analyzed by 2DIEF. The intensities of the spots can be compared between strips. Lysates were immunoprecipitated with p27 antibodies, followed by p27 (lanes 1, 2, 5, 6, 7, and 9) or phosphotyrosine (Y phos) (lanes 3, 4, and 8) immunoblot analysis. The pHs are indicated at the top. p27 immunoprecipitates were treated with phosphatases, namely, PAP (lane 5) and PTP (lanes 7 and 8), prior to 2DIEF. p27 immunoprecipitations were boiled in 1% SDS, reimmunoprecipitated with p27 antibodies, and then PAP treated (lane 6) or PAP-PTP treated (lane 9).
While other forms of modification exist, it is known that p27 is phosphorylated on multiple residues in vivo. Others have recently described the tyrosine phosphorylation of p27, which appears to affect its ability to inhibit cdk2 (15, 19, 22). To determine whether Y phosphorylation might be responsible for AHis-p27's lack of inhibitory activity, we tried to dephosphorylate AHis-p27 with PTP, a Y-specific phosphatase. We incubated AHis-p27 and G0His-p27 with recombinant cyclin D1-cdk4 in vitro and isolated both cdk4-associated and p27-associated complexes by immunoprecipitation (Fig. 4C). These complexes were then treated with PTP and then used in in vitro kinase assays. While mock-treated AHis-p27 did not inhibit cdk4-associated kinase activity, PTP-treated AHis-p27 was a potent inhibitor (Fig. 4C, top panel). PTP treatment did not affect G0His-p27's inhibitory activity (Fig. 4C). PTP appeared to specifically remove a Y phosphorylation on p27, as PTP treatment of cyclin D1-cdk4 alone did not affect its kinase activity. Consistent with this result, kinase activity was seen with AHis-p27-associated complexes, but PTP treatment resulted in the loss of this kinase activity (Fig. 4C, bottom panel). p27-associated G0His-p27 complexes were always inactive. This suggests that p27's inability to inhibit cyclin D1-cdk4 is due to its Y phosphorylation, and loss of this phosphorylation by phosphatase treatment creates a more inhibitory or more G0-like p27.
p27 is differentially phosphorylated in vivo.
To directly demonstrate whether p27 was differentially phosphorylated in vivo in A and G0 cells, we performed 2DIEF on p27 immunoprecipitates. We standardized the amounts of p27 in the A and G0 cell lysates by immunoblot analysis and loaded equal amounts of p27 on the 2DIEF strip (Fig. 4D, lanes 1 to 9). The 2DIEF gels were immunoblotted with p27 and p27 phospho-specific antibodies to detect differently modified species (Fig. 4D). We consistently detected eight different phosphoforms of p27 (Fig. 4D, spots 1, 2, 4, 5, 6, 7, 8, and 9 in lanes 1 and 2), and several additional weaker spots were not always observed (spots 3 and 10).
To determine the identities of the p27 isoforms, we used commercially available p27 phospho-specific antibodies (S10, T157, and T187), and most spots were recognized by these antibodies (see the supplemental material). In contrast, spots 5, 7, and 9 were not recognized by any of the known p27 phospho-specific antibodies. To examine tyrosine phosphorylation, we used two different phosphotyrosine antibodies and found that they recognized these spots only in A cell lysates (Fig. 4D, lanes 3 and 4), suggesting that p27 was tyrosine phosphorylated preferentially in cycling cells.
To confirm the integrity of the phospho-Y antibodies, we used two different phosphatases. Potato acid phosphatase (PAP) treatment prior to 2DIEF analysis, which removes S, T, and some Y phosphorylation, reduced the number of p27 isoforms to three (spots 5, 7, and 9 remained in Fig. 4D, lane 5). Spots 1, 2, 4, 6, and 8 were lost, consistent with the known preference of PAP for phospho-S and -T and the presence of S10, T157, or T187 phosphorylation (see the supplemental material). When the p27 immunoprecipitates were boiled in 1% SDS prior to PAP treatment, spot 9 disappeared as well (Fig. 4D, lane 6). Some Y phosphorylation events are known to be resistant to PAP. Thus, we additionally treated with PTP, an exclusive Y phosphatase. Under this treatment, spot 5 disappeared, further suggesting that spot 5 contained a phosphorylated Y residue (lane 7). Spots 4, 6, 7, and 9 were resistant to PTP treatment, as expected (lane 7); spots 4 and 6 correspond to S/T phosphorylation, and spot 9 is sensitive only to phosphatase treatment following prior boiling in 1% SDS. When this strip was probed with phosphotyrosine antibodies (lane 8), only spot 9 was observed (spot 5; the other Y-specific spot was lost by PTP treatment [lane 7]). Boiling in SDS, followed by combined PAP and PTP treatment, caused the loss of all spots, except spot 7, suggesting that this spot is phosphatase resistant and may be the nonphosphorylated form (lane 9). This suggested that p27 was Y phosphorylated in vivo and that this occurred predominately in cycling cells.
p27 Y phosphorylation inactivates the inhibitory activity of cyclin D1-cdk4-bound p27 in vitro.
