Abstract
The aim of this study was to evaluate the diagnostic value of MRI and 18FDG-PET in bone marrow infiltration of the spine due to metastases of solid tumours and lymphoma in cancer patients. In 35 cancer patients (solid tumours n = 26, lymphoma n = 9) MRI of the spine and 18FDG-PET were reviewed and the detectability of metastases, infiltration of the spine, extent of disease, and therapeutic implications were compared. In 8/35 cases (23%) imaging technique showed concordantly no bone marrow infiltration. In 19/35 patients (54%), both MRI and 18FDG-PET revealed bone marrow infiltration of the axial skeleton. In 12/19 patients (63%), MRI showed more extensive disease which lead to subsequent therapy. The imaging findings of MRI and 18FDG-PET were discordant in 8/35 cases (23%). 18FDG-PET was false positive in two patients. In six patients, 18FDG-PET failed to detect bone metastases and bone marrow infiltration of the spine, which was detected by MRI and proven by clinical follow-up with subsequent therapy in two cases. MRI is more sensitive and specific than 18FDG-PET detecting bone marrow metastases and infiltration of the spine and has a great impact in staging cancer patients.
Keywords: Bone marrow infiltration, Spine, Lymphoma, Solid tumour, MRI, 18FDG-PET
Introduction
The initial site of metastatic disease in bone is the bone marrow in patients with solid tumours with good prognosis [1]. Bone marrow involvement reveals a wide range in lymphoma patients, depending on the subclassification of lymphoma [4]. Early detection of bone marrow metastases and infiltration is crucial for tumour screening and staging to enable faster and accurate therapy and to decrease morbidity due to pain or complications [3–5]. Metastatic bone marrow involvement is found much more frequently (approximately 50–85%) at autopsy than in routine staging procedures [1].
The diagnosis of bone marrow metastasis and infiltration in cancer patients is most commonly addressed with bone scintigraphy, which has the advantage of generally high sensitivity and allowing a complete evaluation of the entire skeleton at relatively low cost [6, 20]. MRI may increase the sensitivity for detection of bone marrow involvement and bone marrow metastasis in patients with malignant lymphoma and solid tumours [2, 3, 5, 15]. There has been no previous study so far comparing 18FDG-PET to MRI with respect to bone marrow infiltration caused by lymphoma and solid tumours, although both imaging techniques are well-established in daily clinical routine for cancer patients [7, 15]. 18FDG-PET is increasingly utilised for enquiries that had been reserved for skeletal scintigraphy, such as the detection of metastases and diagnosis of primary bone tumours [8–12]. Furthermore, 18FDG-PET successfully administered the evaluation of musculoskeletal and intraosseous tumours and in detecting of osseous metastases of breast carcinoma and bone marrow involvement due to malignant lymphoma [8, 12, 14–17].
The purpose of this study was thus to evaluate the diagnostic value of 18FDG-PET and MRI in detecting bone marrow infiltration of the spine in cancer patients.
Materials and methods
Patient population
A total of 35 cancer patients with various solid tumours (n = 26) and lymphoma (n = 9) underwent MRI examination of the spine to stage, follow-up known metastases or evaluate new bone pain or neurological symptoms. 18FDG-PET was avaible in all these patients. Inclusion criterion was a timeframe of less than 1 month between 18FDG-PET and MRI (mean 8.3 days, range 0–31 days, median 10.1 days).
Patients treated by chemotherapy or radiation therapy during the two examinations or 2 months prior to 18FDG-PET, were excluded
The solid tumours consisted of the following: breast carcinoma n = 7, lung carcinoma n = 5, esophageal carcinoma n = 2, parotis carcinoma n = 2, hypopharynx carcinoma n = 2, cervical carcinoma n = 2, gastric carcinoma n = 1 pancreatic carcinoma n = 1, malignant melanoma n = 1, thyroid cancer n = 1, merkel-cell carcinoma n = 1, leiomyosarcoma n = 1. The lymphoma group consisted of: Non-Hodgkins lymphoma n = 5, Hodgkin lymphoma n = 3, plasmocytoma n = 1.
