Abstract
Intervertebral disc (IVD) degeneration is frequently characterized by increased cell proliferation, probably as a tissue regenerative response. Although many growth factors and their receptors have been shown to be expressed normally in the disc, and generally to be over-expressed during degeneration, not all of them have been thoroughly studied concerning their effects on IVD cell proliferation. In the present report, three potent mitogens, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF) and insulin-like growth factor-I (IGF-I) are examined regarding their capacity to induce proliferation in vitro of bovine coccygeal nucleus pulposus (NP) and annulus fibrosus (AF) cells, as well as to activate major intracellular signal transduction pathways. PDGF, bFGF and IGF-I were found to induce DNA synthesis in quiescent IVD cells in a dose-dependent manner. Maximum stimulation was induced by PDGF, while stimulation by all three factors simultaneously exceeded only slightly that caused by PDGF alone. All three growth factors were shown to phosphorylate immediately extracellular-signal regulated kinases (ERKs), while the stimulation by bFGF especially resulted in sustained ERK phosphorylation. Furthermore, all three growth factors induced phosphorylation of Akt in both Thr308 and Ser473 residues immediately after stimulation, although bFGF-induced phosphorylation was much weaker than that provoked by PDGF and IGF-I. In addition, the MEK inhibitor PD98059 and the PI 3-K inhibitor wortmannin were shown to block growth factor-induced ERK- and Akt-phosphorylation, respectively, in IVD cells. Inhibition of the MEK/ERK or the PI 3-K/Akt pathways provoked a significant decline of the proliferative effects of PDGF, bFGF or IGF-I on IVD cell cultures, while the simultaneous inhibition of both signaling pathways abolished completely the mitogenicity of these growth factors. The above effects of the three growth factors were reproduced similarly in both NP and AF cell cultures. Overall, the above results indicate that PDGF, bFGF and IGF-I stimulate the proliferation of IVD cells via the ERK and Akt signaling pathways.
Keywords: Intervertebral disc, Cell proliferation, Growth factors, ERK, Akt
Introduction
Low back pain, an important health problem for the Western societies [4], is strongly associated with degeneration of the intervertebral discs (IVDs) [22]. IVD degeneration is encompassing a decline of cell viability and alterations in the synthesis and distribution of extracellular matrix (ECM) molecules, which eventually lead to major changes in the architecture and the properties of the disc [46]. On the other hand, clusters of cells, particularly in the nucleus pulposus (NP), are frequently found in disc degeneration due to local cell replication [18], possibly as a physiological response towards tissue repair. In an avascular tissue such as IVD, cell replication should be regulated by local growth factor production. Indeed, there are numerous reports in the literature on the expression of various growth factors and cytokines, as well as, their receptors in the disc. Basic fibroblast growth factor (bFGF) has been detected in rat [29], ovine [27] as well as in human IVDs [8, 43, 45], mostly associated with vascular ingrowth in herniated discs, although its receptor FGFR3 was equally expressed in both normal and degenerated samples [20]. Insulin-like growth factor-I (IGF-I) and its receptor have been found to be expressed in human [20, 39], as well as in rabbit discs [28] and in cells from the bovine coccygeal IVD, where moreover specific binding of IGF-I to its receptor has been demonstrated [31]. Platelet-derived growth factor (PDGF) has also been detected in herniated human IVDs [44, 45]. Furthermore, members of the superfamily of transforming growth factor-β (TGF-β), including bone morphogenetic proteins (BMPs), as well as their receptors are expressed in IVD (see [24] and references therein).
The response of IVD cells to the above growth factors has been studied in both in vitro and in vivo systems, since it has been proposed that the clinical application of growth factors could represent a new therapeutic intervention against disc degeneration [7, 24, 47]. Most of the studies, however, are focusing on the effect of growth factors on the production and/or degradation of ECM, and only some of them are dealing with growth factor-induced cell proliferation. For example, proliferation of IVD cells has been shown in response to BMP-2 [42], BMP-7 [48], GDF-5 [7] and TGF-β1 [13], all of them belonging to the TGF-β-superfamily. Furthermore, regarding the effects of classical potent mitogens such as PDGF, bFGF and IGF-I [50] on the proliferation of IVD cells in vitro, in some cases the data are indirect [1, 8] or contradictory (e.g., regarding the effects of IGF-I on annulus fibrosus cells [14, 41]), possibly due to the variety of the assay systems used. Hence, we aimed at a systematic study of these three mitogens under the same experimental conditions.
