Abstract
Degenerative mechanisms for the intervertebral disc are unclear, particularly those associated with cumulative trauma. This research focuses on how mechanical loading at levels below those known to cause acute trauma can lead to cellular injury. Mouse-tail discs were subjected to static bending for 1 week, then allowed to recover unloaded for 3 weeks and 3 months. Discs were analyzed using histology, in situ hybridization (collagen and aggrecan gene expression), TUNEL assay for apoptotic cell death, and biomechanics. The bent discs demonstrated loss of annular cellularity on the concave (compressed) side, while the nucleus and convex annulus appeared normal. Chondrocyte-like cells were apparent within the inner, concave annulus on the recovered discs, with evidence of proliferation at the annulus/endplate interface. However, annular architecture and biomechanical properties for the recovered discs were not different from controls, suggesting that restoration of physiologic tissue stress prevents the inner annular degradation noted in previous compression-induced degeneration models. These data demonstrate that cellular injury can be induced by transient compressive stress, and that recellularization is slow in this avascular tissue. Taken together, this suggests that cellular damage accumulation may be an important injury mechanism that is distinct from acute mechanical failure.
Keywords: Intervertebral disc, Degeneration, Animal models, Trauma, Biomechanics
Introduction
Excessive spinal loading is recognized to play a key role in intervertebral disc degeneration. We have previously demonstrated that the load/degeneration pathway is mediated, in part, by local changes in intradiscal stress that stimulate catabolic cellular processes. The local effects of tissue stress, rather than other global effects of altered loading such as nutrition or immobilization, is underscored by studies of asymmetric static stress (bending) that induces asymmetric changes in cellular activity and annular morphology [3]. Symmetric compression predominantly affects the nucleus, inducing volume loss and cell death that ultimately result in a degenerated phenotype. With bending, however, compressive stress is principally borne by the concave annulus causing local cell death while sparing the nucleus. Consequently, with bending nuclear volume is maintained and global disc degeneration is avoided in the short term. What remains unclear, however, is the rate and mechanism of healing after local annular damage is induced. Since the disc is avascular, damage from repeated episodes of hyperphysiologic bending may ultimately accumulate and lead to overall degeneration as has been noted in several animal models of spinal instability [14, 15, 24]. The ability of the annulus to heal, and the rate at which this occurs, is relevant to many clinical situations where human discs are subjected to excessive acute or chronic asymmetric stress with subsequent restoration of normal mechanical conditions—such as whiplash in the cervical spine, lifting exposure in the workplace, or correction of a scoliotic curve when the spinal balance is restored surgically. The goal of this study was to investigate whether annular damage induced by hyperphysiologic bending is reversible upon restoration of the normal mechanical environment. To accomplish this, we utilized a previously-reported murine model of disc degeneration induced by mechanical loading. Tail discs were bent for 1 week and recovered for either 3 weeks or 3 months after load removal. Disc tissues were analyzed by routine histology, TUNEL assay for cell death, in situ hybridization for collagen and aggrecan gene expression and mechanical testing.
Materials and methods
Twenty-five Swiss Webster mice, male aged of 12 weeks (young adults) were used in this study (approved by UCSF Committee on Animal Research). The mice were randomly assigned into four groups:
Group I (n = 5) mice discs were subjected to 1 week of bending (as defined below). Then the bending was removed and the mice were allowed free unrestricted activity in cages for 3 weeks and sacrificed.
Group II (n = 5) mice discs were subjected to 1 week of bending, then after the bending was removed, the mice were allowed free unrestricted activity for 3 months and sacrificed.
Group III (n = 10) mice discs were subjected to the same surgical procedure as in groups I and II but without subsequent hardware loading. The bending device was removed after 1 week and the animals were sacrificed at either 3 weeks or 3 months (sham group).
Group IV (n = 5) mice were untreated and sacrificed at 3 weeks or 3 months.
For each disc the concave side (submitted to compressive stress) and the convex side (submitted to tensile stress) were compared.
Surgical procedure
The mice in group I, II and III were anesthetized with 0.6 ml of 4% Avertin intraperitoneally. With an electric drill, a 0.4 mm diameter stainless steel pin was inserted percutaneously into the ninth and into the tenth caudal vertebrae. Using a specially-designed alignment jig, the distance between pins was set to 4.42 mm. To apply a compressive stress to one side of the disc, a 1.4 mm thick elastic band was inserted between the two pins on the left side of the mouse tail in groups I and II. The elastic band was calibrated to induce a compression force of 1.7 N applied at 2.5 mm from the disc center. Elastic calibration consisted of cutting rubber tubing to widths (1.4 mm) defined by preliminary mechanical studies that characterized width/stiffness relations. Based on this relationship, 1.7 N is generated at 4.42 mm (set by the alignment jig, as noted above). This bending moment produced 42° of disc bending after 1 week (Fig. 1). The device was protected with a plastic shield attached with hooks on the proximal pin. In group III (sham group), the elastic band was not inserted between the pins but the shield was attached to the pins similarly to group I and group II. Group IV served as a non-operated control and did not undergo any surgical procedure.