Imaging
Magnetic resonance imaging was performed on high-field strength tomographs (1.5T) (Magnetom Symphony, Vision, Siemens Medical Solutions, Erlangen, Germany). Sagittal T1-W SE (TR 400–600 ms, TE 12–15 ms) and STIR sequence (TR 3,600–4,800 ms, TE 120 ms, TI 160 ms) were available with a matrix size of 256 or 512, rectangular field-of-view, number of acquisitions between 2 and 4, and a slice thickness between 4 and 6 mm.
Intraveneous contrast media was used at a regular dosage of 0, 1 mmol Gd-DTPA (Magnevist, Schering, Berlin, Germany) per kg body weight in all cases. T1-weighted fat-suppressed spin echo sequences were performed following iv. contrast media application.
18FDG-Positron emission tomography was performed as a routine staging procedure for the patient after referral by the clinician in charge. Patients fasted for at least 12 h prior to injection of the radiopharmaceutical to provide optimal conditions for tracer uptake. Blood glucose levels were measured in all patients and did not exceed 110 mg/dl (6.1 mmol/l). After intravenous injection of 360 MBq ± 30 fluorine-18, deoxyglucose (FDG) emission scans were acquired 90 min later to optimise the tumour-to-background ratio. A two-dimensional ring scanner (Ecat Exact; Siemens/CTI, Knoxville, Tenn) equipped with a rod source for post-injection segmented attenuation correction was used. Eight to ten bed positions with an 11-cm transverse field of view were measured (2 min transmission and 8 min emission per position). Images were reconstructed by iteration with ordered subsets (ordered subset-expectation maximization, or OSEM, two iterations, eight subsets), no pre- or postfiltering was used, and final reconstruction resolution of the images was 6 mm. The reconstructed images were assessed on a computer monitor at all three levels in axial, coronal and sagittal views.
Data analysis
Magnetic resonance imaging
Two radiologists (NG, CL) experienced in musculoskeletal imaging were blinded to both 18FDG-PET findings and clinical results. They independently evaluated axial skeleton bone marrow in MRI. Again, disagreement was resolved by consensus reading in only two cases.
Criteria for a positive MRI finding were focal, multifocal or diffuse areas of decreased signal intensity in T1-W images with a relative increase in signal intensity in STIR sequences, and soft tissue tumours with continuity to bone marrow pathologies after exclusion of osteoporotic or traumatic fractures or spondylodiscitis. Focal areas of low signal in both images were interpreted as osteoblastic metastasis if there was a contrast media enhancement after iv. contrast media application.
Positron emission tomography
Two nuclear physicians (IB, TK) blinded to the MRI results read the PET scans independently and prospectively by consensus and determined the presence, extent, and location of metastases and marrow infiltration of the spine. Criteria for bone marrow infiltration and metastases in 18FDG-PET were focal areas of increased tracer uptake or a diffusely increased uptake. Findings of extraspinal disease were excluded.
Comparison of 18FDG-PET and MRI
One or more of these bone marrows were investigated depending on the clinical indications: cervical spine (n = 7), thoracic spine (n = 13) or lumbar spine with sacral bone (n = 15). Only these anatomical regions were compared to 18FDG-PET findings.
Patients with no lesions on PET and MRI exams were regarded as truly negative. Bone marrow infiltration or metastastic disease was assumed according to the criteria mentioned above in all cases of concordantly positive MRI and 18FDG-PET findings. Discordant imaging results were resolved by clinical follow-up, comparison to CT or follow-up MRI, 18FDG-PET and skeletal or bone marrow scintigraphy. Follow-up investigations were used to determine the true or false positive or the true or false negative nature of imaging findings in patients with discordant imaging results and for all discordant lesions. Further, in these patients with discordant extent of bone marrow infiltration and bone marrow metastases on 18FDG-PET and MRI, a lesion-by-lesion b analysis was performed in cases of progressive disease showing a lesion unequivocally increasing in size on follow-up.
Sensitivities, specificities and diagnostic accuracies were determined on a patient-by-patient basis. The evaluation focused on the presence and type (uni- or multifocal, or diffuse) infitration pattern due to metastases or lymphoma, relative number of metastases compared to 18FDG-PET, morphological criteria of affected vertebral bodies (fractures due to infiltration, involvement of posterior part of the vertebral body including the vertebral arcs and pedicles, spinal canal stenosis, spinal cord compression and paravertebral soft tissue tumors), as well as spinal cord metastases. 18FDG-PET evaluation also included presence and frequency of bone marrow metastases and infiltration of the axial skeleton.