PDGF, originally purified form human platelets, is the prototype mitogen for connective tissue cells [15, 37]. bFGF—first described as a mitogenic factor for fibroblastic cells—and IGF-I have pleiotropic roles in many cell types and tissues [10, 19, 30]. All these growth factors exert their effects on cells through transmembrane receptors with tyrosine kinase activity, which in turn activate a variety of intracellular signaling cascades, in order to regulate cellular functions. Concerning cell proliferation, probably the two most important signaling pathways are that of mitogen-activated protein kinases (MAPKs)—especially the group of extracellular signal regulated kinases (ERKs)—and that of phosphatidylinositol 3-kinase/Akt (PI 3-K/Akt). The ERKs 1 and 2 are components of a three-kinase phosphorelay module encompassing also the MAPK kinase (MKK) MEK and the MAPK kinase kinase (MKKK) c-Raf [6]. The PI 3-K/Akt pathway has been reported to be involved in the signal transduction of the majority of growth factors studied [5, 9]. Activation of PI 3-K by growth factors generates phosphatidylinositol-3,4,5-triphosphate which recruits the protein kinase B (PKB)/Akt to the cell membrane to be phosphorylated/activated, initiating a cascade of events regulating cell proliferation, as well as several other functions, e.g., apoptosis or glucose metabolism. However, the contribution of these pathways in growth factor-induced proliferation of IVD cells has not been studied yet.
Accordingly, in the present study we have investigated the effect of PDGF, bFGF and IGF-I on IVD-cell proliferation in vitro, as well as, the involvement of the MEK/ERK and the PI 3-K/Akt signaling pathways in this process, in an effort towards the in depth understanding of disc cell physiology, and especially of the mechanisms underlying disc regeneration.
Materials and methods
Human recombinant (h.r.) PDGF-BB (the PDGF-isoform considered to represent the universal ligand for all PDGF-receptor subtypes [40]), h.r. bFGF and h.r. IGF-I were purchased from R&D Systems (Minneapolis, MN, USA). PD98059, wortmannin, LY294002, protease and phosphatase inhibitor cocktails, as well as goat anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from Sigma (St Louis, MO, USA). 5-Bromo-2′-deoxyuridine (BrdU), monoclonal Anti-BrdU antibody (clone BU-33), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and goat anti-mouse FITC-conjugated IgG were also purchased from Sigma. Rabbit anti-phospho-Akt (Ser473), anti-phospho-Akt (Thr308) and anti-Akt1/2/3 antibodies were obtained from Cell Signaling Technology (Hertfordshire, UK), while mouse anti-phospho-ERK1/2 antibody that recognizes phosphorylated Thr202/Tyr204 and mouse anti-pan-ERK antibodies from BD Transduction Laboratories (Bedford, MA, USA). [Methyl-3H]-Thymidine was from Amersham Biosciences (Buckinghamshire, UK). Crude collagenase, all cell culture media, antibiotics and sodium pyruvate were purchased from Biochrom KG (Berlin, Germany). Low glucose (1,000 mg/l) formulation of Dulbecco’s minimal essential medium (DMEM), trypsin and fetal bovine serum (FBS) were from Gibco BRL (Paisley, UK).
Cell isolation and cell culture conditions
Tails from young steers (8-12 months of age) were obtained from a local slaughterhouse and they were processed within 8 h after slaughter, as follows: The IVDs from each tail were aseptically dissected from their adjacent vertebral bodies and divided into nucleus pulposus (NP), inner annulus fibrosus and outer annulus fibrosus (AF). In the present study no inner AF-derived cells were used; from this point on AF refers to outer AF. Each part was further minced in small pieces (approx. 1 mm3), which were subjected to an overnight digestion with a crude collagenase solution in DMEM (1 mg/ml for NP and 3 mg/ml for AF). Cells were recovered by centrifugation and they were routinely cultured in DMEM (high glucose formulation, i.e., 4,000 mg/l) supplemented with penicillin and streptomycin, sodium pyruvate, l-glutamine, and 10% FBS in a humidified atmosphere of 5% CO2 at 37°C. Cells were routinely subcultured when confluent by using a trypsin/citrate (0.25/0.30% w/v) solution. Cell counting, after trypsinization, was performed by using a Coulter counter (Beckman-Coulter, Fullerton, CA). Cells were tested periodically and found to be mycoplasma-free.
Tritiated thymidine incorporation assay
Cells were plated at a density of 2 × 104 cells/cm2, in DMEM containing 10% FBS, and left to grow until they were fully confluent (approximately 2 weeks) with medium renewal twice a week. Then the medium was changed to low glucose formulation of DMEM containing 0.1% FBS for another 4 days (with a medium renewal at 48 h). Then the growth factors to be tested were added to the quiescent cultures, along with methyl-[3H]-thymidine (0.2 μCi/ml, 25 Ci/mmol). After 24 h of incubation, the culture medium was aspirated, the cells were washed with PBS, fixed with 10% ice-cold trichloroacetic acid (TCA), washed extensively under running tap water and air-dried. DNA was solubilized by the addition of 0.3 N NaOH/1% (w/v) SDS and the lysates were subjected to scintillation counting, as previously described [33]. When indicated, the cells were pre-incubated with the appropriate concentrations of kinase inhibitors for 45 min before growth factor treatment.