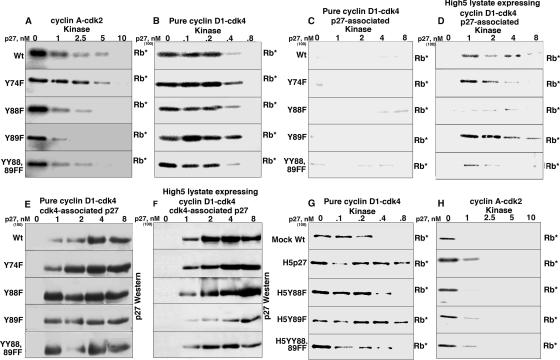
There are three conserved Y's in mouse, human, and mink p27s: Y74, Y88, and Y89. We mutated these to nonphosphorylatable phenylalanines; expressed and purified Y74F, Y88F, Y89F, and YY88 89FF mutant p27s from E. coli; and assayed their ability to inhibit cyclin-cdk's in vitro. All the mutants were able to inhibit purified cyclin A-cdk2 and cyclin D1-cdk4 in the modification-free reaction mixture (Fig. 5A and B), suggesting that these mutations did not globally affect their inhibitory activities. We performed p27-associated kinase assays under the modification-free and modification-inducing conditions, using the pure cyclin D1-cdk4 (Fig. 5C) and the High5 cell lysate expressing cyclin D1-cdk4 (Fig. 5D), to assay for cdk4 activity when cdk4 is bound by p27. p27-associated kinase activity was not detected with any of the mutants on pure cyclin D1-cdk4, demonstrating that, when not modified, they behaved like Wt p27 and were bound inhibitors. When assayed with the unpurified High5 cell lysate expressing cyclin D1-cdk4, Y74F p27- and Y89F p27-associated kinase activities were similar to that seen with Wt p27, suggesting that they had been modified to the bound, noninhibitory form during this reaction. However, Y88F p27- and YY88 89FF p27-associated kinase activity was reduced significantly, suggesting that these mutants were still bound inhibitors and were resistant to the modification that occurred in the High5 cell lysate. All of the mutants associated with cyclin D1-cdk4 assayed by cdk4 immunoprecipitation from the pure cyclin D-cdk and High5 cell lysate expressing cyclin D1-cdk4 (Fig. 5E and F), suggesting that the lack of p27-associated kinase activity was not due to a lack of association.
FIG. 5.
p27-associated kinase activity of the YF p27 mutants in vitro. Increasing amounts of recombinant Wt and mutant p27s were mixed with pure cyclin A-cdk2, pure cyclin D1-cdk4, or High5 cell lysate expressing cyclin D1-cdk4. (A) In vitro kinase assay with p27 and pure cyclin A-cdk2; (B) in vitro kinase assay with p27 and pure cyclin D1-cdk4; (C) p27-associated kinase assay with p27 and pure cyclin D1-cdk4; (D) p27-associated kinase assay with p27 and High5 cell lysate expressing cyclin D1-cdk4; (E) cdk4 immunoprecipitation, followed by p27 immunoblot analysis with pure cyclin D1-cdk4; (F) cdk4 immunoprecipitation, followed by p27 immunoblot analysis with High5 cell lysate expressing cyclin D1-cdk4; (G and H) Wt and YF mutant p27s preincubated in High5 cell extract in the presence of an ATP-regenerating system, recovered by metal agarose chromatography in the presence of urea and used in in vitro kinase assays with pure cyclin D1-cdk4 (G) and cyclin A-cdk2 (H). Due to the lower specific activity and inherent instability of the cyclin D1-cdk4 complex, more cyclin D1-cdk4 is used than cyclin A-cdk2, and the results can be compared only down each column. The higher concentrations of cyclin D1-cdk4 were used in the immunoprecipitation-immunoblot and immunoprecipitation-kinase assays. Rb*, phosphorylated Rb.
We incubated recombinant, bacterially purified p27 and the YF mutants in High5 cell extract, repurified them as described above, and tested the ability of these potentially “modified” p27s to inhibit pure cyclin D1-cdk4 and cyclin A-cdk2 (Fig. 5G and H). Preincubation in High5 cell extract did not affect the mutants' ability to inhibit cyclin A-cdk2 (Fig. 5H). As shown in Fig. 3B, mock-incubated p27 was able to inhibit cyclin D1-cdk4, while H5-incubated p27 became a poor inhibitor of pure cyclin D1-cdk4. Similarly, Y89F p27 became a poor inhibitor after preincubation, but Y88F and YY88 89FF p27s were unaffected by the preincubation and inhibited cyclin D1-cdk4 as well as did mock-incubated p27. These data suggested that when residue Y88 in Wt p27 was phosphorylated by a kinase in the High5 cell lysate, p27 was able to bind but unable to inhibit cyclin D1-cdk4. However, when not phosphorylated, due to the lack of the kinase in the modification-free reaction mixture, p27 was able to bind and inhibit cyclin D1-cdk4. The Y88F and YY88 89FF mutants were always bound inhibitors because they were resistant to the modification.