Results
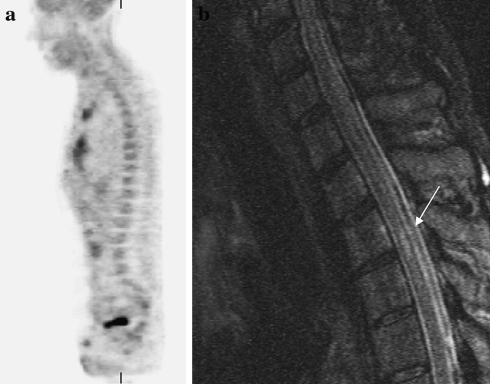
MRI and 18FDG-PET findings in the spine were concordant in 27/35 patients (77%). In 8/35 cases (23%) MRI and 18FDG-PET revealed no metastatic disease in solid tumours or bone marrow infiltration in lymphoma. In one these eight patients with concordant negative imaging findings of the axial skeleton, MRI depicted a spinal cord metastasis of a lung carcinoma (Fig. 1). In another patient with a solid tumour, neither imaging technique revealed any bone marrow infiltration due to metastases, although MRI detected one intramedullary metastasis due to a Merkel cell tumor.
Fig. 1.
A 60-year-old male with a lung carcinoma; time frame between MRI and 18FDG-PET was 28 days. No previous therapy has been performed. 18FDG-PET (a) shows no evidence of metastatic disease. In correlation with the 18FDG-PET, MRI of the spine reveals no metastatic disease in the cervical spine. However, a solitary intramedullary metastasis is clearly seen on the T2-STIR weighted image (arrow) with subsequent therapy
In 19/35 cancer patients (54%) MRI and 18FDG-PET concordantly revealed bone marrow metastases and infiltration of the spine (Figs. 2, 3). In 12 of those 19 patients (63%) MRI showed more metastatic disease or bone marrow infiltration of the spine (Fig. 2). In seven cases of positive concordant imaging findings, the extent of metastatic disease and bone marrow infiltration was similar described by MRI and 18FDG-PET.
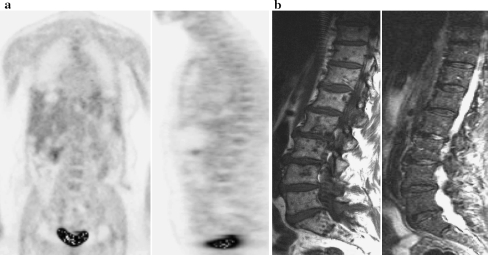
Fig. 2.
A 60-year-old female with malignant melanoma. Time frame between MRI and 18FDG-PET was only 4 days. a18FDG-PET reveals multifocal bone marrow involvement due to metastatic disease in the lumbar spine. b MRI demonstrates multifocal bone marrow infiltration due to metastatic disease in the lumbar spine and at level S1. (left T1-weighted image, right STIR image)
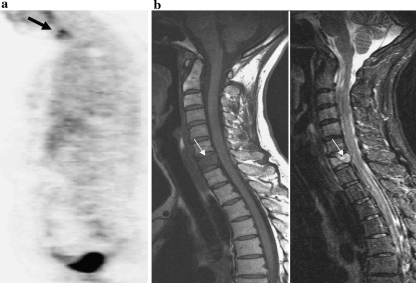
Fig. 3.
A 54-year-old female after surgery of a lung carcinoma. MRI and 18FDG-PET were performed after skeletal scintigraphy revealed a solitary increased uptake of the cervical spine. a Concordantly with the bone scintigraphy, 18FDG-PET reveals a solitary osseous metastasis in the cervical spine. b MRI demonstrates a diffuse hypointense vertebra at level C7 on a T1-weighted image (left side) corresponding to a signal increase on the STIR sequence (right side). Note the small hypointense oblique line indicating a pathological fracture. There is a retropulsion of the posterior bony fragment into the spinal canal. This patient underwent subsequent radiation therapy
Yielding additional morphological information in 13/19 patients (68%) with concordant positive imaging findings MRI had a therapeutic impact in 7 of those 13 (54%).