Bromodeoxyuridine incorporation assay
In addition to [3H]-thymidine incorporation, cells synthesizing DNA were also identified after dual labeling with 5-bromo-2′-deoxyuridine and 4′,6-diamidino-2-phenylindole (DAPI) dihydrochloride, using a modification of the method described by Hsieh et al. [16]. In brief, cells plated overnight on glass coverslips at a density of 2 × 104 cells/cm2, in DMEM containing 10% FBS were growth-arrested for 4 days as above (tritiated thymidine incorporation assay) and then stimulated with the growth factors along with 50 μM bromodeoxyuridine for 24 h. The cells were fixed in freshly prepared solution of 4% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 in PBS, treated with 2 N HCl, incubated with anti-bromodeoxyuridine mAb (1 h) followed by a FITC-conjugated anti-mouse IgG (1 h), and then stained with 1 mg/ml DAPI in PBS (10 min) in the dark at room temperature. Cells were washed three times with PBS at each step. DAPI- and bromodeoxyuridine-positive nuclei were observed on a Zeiss Axiopan 2 fluorescence microscope with a 40× objective; a field containing approximately 200 cells was used for quantification purposes.
Western analysis
Bovine IVD cell cultures arrested in DMEM 0.1% FBS were treated with growth factors in the presence or absence of kinase inhibitors as described (tritiated thymidine incorporation assay, see above) for various time periods. Then, they were washed with ice-cold PBS, scraped into 2× hot SDS-PAGE sample buffer [125 mM Tris–HCl pH 6.8, 5% (w/v) SDS, 20% (v/v) glycerol, 125 mM β-mercaptoethanol, 0.02% (w/v) bromophenol blue, supplemented with protease- and phosphatase-inhibitor cocktails], sonicated for 2 × 10 s, boiled for 5 min, clarified by centrifugation, and stored at −80°C until use. The lysates were separated on SDS-PAGE and the proteins were transferred to PVDF membranes (Amersham Biosciences). The membranes were blocked with 5% (w/v) non-fat dried milk in 10 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.05% Tween-20 (TTBS) buffer and incubated with the appropriate primary antibodies. After washing with TTBS, the membranes were incubated with the respective secondary antibody for 1 h, washed again with TTBS and the immunoreactive bands were visualized on Kodak-X-OMAT-AR film by chemiluminescence (ECL kit) according to the manufacturer’s (Amersham Biosciences) instructions.
Statistics
Data presented are the mean of quadruplicate observations (±SD). Differences were considered significant when P < 0.05 (Student’s t test).
Results
Proliferative response of IVD cells to PDGF, bFGF and IGF-I
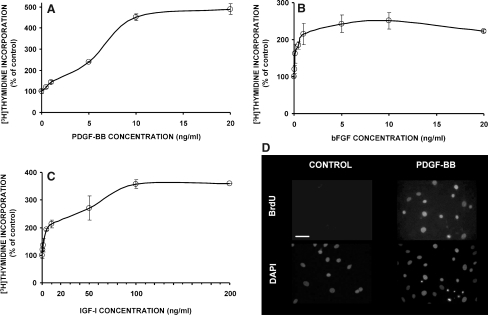
In order to determine the response of IVD cells to the growth factors under study (i.e., PDGF, bFGF, and IGF-I), we have developed primary cultures of bovine coccygeal nucleus pulposus and annulus fibrosus cells. The cells were cultured in the presence of 10% FBS for approximately 2 weeks, and then they were rendered quiescent by serum starvation, as described under materials and methods. In these cultures less than 5% of the cells were in the S-phase of the cell cycle, as determined by flow cytometry after propidium iodide staining (not shown here). As shown in Fig. 1, all three growth factors stimulated DNA synthesis in NP cultures in a dose-dependent manner, as judged by the incorporation of tritiated thymidine. Specifically, PDGF starts to provoke a statistically significant stimulation from 1 ng/ml, while the maximum stimulation—approximately fivefold compared to the control—is observed from 10 ng/ml upward (Fig. 1a). Basic FGF is stimulatory from concentrations as low as 0.1 ng/ml, while it reaches a plateau—approximately 2.5-fold compared to the control—at 5 ng/ml (Fig. 1b). The lowest concentration of IGF-I which significantly stimulates DNA synthesis is 5 ng/ml, and its plateau—corresponding to stimulation of approximately 3.5-fold in comparison with the control—starts at 100 ng/ml (Fig. 1c). Based on these dose–response curves, all following experiments were performed at the lowest concentrations of the three growth factors that induce a maximum stimulation, i.e., 10 ng/ml PDGF, 5 ng/ml bFGF and 100 ng/ml IGF-I.