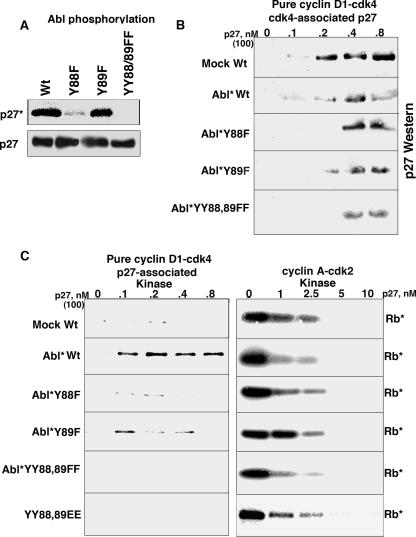
Residues Y88 and Y89 are found within two consecutive consensus Src homology 2 motifs (YXXP) (58), where Y is potential phosphorylation sites for nonreceptor tyrosine kinases, such as Abl, Lck, Itk, and Nck (7, 10). We incubated recombinant Abl kinase with the bacterially purified p27s (Fig. 6A). While Abl was able to phosphorylate Wt p27 and Y89F p27, it was unable to phosphorylate either Y88F or YY88 89FF p27. This tyrosine-phosphorylated material was repurified via the histidine tag on the p27s. All of the Abl-treated p27s associated with cdk4 to an extent similar to that of mock-treated p27, as seen by cdk4 immunoprecipitation and p27 immunoblot analysis (Fig. 6B). These p27s were used in p27-associated kinase assays with pure cyclin D1-cdk4 (Fig. 6C). p27-associated kinase activity was not seen with the mock-phosphorylated p27, while Abl-phosphorylated p27 was able to bind without causing inhibition, permitting p27-associated kinase activity to be detected (Fig. 6C, left). Y89F mutant p27 resulted in p27-associated kinase activity, consistent with the Abl phosphorylation detected with this mutant (Fig. 6A). This activity was weaker than that detected with Abl kinase-treated Wt p27, due to the lower stoichiometry of phosphorylation for this mutant (data not shown). Y88F or YY88 89FF p27, however, still bound and inhibited cyclin D1-cdk4, consistent with the observation that these mutants were resistant to Abl phosphorylation. Thus, Abl can phosphorylate p27 on residue Y88 and convert p27 from a cyclin D1-cdk4 inhibitor to a noninhibitor. This conversion was specific for cyclin D1-cdk4, as Abl-phosphorylated p27 was as inhibitory as nonphosphorylated p27 on cyclin A-cdk2 (Fig. 6C, right).
FIG. 6.
Abl kinase can phosphorylate p27 and convert it to a bound noninhibitor in vitro. (A) Equal amounts of His-Wt and YF mutant p27s were incubated with purified Abl kinase, followed by immunoprecipitation with Talon metal affinity resin. The recovered p27s were then subjected to p27 immunoblot analysis, and phosphorylation was directly visualized by autoradiography of the same membrane. (B) Wt p27 and Abl-phosphorylated (Abl*) p27s were mixed with recombinant pure cyclin D1-cdk4, followed by cdk4 immunoprecipitation and p27 immunoblot analysis. (C) Wt p27 and Abl-phosphorylated p27s were mixed with recombinant pure cyclin D1-cdk4, followed by p27 immunoprecipitation and in vitro kinase assay to assay p27-associated complexes (left). Wt p27 and Abl-phosphorylated p27s were mixed with cyclin A-cdk2 followed directly by in vitro kinase assay (right). Rb*, phosphorylated Rb.
In an attempt to mimic constitutive phosphorylation of residues 88 and 89, we generated a YY88 89EE mutant and tested its ability to inhibit pure cyclin D1-cdk4 and cyclin A-cdk2 (Fig. 6C). The YY88 89EE mutant was still a bound inhibitor of cyclin D1-cdk4, as demonstrated by its association with cdk4 (data not shown) and by its lack of p27-associated kinase activity detected with pure cyclin D1-cdk4 substrates. The mutant, however, inhibited cyclin A-cdk2 to an extent similar to that of Wt p27, suggesting that the EE replacement did not globally affect p27's inhibitory activity.
p27 Y phosphorylation inactivates the inhibitory activity of cyclin D-cdk4-bound p27 in vivo.