MRI and 18FDG-PET imaging findings were discordant in 8/35 cases (23%). 18FDG-PET was false negative in six patients (lymphoma n = 4, solid tumors n = 2) and failed to detect metastases and bone marrow infiltration later proven in MRI and the clinical follow up resulting in 2/6 cases in subsequent radiation therapy (Fig. 4). In two patients with lymphoma 18FDG-PET was false positive, whereas the MRI of the spine demonstrated in both cases no evidence of bone marrow infiltration in either patient proven by clinical follow up. MRI was superior in six patients regarding the detection of bone marrow metastasis and infiltration due to solid tumors (n = 3) and lymphoma (n = 3).
Fig. 4.
A 50-year-old female with breast cancer presenting with back pain. a18FDG-PET reveals no metastatic disease in the spine. In whole-body investigation, there was no relapse and no sign of distant metastases. b MRI shows a pathological bone marrow signal with a combined diffuse and multifocal infiltration pattern proven by a progressive metastastic disease. The metastases appear hypointense on T1-weighted images and exhibit a signal increase on T2-weighted STIR sequences (right T1-weighted image, left STIR sequence image)
MRI was positive, whereas 18FDG-PET failed to detect bone marrow infiltration. MRI detected a multifocal bone marrow infiltration of the spine in three cases; all of those findings were proven in clinical follow-up and subsequent investigations (MRI n = 1, CT n = 1, skeletal scintigraphy n = 2, bone marrow scintigraphy n = 2).
In three patients with lymphoma (Hodgkin lymphoma n = 1, NHL n = 2), diffuse bone marrow infiltration was depicted by MRI, in one case in combination with a soft-tissue tumour. One of those patients underwent surgery due to neurological symptoms caused by a soft-tissue tumor.
Three patients had discordant findings concerning bone marrow metastases from solid tumors. One of them suffered from lung carcinoma and two from breast carcinoma.
A diffuse bone marrow infiltration due to metastatic disease was observed in two of those three patients. A unifocal metastasis was seen in the third patient in combination with a medullary metastasis later proven in clinical follow-up.
MRI and 18FDG-PET concordantly indicated the presence of bone marrow metastases and infiltration of the spine in 19/35 (54%) patients. In 12 of those with positive concordant imaging findings (64%), MRI revealed the following additional morphological information: spinal canal stenosis (n = 8), involvement of posterior parts of the vertebral body, including the vertebral arcs or pedicles (n = 9), paravertebral and intraspinal soft tissue tumours (n = 7), fractured vertebra (n = 1), codfisch vertebra (n = 2) and spinal cord metastases (n = 1). Local therapy was initiated in 7/12 patients [local radiation therapy (n = 6), surgical treatment (n = 1)].
Regarding bone marrow infiltration in lymphoma and solid tumor patients, MRI showed a disseminated pattern in 10/19 patients (53%), multifocal infiltration in 6/19 patients (32%), and unifocal metastases in 3/19 patients (16%). Conversely, 18FDG-PET demonstrated a unifocal pattern in 6/19 (32%) patients, multifocal infiltration in 9/19 (47%) patients, and disseminated spine disease of the spine in 4/19 patients (21%).
Discussion
First of all, we have to stress that bone marrow infiltration means a pre-radiological lesion due to metastastic or lymphoma disease, which can be only detected by MRI, PET (PET/CT) and bone marrow scintigraphy.
Few studies exist which directly compare MRI and 18FDG-PET with respect to bone marrow metastases of solid tumours and bone marrow infiltration due to lymphoma [9, 14]. In contrast to previously published data, this study reveals MRIs greater sensitivity (78%), specificity (88%), and diagnostic accuracy (82%) than 18FDG-PET in detecting bone marrow metastasis and infiltration in cancer patients.
Daldrup-Link et al. investigated 39 children and young adults with different tumour entities including sarcoma and lymphoma [9]. In detecting bone marrow metastasis and infiltration in cancer patients, they observed the following degrees of sensitivity: 90% 18FDG-PET, 82% MRI and in 71% skeletal scintigraphy. 18FDG-PET was demonstrated to be more sensitive than MRI and skeletal scintigraphy. When comparing 18FDG-PET with bone scintigraphy, initial data suggest that 18FDG-PET is more sensitive than conventional bone imaging [9, 13, 19]. However, some authors report that 18FDG-PET is less sensitive than bone scintigraphy in identifying osseous metastases, especially in prostate cancer and metastases of the osteosarcoma [12, 14].