Fig. 1.
Stimulation of DNA synthesis in bovine coccygeal nucleus pulposus cells by growth factors. Cells were rendered quiescent after 4 days in low serum as described in “Materials and methods”; then in a–c, they were treated with the indicated concentrations of growth factors along with tritiated thymidine, and DNA synthesis was estimated as percentage of that of the untreated culture (mean of three independent experiments); in d, medium containing PDGF-BB (10 ng/ml) or not (control) was added along with BrdU, and DNA synthesis was observed by immunofluorescence (one representative field of each sample is depicted—counterstaining of the nuclei with DAPI in the same field is shown below; the white bar corresponds to 55 μm)
Experiments with 5-bromo-2′-deoxyuridine (BrdU) incorporation confirmed that the cultures before growth factor-treatment were indeed quiescent, since they did not incorporate BrdU over a 24-h period (see control cells in the left panel of Fig. 1d), while cell nuclei in PDGF-treated cultures were labeled with BrdU at a percentage of 54% (see Fig. 1d, right panel). IGF-I- and bFGF-treated cultures incorporated BrdU at percentages of 42 and 36%, respectively (not shown).
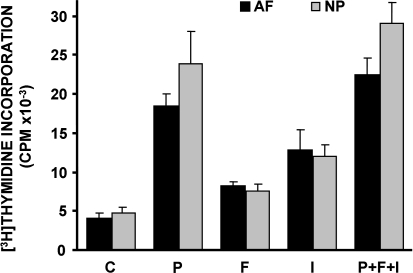
Similar proliferative responses to all three growth factors have been observed with cell cultures derived from various donors. Furthermore, as depicted in Fig. 2, annulus fibrosus (AF) and nucleus pulposus (NP) cell cultures derived from the same donor respond similarly to the growth factors studied. In all cases, PDGF was the most potent of the three growth factors tested. Moreover, when all three growth factors were applied simultaneously to the cell cultures the stimulation was only slightly higher than the stimulation induced by PDGF alone (not statistically significant; Fig. 2).
Fig. 2.
Stimulation of DNA synthesis in bovine coccygeal nucleus pulposus (NP) and annulus fibrosus (AF) cells by growth factors. Quiescent cells were stimulated with 10 ng/ml PDGF-BB (P), 5 ng/ml bFGF (F), 100 ng/ml IGF-I (I) or a combination of all three of them (P + F + I) along with tritiated thymidine, and DNA synthesis was estimated (a representative experiment out of three similar ones is depicted)
Activation of ERK and Akt in IVD cells by PDGF, bFGF and IGF-I
As has been shown in other cell systems, growth factors transduce their mitogenic signals through intracellular signaling pathways, MEK/ERK and PI 3-K/Akt being among the most important. Accordingly, we have studied in IVD cells the activation of these pathways by monitoring their phosphorylation status after growth factor-stimulation. For ERK, we have used an antibody recognizing both ERK1 and ERK2 dually phosphorylated at Thr202 and Tyr204. On the other hand, for Akt we have used two different antibodies recognizing either pAktThr308 or pAktSer473, as the role of these two phosphorylation sites in the induction of cell proliferation is still under study (see “Discussion”).
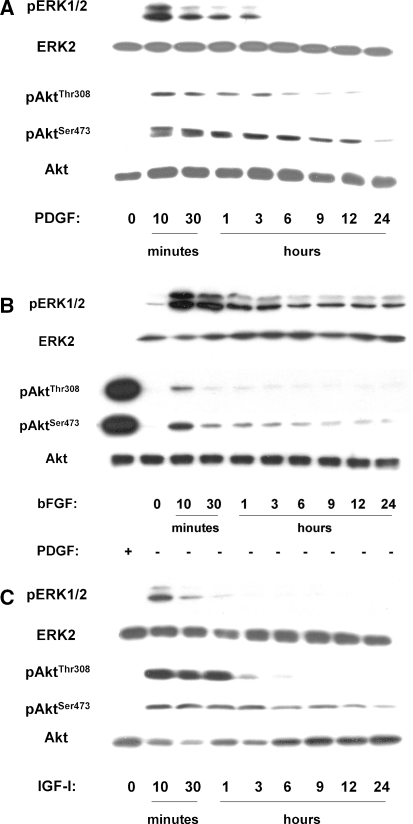
In response to PDGF, ERK was transiently phosphorylated for approximately 3 h, with a maximum observed immediately (10 min) after stimulation (Fig. 3a). On the other hand, Akt, after an acute activation at 10 min, exhibited a more sustained phosphorylation in both sites (Thr308 and Ser473) lasting up to 12 h, while especially pAktSer473 was detectable even 24 h after induction by PDGF (Fig. 3a).