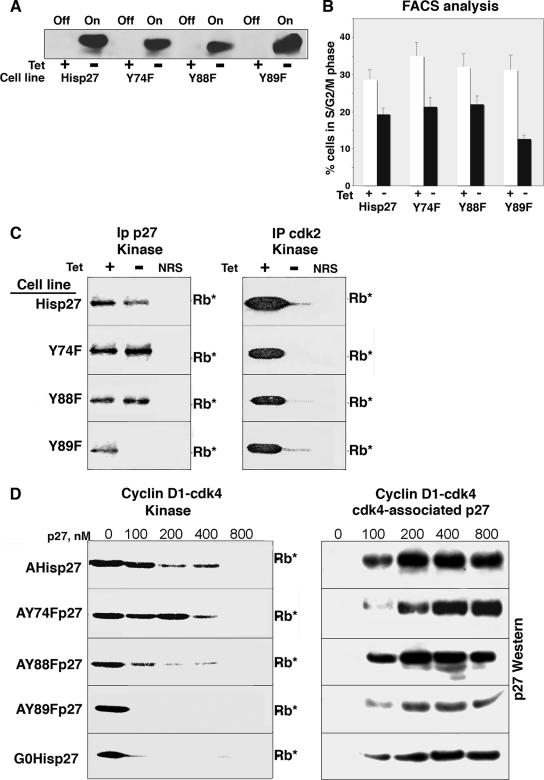
To determine whether the lack of Y phosphorylation would also convert p27 into a bound inhibitor in vivo, we generated Tet-repressible lines in Mv1Lu cells that inducibly expressed the YF mutants (Fig. 7). When Tet was removed from the medium, all of the mutant p27s were induced to similar extents (Fig. 7A), and expression of the mutants was predominantly nuclear (data not shown). We tried to express the YY88 89FF mutant in Mv1Lu cells, but this mutant appeared to be unstable and the percentage of full-length p27 was always much lower than that of the single mutants, preventing accurate analysis. Expression of all of the mutants arrested the cells in the G1 phase (Fig. 7B). The Y89F mutant, however, appeared to cause a more severe growth arrest, with 87% of the cells being detected in G1 phase. As shown in Fig. 2, Wt p27's inhibition of cdk2 kinase activity was sufficient to cause a potent G1 arrest. All of the YF mutants also inhibited cdk2 kinase activity, as detected by cdk2 immunoprecipitation followed by in vitro Rb kinase assays (Fig. 7C, right). When we analyzed p27-associated kinase activity by p27 immunoprecipitation from the YF mutant cells grown in the absence of Tet, differences in the abilities of the mutants to inhibit cdk4 were detected (Fig. 7C, left). While p27-associated kinase activity was detected from His-p27, Y88F p27, and Y74F p27 cells, p27-associated kinase activity was not detected with the Y89F mutant, suggesting that this p27 potently inhibited cdk4/6. This suggests that the increased G1 arrest detected with the Y89F mutant is due to the inhibition of both cdk2 and cdk4, rather than to the inhibition of cdk2 alone, as occurs with His-p27.
FIG. 7.
In vivo analysis of the YF p27 mutants. Tet-Y74F p27, Tet-Y88F p27, Tet-Y89F p27, and Tet-His-p27 cells were grown with or without Tet for 20 h. (A) Lysates were analyzed by direct p27 immunoblot analysis. Off, p27 expression off; On, p27 expression on. (B) Cells were harvested for flow cytometric analysis of DNA content. The percentages of cells in S, G2, and M phase are plotted. Bars represent the averages from four individual experiments, and standard deviations are noted. White bars indicate conditions with Tet, and black bars indicate conditions lacking Tet. (C) Lysates from the different cell lines indicated on the left were immunoprecipitated (IP) with p27 (left) or cdk2 (right) antibodies, followed by in vitro kinase assays (Rb*). In all experiments, NRS served as a negative control. (D) A or G0 Tet-His-p27 or Tet-YF mutant p27 cells were cultured in the absence of Tet for 20 h. His-p27s were purified by metal agarose chromatography to produce G0His-p27, AHis-p27, A-Y74F p27, A-Y88F p27, or A-Y89F p27. Increasing amounts of these p27s were mixed with pure cyclin D1-cdk4 and used in Rb kinase assays in vitro (Rb*) (left) or immunoprecipitated with cdk4 antibodies, followed by p27 immunoblot analysis (right).
We purified the mutant histidine-tagged p27s from A cells by metal agarose chromatography in urea as described above and assayed them for their ability to inhibit recombinant cyclin D1-cdk4 phosphorylation of exogenous Rb substrates in vitro (Fig. 7D). While p27 isolated from contact-arrested cells (G0His-p27) was a more potent inhibitor than p27 isolated from proliferating cells (AHis-p27), the Y89F mutant and, to a lesser extent, the Y88F mutant isolated from A cells were potent inhibitors and had inhibitory activities comparable to that seen with G0His-p27. The Y74F mutant isolated from A cells was a poor cyclin D1-cdk4 inhibitor and had activity similar to that of AHis-p27. All of the mutants bound recombinant cyclin D1-cdk4, as demonstrated by cdk4 immunoprecipitations followed by p27 immunoblot analysis. Thus, these data suggest that the inability of Y89F p27 and, to a lesser extent, Y88F p27 to be phosphorylated in a cycling cell created better bound inhibitors when the p27s were assayed in vivo and in vitro.
DISCUSSION
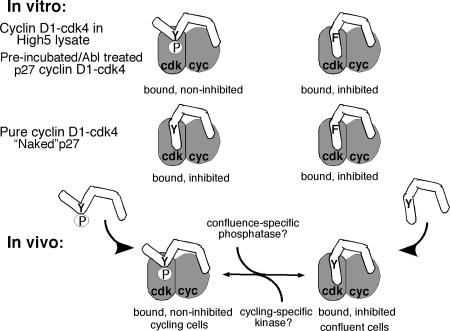
These are the first studies that demonstrate that p27 can interconvert between a cyclin D-cdk4-bound inhibitor and a noninhibitor in a growth-state-dependent manner. Tyrosine kinase-dependent phosphorylation of residue Y88 or Y89 in p27 under cycling conditions created a cdk4-bound noninhibitor, whereas lack of this phosphorylation following confluence due to lack of a kinase or the presence of a confluence-specific phosphatase removed Y phosphorylation and permitted cyclin D1-cdk4 inhibition (Fig. 8). In this way, signals from contact-arrested or growing cells do not alter p27's ability to bind cyclin D-cdk4 but act as a switch to affect its ability to inhibit the complex once bound. This has important implications in terms of p27's role in normal cellular homeostasis as well as in tumor progression.