Kao et al. investigated 24 patients with a biopsy-proven malignancy and suspected bone metastasis [14]. They found that 18FDG-PET was negative in 11 metastatic and 20 benign bone lesions with a positive bone-scan finding. This indicates less sensitivity in detecting malignant bone metastases, but they concluded that 18FDG-PET showed better specificity than bone scans [14].
Moog et al. studied 56 patients in primary staging of malignant lymphoma, (34 Hodgkin’s disease, 22 NHL) [15]. Both imaging techniques revealed positive concordant imaging findings in 12/56 of them, although 18FDG-PET was superior to skeletal scintigraphy in detecting bone marrow infiltration in 30 regions and whereas skeletal scintigraphy only revealed bone marrow infiltration in 20 regions. These findings were confirmed by biopsy, CT, MRI and skeletal scintigraphy. Moog et al. found that 18FDG-PET is suitable for identifying osseous involvement in malignant lymphoma and that it is more sensitive and specific than bone scintigraphy [15]. However, they did not directly compare 18FDG-PET to MRI in the assessment of bone and bone marrow lesions. Ghanem et al. carried out a retrospective examination of 38 patients, noting eight discordant findings between MRI and 18FDG-PET in the spine [13]. In seven of their eight cases, the 18FDG-PET was false negative and a therapeutic measure was carried out in two of the seven cases. In one case the 18FDG-PET finding were false positive [13].
False-positive 18FDG-PET results are caused by sacral fractures [9]. Fayad et al. studied three patients with colorectal cancer who had insufficiency fractures; however, cross-sectional CT or MRI imaging can differentiate the FDG-uptake caused by fractures and the FDG-accumulation due to tumoural infiltration [9].
In addition to skeletal scintigraphy, PET is an imaging modality increasingly being used to successfully diagnose skeletal metastases. In the latter method, the radiotracer 18fluoride is used in addition to 18F-FDG [17]. However, 18FDG-PET was not primarily indicated solely for the assessment of bone metastases. In cases when 18F-FDG-PET was carried out, the additional findings of bone metastases were made via the non-specific phenomenon of increased glucolysis in malignant cells [6].
Few studies exist which compare 18F-fluoride-PET with MRI. Schirrmeister et al. [17] compared 18F-fluoride-PET with skeletal scintigraphy, whereby 18F-fluoride-PET detected 64 skeletal metastases in 17 of the 34 patients [17]. Skeletal scintigraphy findings, positive in only 11 of the 34 patients, detected 29 metastases. Some authors suggest that 18FDG-PET will eventually replace skeletal scintigraphy [6]. Schirrmeister et al. [17] compared 18F-fluoride-PET with SPECT and planar skeletal scintigraphy in breast and lung cancer patients and showed the definite superiority of 18F-fluoride-PET to planar skeletal scintigraphy with and without the incorporation of the SPECT technique [17].
So finally, the heterogenity of our patient population might create a certain bias in our direct comparison. We included fast and slow growing tumours in our retrospective study, leading to a certain tendency. Further prospectiev studies with a selected patient group are necessary.
In conclusion, we observed MRI to be superior to 18FDG-PET in detecting bone marrow metastases and marrow infiltration of the spine in patients with solid tumours and lymphoma. MRI reveals important additional information such as like pathological fractures, spinal canal involvement and intraspinal and medullary metastases, having therapeutic consequences. MRI is more sensitive and specific in visualising bone marrow metastasis and infiltration of the spine. In cases of false positive PET scans, MRI of the spine depicts no bone marrow infiltration. In cancer patients with bone pain and neurological symptoms, and in cases of a negative 18FDG-PET-scan MRI of the spine should be performed to document actual and potential complications of bone marrow metastasis or infiltration.