Fig. 3.
Western analysis of growth factor-induced ERK and Akt phosphorylation in bovine NP cells. Lysates of quiescent cells treated with 10 ng/ml PDGF-BB, 5 ng/ml bFGF or 100 ng/ml IGF-I for the indicated time-intervals were subjected to immunoblotting for the indicated phosphoproteins or the corresponding unphosphorylated proteins (a representative experiment out of three similar ones is depicted)
In response to bFGF the phosphorylation of ERK peaked again at 10 min, but it was sustained throughout a 24-h period (Fig. 3b). On the other hand, phosphorylation of Akt by bFGF was much weaker compared to that provoked by PDGF, used as positive control (Fig. 3b). Nevertheless, phosphorylation in both Thr308 and Ser473 peaked at 10 min, while again activation of pAktSer473 was more sustained.
The response to IGF-I resembled more that provoked by PDGF: pERK was transiently activated, while phosphorylation of Akt was immediate in both residues, but pAktThr308 was detectable up to 6 h and pAktSer473 up to 24 h (Fig. 3c).
When the above experiments were repeated with cell cultures originating from annulus fibrosus, a similar pattern of activation of both signaling cascades was observed (data not shown).
Involvement of MEK/ERK and PI 3-K/Akt in the proliferative response of IVD cells to PDGF, bFGF and IGF-I
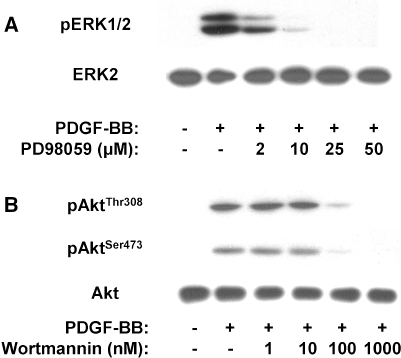
Having observed that the pathways of ERK and PI 3-K/Akt are activated in response to PDGF, bFGF and IGF-I, we proceeded to investigate the role of this activation in growth factor-induced proliferation. To this end, we have used two established pharmacologic, cell permeable inhibitors: PD98059, which is specific for the MEK/ERK pathway [2] and wortmannin, which is specific for the PI 3-K/Akt pathway [49]. Initially, we identified the optimal concentrations of the two inhibitors to be used in IVD cell cultures, i.e., the lowest ones provoking maximum inhibition. As shown in Fig. 4a, PD98059 starts to inhibit the PDGF-induced ERK phosphorylation of NP cells at 2 μM; complete inhibition is achieved at 25 μM. Since in the literature concentrations of PD98059 below 50 μM are considered specific for MEK inhibition [2], and we did not observe any inhibition of PDGF-induced Akt phosphorylation at 25 μM of PD98059 (not shown), we have chosen this as the optimal concentration for subsequent experiments.
Fig. 4.
Western analysis indicating inhibition of ERK and Akt phosphorylation by PD98059 and wortmannin, respectively. Lysates of quiescent cells pretreated with the indicated concentrations of inhibitors for 45 min and then treated with 10 ng/ml PDGF-BB for additional 10 min were subjected to immunoblotting for the indicated phosphoproteins or the corresponding unphosphorylated proteins (a representative experiment out of two similar ones is presented)
Furthermore, wortmannin at a concentration of 100 nM inhibits effectively Akt phosphorylation at both Thr308 and Ser473 (Fig. 4b). Although at 1,000 nM inhibition is total, there are data in the literature indicating unspecific effects of wortmannin over 300 nM [11]. Nevertheless, in IVD cells wortmannin at 100 nM did not inhibit PDGF-induced ERK phosphorylation (not shown), hence we have chosen this as the optimal concentration for subsequent experiments. We have also observed that PD98059 (25 μM) completely abolishes bFGF- and IGF-I-induced ERK phosphorylation, while wortmannin (100 nM) annuls the pAktThr308 and pAktSer473 increase provoked by bFGF and IGF-I (not shown).