FIG. 8.
Model of p27 modification. In vitro, p27 is phosphorylated by assaying in the High5 cell lysate expressing cyclin D1-cdk4 or when preincubated with High5 extract or modified with Abl, which converts p27 to a bound noninhibitor. “Naked” p27 assayed in the pure cyclin D1-cdk4 reaction is a bound inhibitor. The Y88F mutant is resistant to these types of modification and remains a bound inhibitor in all reactions. In vivo, p27 is a bound inhibitor in G0 cells and a bound noninhibitor in A cells. p27 is tyrosine phosphorylated in growing cells, either before or after binding to cyclin D1-cdk4, preventing inhibition of cyclin D1-cdk4. p27 is dephosphorylated either before binding or while bound to cyclin D-cdk4 in order to inhibit kinase activity in contact-arrested cells. The mutant Y89F is resistant to Y phosphorylation in cycling cells and remains a bound inhibitor. cyc, cyclin D1.
How could p27 associate with cyclin D-cdk4 without causing inhibition? The three-dimensional structure of other p27-bound complexes demonstrate that residues Y88 and Y89 are part of the 3-10 helix (residues 85 to 90), which appears to insert itself directly within the catalytic cleft of cdk2 (20, 39, 44). This binding fills up the catalytic cleft, eliminating possible ATP binding. Nuclear magnetic resonance analysis demonstrated that when Y phosphorylated on residue Y88, the tail of p27 was pushed out of the catalytic cleft. p27 forms extensive contacts with cyclin A through the LFG domain (residues 37 to 59), suggesting that similar contacts may be made with cyclin D.
In vivo, it is possible that multiple kinases might switch p27 from a cdk4 inhibitor to a noninhibitor by phosphorylating Y88 or Y89, depending on the cell type and/or condition. Consistent with this idea, we found that the Y89F mutant was a better cdk4 inhibitor in vivo and that the Y88F mutant was more inhibitory in vitro, suggesting that some level of redundancy between the two sites must exist and that the cell might employ different mechanisms to push p27's tail out of the catalytic cleft. While we have shown that Abl can directly phosphorylate these sites in vitro, others have demonstrated that when overexpressed, Src, Lyn, or Yes is also able to phosphorylate p27 on residue Y88, Y89, or Y74 (15, 19). Nevertheless, it remains unclear which one is the growth-state-dependent kinase that alters p27's cyclin D1-cdk4-inhibitory activity in Mv1Lu cells under normal cell cycle conditions.
Our data highlight an important point and suggest that modulation of the ratio of phosphorylated Y forms relative to nonphosphorylated Y forms may be employed by the cell to increase cdk4 activity at specific times. While we found that p27 is preferentially Y phosphorylated in proliferating cells, tyrosine-phosphorylated p27 is a very low abundance species. Related to this issue, a significant finding was that Y-phosphorylated p27 did not bind preferentially to cdk4, suggesting that only a small pool of the total p27-cyclin D1-cdk4 complex is active in a cycling cell. We demonstrated that every cyclin D1-cdk4 complex was p27 bound (Fig. 1), so when the Y89F mutant was overexpressed in vivo, we shifted the balance from Y-phosphorylated to non-Y-phosphorylated p27, and all the cyclin D1-cdk4 was inhibited. This has implications for the way p27 works. Evidence suggests that the catalytic function of cdk4 may be especially required during the G0-to-G1-phase transition (55). The level of Y-phosphorylated p27 may be specifically increased following release from G0 and the reactivation of tyrosine kinases, resulting in a burst of cdk4 kinase activity. During asynchronous cell growth, only residual or maintenance levels of cyclin D1-cdk4 kinase activity may be required, accounting for the low level of p27 tyrosine phosphorylation detected here. Thus, the total level of p27 would dictate how much cyclin D1-cdk4 complex is formed, while the level of Y phosphorylation would dictate the activity of the complex.
While others have suggested that in the presence of overexpressed Y kinases, similar noninhibitory interactions occur between Y-phosphorylated p27 and cyclin A-cdk2 (15, 19), p27-cyclin A-cdk2 complexes that contain kinase activity are not detected under normal cellular conditions. In our experiments, Y-phosphorylated p27 still inhibited cyclin A-cdk2, suggesting that the presence or absence of Y phosphorylation did not affect its interaction with cyclin A-cdk2 and that this was a cyclin D1-cdk4-specific effect. Thus, it seems likely that the ability of Y-phosphorylated p27 to inhibit cdk2 may be detected only under more-specialized conditions, such as occur in cancer when tyrosine kinases are greatly overexpressed. It is possible that p27 is not Y phosphorylated in a growth-state-dependent manner to the levels necessary to detect differences in its ability to inhibit cdk2, and the primary role of Y-phosphorylated p27 during normal cell cycle regulation is to modulate cdk4 activity. This would help to ensure the temporal order of cdk activity following release from G0 arrest, where cdk4 is activated before cdk2, and would explain why rapid tyrosine kinase activation does not result in immediate cdk activation and entry into S phase. An increase in tyrosine kinase activity following G0 release would quickly negate p27's cdk4-inhibitory activity, independently of the signals and time required to degrade p27 and reactivate cdk2. In the time needed to affect p27's cytoplasmic transport and degradation, required for cdk2 activation, the reactivated p27-cyclin D-cdk4 complexes could phosphorylate Rb and increase cyclin E expression. Premature activation of cdk2 would be detrimental, as its range of substrates is much broader and includes direct members of the DNA replication machinery. p27's quick reactivation of cdk4 by tyrosine kinase phosphorylation ensures that the cell is metabolically ready for cdk2 activation and commitment to S phase.