References
- 1.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:336–340. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Altehoefer C, Blum U, Bathmann J, Wüstenberg C, Uhrmeister P, Laubenberger J, Lange W, Schwarzkopf J, Moser E, Langer M. Comparative accuracy of magnetic resonance imaging and immunoscintigraphy for detection of bone marrow involvement in patients with malignant lymphoma. J Clin Oncol. 1997;15:1754–1760. doi: 10.1200/JCO.1997.15.5.1754. [DOI] [PubMed] [Google Scholar]
- 3.Altehoefer C, Ghanem N, Högerle S, Moser E, Langer M. Comparative detectability of bone metastases and impact on therapy of magnetic resonance imaging and bone marrow scintigraphy in patients with breast cancer. Eur J Radiol. 2001;40:16–23. doi: 10.1016/S0720-048X(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 4.Baur A, Stäbler A, Nagel D, et al. Magnetic resonance imaging as a supplement for clinical staging system of Durie and Salmon ? Cancer. 2002;95:1334–45. doi: 10.1002/cncr.10818. [DOI] [PubMed] [Google Scholar]
- 5.Bares R. Skeletal scintigraphy in breast cancer management. Q J Nucl Med. 1998;42:43–48. [PubMed] [Google Scholar]
- 6.Brink I, Schumacher T, Mix M, et al. FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614–1620. doi: 10.1007/s00259-004-1606-x. [DOI] [PubMed] [Google Scholar]
- 7.Cook GJ, Houston S, Rubens RD, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 8.Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jurgens H, Schober O, Rummeny EJ. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG-PET. AJR Am J Roentgenol. 2001;177:229–236. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 9.Fayad LM, Cohade C, Wahl RW, Fishman EK. Sacral fractures: a potential pitfall of FDG-PET. AJR Am J Roentgenol. 2003;181:1239–1243. doi: 10.2214/ajr.181.5.1811239. [DOI] [PubMed] [Google Scholar]
- 10.Franzius C, Daldrup-Link HE, Wagner-Bohn A, Sciuk J, Heindel WL, Jürgens H, Schober O. FDG-PET for detection of recurrences from malignant primary bone tumors and comparison with conventional imaging. Ann Oncol. 2002;13:157–160. doi: 10.1093/annonc/mdf012. [DOI] [PubMed] [Google Scholar]
- 11.Franzius C, Sciuk J, Daldrup-Link HE, Jurgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med. 2000;27:1305–1311. doi: 10.1007/s002590000301. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem N, Altehoefer C, Högerle S et al (2002) MRI and FDG-PET of the axial skeleton in cancer patients. ESSR 2002, IX Annual Meeting. pp 191–192
- 13.Ghanem N, Altehoefer C, Högerle S, Schäfer O, Winterer J, Moser E, Langer M. Comparative diagnostic value and therapeutic relevance of magnetic resonance imaging and bone marrow scintigraphy in patients with metastastic solid tumors of the axial skeleton. Eur J Radiol. 2002;43:256–261. doi: 10.1016/S0720-048X(01)00477-6. [DOI] [PubMed] [Google Scholar]
- 14.Kao CH, Hsieh JF, Tsai SC, Ho YJ, Yen RF. Comparison and discrepancy of 18F-2-deoxyglucose positron emission tomography and Tc-99m MDP bone scan to detect bone metastases. Anticancer Res. 2000;20:2189–2192. [PubMed] [Google Scholar]
- 15.Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med. 1999;40:1407–1413. [PubMed] [Google Scholar]
- 16.Munker R, Hasenclever D, Brosteanu O, et al. Bone marrow involvement in Hodgkin`s disease: an analysis of 135 consecutive cases. J Clin Oncol. 1995;13:403–409. doi: 10.1200/JCO.1995.13.2.403. [DOI] [PubMed] [Google Scholar]
- 17.Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 18.Sherry MM, Greco FA, Johnson DH, Hainsworth ID. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favourable prognosis. Cancer. 1986;58:178–182. doi: 10.1002/1097-0142(19860701)58:1<178::AID-CNCR2820580130>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Shreve PD, Barton GH, Gross MD, Wahl RL. Metastatic prostate cancer: Initial findings of PET with 2-Deoxy-2-(F-18)fluoro-d1-glucose. Radiology. 1996;199:751–756. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 20.Wikenheiser KA, Silberstein EB. Bone scintigraphy screening in stage I-II breast cancer: Is it cost-effective? Clevel Clin J Med. 1996;63:43–44. doi: 10.3949/ccjm.63.1.43. [DOI] [PubMed] [Google Scholar]