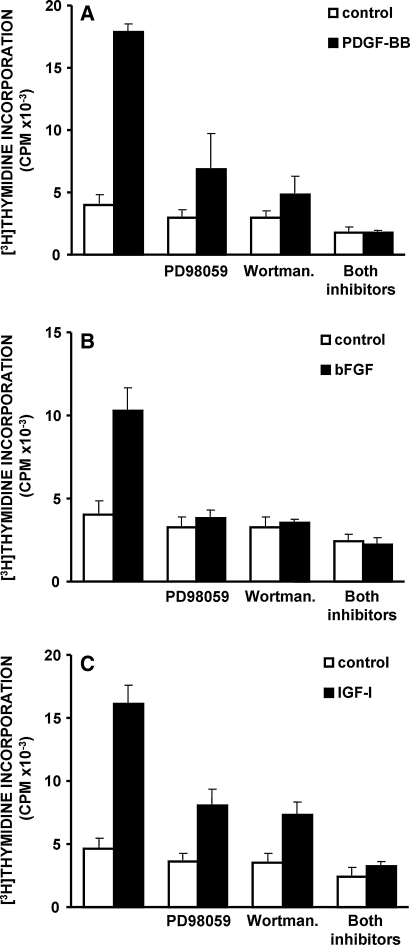
Subsequently, we proceeded with functional studies using the two inhibitors. We have shown that PDGF-induced stimulation of DNA synthesis in NP cells is inhibited by PD98059 to approximately 40% of the stimulation in the absence of the inhibitor, and by wortmannin to 15%, while it is completely annulled by the simultaneous use of both inhibitors (Fig. 5a). Regarding the stimulation of DNA synthesis provoked by bFGF, it is inhibited almost completely by PD98059 or wortmannin, as well as by their combination (Fig. 5b). Finally, the proliferative effect of IGF-I is partly inhibited by PD98059 or wortmannin, and virtually completely by their combined use (Fig. 5c). The involvement of the PI 3-K/Akt pathway in growth factor-induced DNA synthesis was confirmed using another PI 3-K inhibitor, LY294002 (data not shown). Furthermore, application of the above inhibitors in AF cell cultures treated with the above three growth factors yielded similar inhibition of DNA synthesis stimulation (not shown).
Fig. 5.
Inhibition of growth factor-induced DNA synthesis in bovine NP cells by pharmacologic inhibitors. Quiescent cells pretreated with PD98059 (25 μM) or wortmannin (100 nM) or a combination of both were stimulated with 10 ng/ml PDGF-BB (P), 5 ng/ml bFGF (F) or 100 ng/ml IGF-I (I), and DNA synthesis was estimated by tritiated thymidine incorporation (a representative experiment out of three similar ones is depicted)
In conclusion, inhibition of the MEK/ERK or the PI 3-K/Akt pathways results in a significant attenuation of the proliferative effects of PDGF, bFGF or IGF-I on IVD cell cultures, while the simultaneous inhibition of both signaling pathways abolishes completely the mitogenicity of these growth factors.
Discussion
Cell proliferation constitutes a feature of disc degeneration [18], probably as a tissue regenerative response, and this is further supported by the fact that growth factors and their receptors are over-expressed during degeneration (see “Introduction”). Although the responses of IVD cells to various growth factors and cytokines in vitro have been studied in terms of cell functions such as collagen and proteoglycan production or specific gene expression (for a comprehensive review see [24]), cell proliferation in vitro, especially in response to typical mitogens expressed in the disc, such as PDGF, bFGF and IGF-I, has not been systematically investigated. The need for such studies is becoming more vital in the prospect of clinical application of growth factors for the treatment of disc pathologies [24, 47].
Our study of the proliferative response of IVD cells to PDGF, bFGF and IGF-I showed that all three growth factors elicited stimulation of DNA synthesis in a dose-dependent manner (Fig. 1), at concentration ranges similar with the ones reported for other connective tissue cell types, such as fibroblasts or chondrocytes [26, 38]. Regarding the maximal induction achieved by each growth factor, clearly the rate of potency was PDGF-BB > IGF-I > bFGF (see Figs. 1, 2). It must be mentioned here, that Thompson et al. [41], at variance to our results, reported that FGF (300 ng/ml) is more potent than IGF-I (20 ng/ml), at least for AF cells. Beyond the differences in the origin or the concentrations of the growth factors, the discrepancy between the two studies could be due to the different donor organism (bovine vs. canine) or to the different culture system (isolated cells vs. whole organ culture).
Interestingly, the stimulation provoked by the simultaneous action of all three growth factors was marginally higher than that induced by the most potent one, i.e., PDGF (Fig. 2), allowing us to assume that all three factors are transducing their mitogenic signals to the nucleus through the same intracellular signaling pathway(s).
The signaling pathways of MEK/ERK and PI 3-K/Akt are known to mediate the effects of classical mitogens in various cell types. However, only limited studies have appeared until now reporting the activation and/or the function of these pathways in IVD cells or tissues [34–36]. Accordingly, we have studied the activation of the MEK/ERK and the PI 3-K/Akt pathways in bovine IVD cells in response to PDGF, bFGF and IGF-I. Moreover we have followed the kinetics of this activation, since this information is crucial in understanding the mechanism of action of a growth factor, as well as in determining its final effect. For example, we have shown recently that delayed ERK activation by TGF-β in human fibroblasts was due to the autocrine induction of bFGF [12]. In the same context, transient ERK activation by EGF can stimulate proliferation in PC12 neuronal cells, whereas in the same cell type sustained ERK activation by NGF can be inhibitory [23]. Furthermore, pivotal signaling molecules, such as Akt, can be phosphorylated in several residues, and differences in phosphorylation can direct to variations in the final outcome. Although full activation of Akt by growth factors encompasses its phosphorylation in both Thr308 and Ser473 [3], a recent work [17] has shown that defective Ser473 phosphorylation due to genetic deletion of an upstream effector affected only a subset of Akt targets mostly involved in survival functions, while it was dispensable for other functions, such as growth factor-induced proliferation. Accordingly, we have studied phosphorylation of Akt in both aminoacid residues.