p27 may also be routinely switched from a cdk4 inhibitor to an activator during normal cellular homeostasis and differentiation. For example, in tissues undergoing repair, such as the adult liver, inactive p27-cyclin D-cdk4 complexes regain kinase activity after partial hepatectomy (26). Likewise, tissues that undergo dynamic cell cycle entry and exit as part of their normal differentiation programs, such as breast epithelium following lactation cessation or granulosa cells in the adult ovary during folliculogenesis, ovulation, or luteinization, all rely on the activities of CKIs, and p27 specifically has been demonstrated to be crucial to these processes (21). p27-cyclin D-cdk4 complexes in quiescent tissues appear to be catalytically inactive, but the complex gains kinase activity following release from the G0 state. Is p27, which is responsible for this arrest, phosphorylated by tyrosine kinases, enabling it to reactivate cyclin D-cdk4 and thus drive the exit from the G0 state? While this remains to be demonstrated, the idea that modulation of the level of p27 Y phosphorylation by tyrosine kinase activity would dictate the activity or inactivity of the cdk4 complex might explain how p27 affects these important changes.
p27's differential switch may also be involved in its role in cancer progression, where, in addition to having a well-characterized tumor suppressor role, p27 may have an oncogenic function (9). In humans, low or cytoplasmic p27 levels correlate with aggressive tumors and poor prognoses for patients with numerous cancers, including breast, prostate, bladder, and thyroid cancers, and this loss of p27 generally results in an increase in cdk2 and cdk1 kinase activity (3, 34). Interestingly, p27 is almost never mutated or silenced at the genetic level, suggesting that optimal cell growth benefits from at least a low level of p27 in the cell. The observation that p27+/− animals are more tumor prone than p27−/− animals in prostate and mammary models supports this idea (17, 33). Persisting levels of p27 would ensure that cyclin D-cdk4 complexes would be assembled. As cyclin D and cdk4 have been implicated as potent oncogenes and cdk4 kinase activity is required to maintain tumorigenesis in several cancer models (6, 48, 59), p27's association with this complex must be activating, rather than inhibiting. It is possible that under these tumor-promoting conditions, p27 is “locked” into the non-cdk4-inhibitory mode by the overexpression of tyrosine kinases, and modulation of the level of p27 Y phosphorylation might be exploited as an effective chemotherapy. Elucidation of the signals and kinases that modify p27 will be important to understand these complex issues. A similar Y residue exists in the related CKI p21, and it will be interesting to see if a similar modification affects its inhibitory activity as well.
Supplementary Material
Acknowledgments
We thank J. Massagué for the generous gift of the Tet-p27 cell line, the Mv1Lu-tTA cells, and antibodies to p27, cdk2, and cdk4. We thank S. Nataraj and C. Roman for valuable discussion.
This work was supported by grants from the Leukemia Research Foundation, the Wendy Will Case Cancer Fund, and the American Cancer Society to S.W.B.
Footnotes
Published ahead of print on 1 October 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adams, P. D., X. Li, W. R. Sellers, K. B. Baker, X. Leng, J. W. Harper, Y. Taya, and W. G. Kaelin, Jr. 1999. Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin-cdk complexes. Mol. Cell. Biol. 191068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, P. D., W. R. Sellers, S. K. Sharma, A. D. Wu, C. M. Nalin, and W. G. Kaelin, Jr. 1996. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 166623-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkarain, A., R. Jordan, and J. Slingerland. 2004. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J. Mammary Gland Biol. Neoplasia 967-80. [DOI] [PubMed] [Google Scholar]
- 4.Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 2778517-8523. [DOI] [PubMed] [Google Scholar]
- 5.Bagui, T. K., S. Mohapatra, E. Haura, and W. J. Pledger. 2003. p27Kip1 and p21Cip1 are not required for the formation of active cyclin D-cdk4 complexes. Mol. Biol. Cell 237285-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughn, L. B., M. Di Liberto, K. Wu, P. L. Toogood, T. Louie, R. Gottschalk, R. Niesvizky, H. Cho, S. Ely, M. A. Moore, and S. Chen-Kiang. 2006. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 667661-7667. [DOI] [PubMed] [Google Scholar]
- 7.Birge, R. B., B. S. Knudsen, D. Besser, and H. Hanafusa. 1996. SH2 and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells 1595-613. [DOI] [PubMed] [Google Scholar]
- 8.Blain, S. W., E. Montalvo, and J. Massagué. 1997. Differential interaction of the cyclin-dependent kinase (cdk) inhibitor p27 with cyclin A-cdk2 and cyclin D2-cdk4. J. Biol. Chem. 27225863-25872. [DOI] [PubMed] [Google Scholar]