Here, we describe for the first time that PDGF, bFGF and IGF-I activate both MEK/ERK and PI 3-K/Akt pathways in IVD cells (Fig. 3). According to our results, in bovine IVD cells all three growth factors studied phosphorylate ERK immediately, although with varying kinetics: the effects of PDGF and IGF-I are transient, while that of bFGF is sustained throughout the whole study-period of 24 h (Fig. 3). Furthermore, we observed that PDGF and IGF-I activate also Akt, and this effect is more sustained than ERK phosphorylation (Fig. 3a, c). Intriguingly, phosphorylation in Ser473 was somewhat more prolonged than in Thr308—by both growth factors. To our knowledge, this is the first report of differential phosphorylation kinetics of the two residues after stimulation with growth factors in any cell type. Whether this difference reflects a role of pAktSer473 in IVD cell survival as opposed with a role of pAktThr308 in their proliferative response, as previously suggested [17], remains speculative for the time. Interestingly, bFGF also provoked phosphorylation of Akt (Fig. 3b) although much weaker than that induced by PDGF and IGF-I, a fact that could possibly be associated with the weaker maximum induction of DNA synthesis by bFGF in comparison with the other two growth factors (Fig. 2).
In an effort to examine whether the activation of the above signal transduction pathways is functionally involved in growth factor-induced DNA synthesis stimulation, we have used cell permeable pharmacological inhibitors that specifically block these pathways. We have chosen the MEK inhibitor PD98059 [2], as well as two widely used PI 3-K inhibitors, wortmannin and LY294002 [9], and we have determined the optimal concentrations for their use in bovine IVD cell cultures (Fig. 4). Using this approach, we have shown attenuation of the stimulation provoked by all three growth factors in the presence of the MEK/ERK pathway inhibitor PD98059 (Fig. 5). Furthermore, inhibition of Akt phosphorylation by PI 3-K inhibitors results in decrease of PDGF- or IGF-I-induced DNA synthesis. In addition, we have observed that although Akt phosphorylation by bFGF in bovine IVD cells is weak, it is necessary for the stimulatory effect of the growth factor, since the latter is attenuated in the presence of wortmannin (Fig. 5b) or LY294002 (not shown). Notably, although it has been shown that bFGF can activate the PI 3-K/Akt pathway in several cell systems [10], this activation is only occasionally linked directly with the proliferative effect of this growth factor [21].
When PD98059 and wortmannin were added simultaneously to IVD cell cultures, they inhibited totally the proliferative effects of PDGF, bFGF and IGF-I. Collectively, for all three growth factors studied, stimulation of DNA synthesis is mediated via activation of both the ERK and the PI 3-kinase/Akt pathways. This could also explain why application of all three growth factors simultaneously does not result in substantially higher stimulation than that obtained with the most potent growth factor alone, i.e., PDGF (Fig. 2, see above).
A general observation throughout our study was that cultures derived from annulus fibrosus responded to PDGF, bFGF and IGF-I similarly to the cultures originating from nucleus pulposus, concerning both the stimulation of DNA synthesis and the activation of intracellular signaling cascades. This is in agreement with most in vitro studies, where the mitogenic responses of AF and NP cells are comparable [7, 25].
The cell system we have chosen for our studies is based on cultures derived from bovine coccygeal discs—a model that is widely used, as it simulates the properties of human lumbar discs [32]—because of the relative paucity of healthy human samples needed for the establishment of cell cultures. Nevertheless, it should be noted here, that human IVD cell cultures originating from surgical specimens respond to the growth factors under study generally similarly to bovine ones (to be shown elsewhere). Furthermore, our cell system for the study of growth factor-induced proliferation makes use of monolayer cultures, a milieu possibly mimicking the conditions of the degenerated disc, i.e., the presence of cell clusters and the reduced restraint by ECM. On the other hand, since ECM components, as well as 3D culture conditions—simulating the architecture of the healthy disc—could be crucial for cell proliferation, we are currently examining the proliferative responses of IVD cells to growth factors in 3D culture model systems (work in progress).
In conclusion, the evidence presented in this work indicate that PDGF, bFGF and IGF-I stimulate the proliferation of IVD cells via the ERK and Akt signaling pathways.