- 9.Blain, S. W., H. I. Scher, C. Cordon-Cardo, and A. Koff. 2003. p27 as a target for cancer therapeutics. Cancer Cell 3111-115. [DOI] [PubMed] [Google Scholar]
- 10.Boggon, T. J., and M. J. Eck. 2004. Structure and regulation of Src family kinases. Oncogene 237918-7927. [DOI] [PubMed] [Google Scholar]
- 11.Castano, E., Y. Kleyner, and B. D. Dynlacht. 1998. Dual cyclin-binding domains are required for p107 to function as a kinase inhibitor. Mol. Cell. Biol. 185380-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J., P. Saha, S. Kornbluth, B. D. Dynlacht, and A. Dutta. 1996. Cyclin-binding motifs are essential for the function of p21Cip1. Mol. Cell. Biol. 164673-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 181571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, M., V. Sexl, C. J. Sherr, and M. Roussel. 1998. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl. Acad. Sci. USA 951091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu, I., J. Sun, A. Arnaout, H. Kahn, W. Hanna, S. Narod, P. Sun, C. K. Tan, L. Hengst, and J. Slingerland. 2007. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyson, N. 1998. The regulation of E2F by pRb-family proteins. Genes Dev. 122245-2262. [DOI] [PubMed] [Google Scholar]
- 17.Gao, H., X. Ouyang, W. Banach-Petrosky, A. D. Borowsky, Y. Lin, M. Kim, H. Lee, W. J. Shih, R. D. Cardiff, M. M. Shen, and C. Abate-Shen. 2004. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. USA 10117204-17209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 895547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimmler, M., Y. Wang, T. Mund, Z. Cilensek, E. M. Keidel, M. B. Waddell, H. Jakel, M. Kullmann, R. W. Kriwacki, and L. Hengst. 2007. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128269-280. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto, Y., K. Kohri, Y. Kaneko, H. Morisaki, T. Kato, K. Ikeda, and M. Nakanishi. 1998. Critical role for the 3-10 helix region of p57(Kip2) in cyclin-dependent kinase 2 inhibition and growth suppression. J. Biol. Chem. 27316544-16550. [DOI] [PubMed] [Google Scholar]
- 21.Jirawatnotai, S., D. S. Moons, C. O. Stocco, R. Franks, D. B. Hales, G. Gibori, and H. Kiyokawa. 2003. The CDK inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J. Biol. Chem. 27817021-17027. [DOI] [PubMed] [Google Scholar]
- 22.Kardinal, C., M. Dangers, A. Kardinal, A. Koch, D. T. Brandt, T. Tamura, and K. Welte. 2006. Tyrosine phosphorylation modulates binding preference to a cyclin-dependent kinase and subcellular localization of p27kip1 in the acute promyelocytic leukemia cell line NB4. Blood 1071133-1140. [DOI] [PubMed] [Google Scholar]
- 23.Kato, A., H. Takahashi, Y. Takahashi, and H. Matsushime. 1997. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J. Biol. Chem. 2728065-8070. [DOI] [PubMed] [Google Scholar]
- 24.Kato, J.-Y., M. Matsuoka, D. K. Strom, and C. J. Sherr. 1994. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol. Cell. Biol. 142713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koff, A., M. Ohtsuki, K. Polyak, J. M. Roberts, and J. Massagué. 1993. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science 260536-539. [DOI] [PubMed] [Google Scholar]
- 26.Kwon, Y. H., A. Jovanovic, M. S. Serfas, H. Kiyokawa, and A. L. Tyner. 2002. p21 functions to maintain quiescence of p27-deficient hepatocytes. J. Biol. Chem. 27741417-41422. [DOI] [PubMed] [Google Scholar]
- 27.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11847-862. [DOI] [PubMed] [Google Scholar]
- 28.Landis, W. M., S. B. Pawlyk, T. Li, P. Sicinski, and W. P. Hinds. 2006. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 913-22. [DOI] [PubMed] [Google Scholar]
- 29.Mahony, D., D. A. Parrry, and E. Lees. 1998. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene 16603-611. [DOI] [PubMed] [Google Scholar]
- 30.Malumbres, M., and M. Barbacid. 2001. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer 1222-231. [DOI] [PubMed] [Google Scholar]
- 31.Massagué, J., S. W. Blain, and R. S. Lo. 2000. TGF-β signaling in growth control, cancer and heritable disease. Cell 103295-309. [DOI] [PubMed] [Google Scholar]
- 32.McConnell, B. B., F. J. Gregory, F. J. Stott, E. Hara, and G. Peters. 1999. Induced expression of p16Ink4a inhibits both cdk4- and cdk2-associated kinase activity by reassortment of cyclin-cdk inhibitor complexes. Mol. Cell. Biol. 191981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muraoka, R. S., A. E. G. Lenferink, B. Law, E. Hamilton, D. M. Brantley, L. R. Roebuck, and C. L. Arteaga. 2002. Erb2/Neu-induced cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 222204-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickeleit, I., S. Zender, U. Kossatz, and N. P. Malek. 2007. p27kip1: a target for tumor therapies? Cell Div. 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nourse, J., E. Firpo, M. W. Flanagan, M. Meyerson, K. Polyak, M. H. Lee, J. Massagué, G. R. Crabtree, and J. M. Roberts. 1994. Rapamycin prevents IL-2-mediated elimination of the cyclin-CDK kinase inhibitor, p27Kip1. Nature 372570-573. [DOI] [PubMed] [Google Scholar]