Acknowledgments
This work was supported by the European Union (“EURODISC” project, contract No QLK6-CT-2002-02582) and by a grant from the AO Fund, Switzerland (05-K68). HP was also partly supported by a postdoctoral fellowship of NCSR “Demokritos”. We would like to acknowledge Dr. Sotirios Moschovitsis, Director Veterinary of Public Health, Ministry of Rural Development and Food, Greece for helping us to obtain bovine tissue specimens.
References
- 1.Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine. 2006;31:959–966. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/S0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 7.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc—in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 8.Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235–241. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Duronio V, Scheid MP, Ettinger S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell Signal. 1998;10:233–239. doi: 10.1016/S0898-6568(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 10.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Ferby IM, Waga I, Hoshino M, Kume K, Shimizu T. Wortmannin inhibits mitogen-activated protein kinase activation by platelet-activating factor through a mechanism independent of p85/p110-type phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:11684–11688. doi: 10.1074/jbc.271.20.11684. [DOI] [PubMed] [Google Scholar]
- 12.Giannouli CC, Kletsas D. TGF-beta regulates differentially the proliferation of fetal and adult human skin fibroblasts via the activation of PKA and the autocrine action of FGF-2. Cell Signal. 2006;18:1417–1429. doi: 10.1016/j.cellsig.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 14.Gruber HE, Norton HJ, Leslie K, Hanley EN., Jr Clinical and demographic prognostic indicators for human disc cell proliferation in vitro: pilot study. Spine. 2001;26:2323–2327. doi: 10.1097/00007632-200111010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh JK, Kletsas D, Clunn G, Hughes AD, Schachter M, Demoliou-Mason C. p53, p21(WAF1/CIP1), and MDM2 involvement in the proliferation and apoptosis in an in vitro model of conditionally immortalized human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:973–981. doi: 10.1161/01.atv.20.4.973. [DOI] [PubMed] [Google Scholar]
- 17.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 19.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/er.16.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol. 2005;207:445–452. doi: 10.1002/path.1862. [DOI] [PubMed] [Google Scholar]
- 21.Lee HT, Kay EP. Regulatory role of cAMP on expression of Cdk4 and p27(Kip1) by inhibiting phosphatidylinositol 3-kinase in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:3816–3825. doi: 10.1167/iovs.03-0147. [DOI] [PubMed] [Google Scholar]
- 22.Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005;17:134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- 23.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 24.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 15):422–432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, Schmid T, Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 26.Matsusaki M, Ochi M, Uchio Y, Shu N, Kurioka H, Kawasaki K, Adachi N. Effects of basic fibroblast growth factor on proliferation and phenotype expression of chondrocytes embedded in collagen gel. Gen Pharmacol. 1998;31:759–764. doi: 10.1016/s0306-3623(98)00105-0. [DOI] [PubMed] [Google Scholar]
- 27.Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 28.Murakami H, Yoon ST, Attallah-Wasif ES, Tsai KJ, Fei Q, Hutton WC. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine. 2006;31:1770–1774. doi: 10.1097/01.brs.0000227255.39896.f3. [DOI] [PubMed] [Google Scholar]
- 29.Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:263–267. doi: 10.1016/S1357-2725(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 31.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 32.Oshima H, Ishihara H, Urban JP, Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11:332–338. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 33.Pratsinis H, Giannouli CC, Zervolea I, Psarras S, Stathakos D, Kletsas D. Differential proliferative response of fetal and adult human skin fibroblasts to transforming growth factor-beta. Wound Repair Regen. 2004;12:374–383. doi: 10.1111/j.1067-1927.2004.12305.x. [DOI] [PubMed] [Google Scholar]
- 34.Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882–889. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 35.Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine. 2005;30:2503–2509. doi: 10.1097/01.brs.0000186326.82747.13. [DOI] [PubMed] [Google Scholar]
- 36.Risbud MV, Di Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ, Shapiro IM. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 37.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14:403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Thompson JP, Oegema TR, Jr., Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, Hutton WC. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 43.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Basic fibroblast growth factor immunoreactivity in blood vessels and cells of disc herniations. Spine. 1995;20:271–276. doi: 10.1097/00007632-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju EO. Platelet-derived growth factor and vascular endothelial growth factor expression in disc herniation tissue: and immunohistochemical study. Eur Spine J. 1997;6:63–69. doi: 10.1007/BF01676576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W, Richter W. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82:126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 49.Wipf P, Halter RJ. Chemistry and biology of wortmannin. Org Biomol Chem. 2005;3:2053–2061. doi: 10.1039/b504418a. [DOI] [PubMed] [Google Scholar]
- 50.Yoon ST. Molecular therapy of the intervertebral disc. Spine J. 2005;5:280S–286S. doi: 10.1016/j.spinee.2005.02.017. [DOI] [PubMed] [Google Scholar]