- 36.Obaya, A. J., I. Kotenko, M. D. Cole, and J. M. Sedivy. 2002. The proto-oncogene c-myc acts through the cyclin-dependent kinase (Cdk) inhibitor p27kip1 to facilitate the activation of Cdk4/6 and early G1 phase progression. J. Biol. Chem. 27731263-31269. [DOI] [PubMed] [Google Scholar]
- 37.Ortega, S., M. Malumbres, and M. Barbacid. 2002. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim. Biophys. Acta 160273-87. [DOI] [PubMed] [Google Scholar]
- 38.Parry, D., D. Mahony, K. Wills, and E. Lees. 1999. Cyclin D-cdk subunit arrangement is dependent on the availability of competing Ink4 and p21 class inhibitors. Mol. Cell. Biol. 191775-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavletich, N. P. 1999. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287821-828. [DOI] [PubMed] [Google Scholar]
- 40.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massagué. 1994. Cloning of p27kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 7859-66. [DOI] [PubMed] [Google Scholar]
- 41.Poon, R. Y. C., H. Toyoshima, and T. Hunter. 1995. Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol. Biol. Cell 61197-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynisdóttir, I., and J. Massagué. 1997. The subcellular location of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11492-503. [DOI] [PubMed] [Google Scholar]
- 43.Reynisdóttir, I., K. Polyak, A. Iavarone, and J. Massagué. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 91831-1845. [DOI] [PubMed] [Google Scholar]
- 44.Russo, A. A., P. D. Jeffrey, A. Patten, J. Massagué, and N. Pavletich. 1996. Crystal structure of the p27Kip1 cyclin-dependent kinase inhibitor bound to the cyclin A-cdk2 complex. Nature 382325-331. [DOI] [PubMed] [Google Scholar]
- 45.Russo, A. A., P. D. Jeffrey, and N. Pavletich. 1996. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat. Struct. Biol. 3696-700. [DOI] [PubMed] [Google Scholar]
- 46.Russo, A. A., L. Tong, J. O. Lee, P. D. Jeffrey, and N. Pavletich. 1998. Structural basis for inhibition of the cyclin-dependent kinase cdk6 by the tumour suppressor p16Ink4a. Nature 395237-243. [DOI] [PubMed] [Google Scholar]
- 47.Sandhu, C., J. Garbe, N. Bhattacharya, J. Daksis, C.-H. Pan, P. Yaswen, J. Koh, J. M. Slingerland, and M. R. Stampfer. 1997. Transforming growth factor β stabilizes p15Ink4B protein, increases p15Ink4B-cdk4 complexes, and inhibits cyclin D1-cdk4 association in human mammary epithelial cells. Mol. Cell. Biol. 172458-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro, G. I. 2006. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 241770-1783. [DOI] [PubMed] [Google Scholar]
- 49.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 131501-1512. [DOI] [PubMed] [Google Scholar]
- 50.Slingerland, J. M., L. Hengst, C. Pan, D. Alexander, M. Stampfer, and S. I. Reed. 1994. A novel inhibitor of cyclin-cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol. Cell. Biol. 143683-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7135-146. [PubMed] [Google Scholar]
- 52.Stepanova, L., X. Leng, S. G. Parker, and J. W. Harper. 1996. Mammalian p50cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes cdk4. Genes Dev. 101491-1502. [DOI] [PubMed] [Google Scholar]
- 53.Toyoshima, H., and T. Hunter. 1994. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 7867-74. [DOI] [PubMed] [Google Scholar]
- 54.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 311-20. [DOI] [PubMed] [Google Scholar]
- 55.Tsutsui, T., B. Hesabi, D. S. Moons, P. P. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of cdk4 delays cell cycle entry with enhanced p27kip1 activity. Mol. Cell. Biol. 197011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlach, J., S. Hennecke, K. Alevizopoulos, D. Conti, and B. Amati. 1996. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 156595-6604. [PMC free article] [PubMed] [Google Scholar]
- 57.Warner, B. J., S. W. Blain, J. Seoane, and J. Massagué. 1999. Myc downregulation by transforming growth factor β required for activation of the p15Ink4b G1 arrest pathway. Mol. Cell. Biol. 195913-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, J. J., D. E. Afar, H. Phan, O. N. Witte, and K. S. Lam. 2002. Recognition of multiple substrate motifs by the c-ABL protein tyrosine kinase. Comb. Chem. High Throughput Screen. 583-91. [DOI] [PubMed] [Google Scholar]
- 59.Yu, Q., E. Sicinska, Y. Geng, M. Ahnstrom, A. Zagozdzon, Y. Kong, H. Gardner, H. Kiyokawa, N. L. Harris, O. Stal, and P. Sicinski. 2006. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 923-32. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, X., M. Ohtsubo, R. M. Bohmer, J. M. Roberts, and R. K. Assoian. 1996. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and the phosphorylation of the retinoblastoma protein. J. Cell Biol. 133391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.