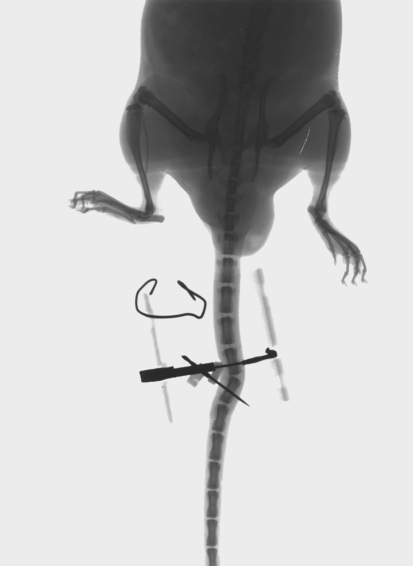
Fig. 1.
X-ray image of bending device placed on rat-tail
After the surgical procedure an X-ray was taken for each tail to check the position of the pins into the vertebrae and to measure the angulation of the deformity. The mice were allowed free unrestricted activity in cages and monitored daily. Two mice in group III (sham group) died during the 3 months of observation. And one mouse in group I was eliminated because a pin was inserted too close to the disc. A total of 27 mice were available for the study.
After 7 days, mice in group I, II and III were anesthetized with 0.6 ml of 4% Avertin intraperitoneally. The bending apparatus described above was removed, the pins were cut close to the skin and a X-ray was taken.
Twenty-one days after the second procedure, mice in group I were sacrificed and the study disc was harvested. To accomplish this, the tail skin was removed and the study disc, with the adjacent vertebral bodies, was isolated and harvested under aseptic conditions and kept in 4% PFA at 4°C for one night.
Three months after the second procedure, mice in groups II, III and IV were sacrificed and the tissues were harvested with the same protocol as described above. For histologic and biologic studies, five specimens in each of these three groups group were kept in 4% para-formaldehyde (PFA) at 4°C for one night before the decalcification procedure.
For the mechanical testing five specimens in group II, and three specimens in group III were frozen in saline solution after harvesting.
Tissue preparation for histology and biology studies
The tissue samples were fixed in 4% PFA, decalcified using EDTA at 4°C for 11 days, embedded in paraffin, and sectioned as previously described [11].
Histology
The sections were stained for routine histology with Safranin-O/fast green (stains proteoglycan red and collagen blue) and hematoxylin-eosin. The stained sections were viewed under brightfield on a Nikon Microphot-FX microscope (×20 magnifications) and digitized.
To quantify the annular morphology we used the method of directed secants [11]. We calculated the mean intercept length (MIL) aspect ratio (ratio of minimum MIL to maximum MIL) of the annulus using in-house and commercial software (IDL). When the aspect ratio is equal to 1 the tissue has no preferred orientation and when it approaches zero the tissue is highly organized.
To calculate cell density (number of cells/mm2) in the annulus, additional sections were stained with Methyl green. Then, sections were digitized and the cell density was calculated using an automated software algorithm (NIH Image 1.6).
Cell death
Apoptosis in the intervertebral disc was examined in tissue sections using the TUNEL reaction (in situ Cell Detection Kit, Fluorescein Mannheim, Mannheim Germany). Briefly the 5 μm sections were deparaffinized and digested with proteinase K (20 μg/ml in 10 mM tris–HCL, pH 7.4–8.0) 15 min at 37°C in humidified chamber. Then, the sections were washed in PBS and the TUNEL reagent was added to the sections. The incubation time was 1 h in humidified chamber in dark at 37°C. Next, slides were washed in PBS and counterstain with Hoechst dye. Then sections were analyzed under fluorescence light on a Leitz DMRB/Leica microscope. While the TUNEL method preferentially labels cells undergoing apoptosis and has been described as the best available assay for apoptosis in vivo [19], it can also highlight DNA fragments from necrotic cells [20]. Other approaches to distinguish apoptosis from necrosis include characterization of morphologic features that include cell shrinkage, pyknotic nuclei with chromatin condensation, and apoptotic bodies [12]. Of these, apoptotic bodies can be observed using high-power optical microscopy, and therefore their presence was used to confirm the TUNEL identification of apoptotic versus necrotic disc cells.
Collagen II and aggrecan gene expression
In situ hybridization analyses were performed to assess changes in collagen and aggrecan gene expression in the disc with a technique previously described [11]. Briefly, the sections were deparaffinized, digested with proteinase K, post-fixed in 4% paraformaldehyde, treated with 0.1% sodium borohydride. Slides were hybridized with 70 μl hybridization solution containing approximately 1.5 × 106 cpm of probe. Slides were washed in 5×SSC (saline sodium citrate), 20 mM ß-mercaptoethanol, then in high stringency wash of 50% formamide, 2× SSC, 40 mM β-mercaptoethanol followed by washes at 37°C: NTE buffer (10 mM Tris–HCL, pH 8, 0.5 M NaCL, 5 mM EDTA), followed by NTE containing 20 μg/ml RNAse A (sigma, St. Louis, MO, USA), then NTE. Slides were dehydrated, coated with Kodak NTB-2 photographic emulsion and exposed for 3 days, developed and counterstained with Hoechst dye. Sections were examined under darkfield and/or fluorescence on a Leitz DMRB/Leica microscope.
Mechanical testing (group II and group III)
We used a four-point bending protocol to assess the mechanical properties of the disc subjected to 1 week of bending and 3 months of recovery (group II) and of sham disc (group III). The tails were thawed and placed into a specially-designed four point-bending device [3]. To test the compressed side of the annulus, the specimens were laid with the convex deformity of the disc in front of the moving cross-head of the materials test system (MTS). The specimens were loaded to failure at a displacement rate of 0.006 mm/s, while the applied force and indentor travel were recorded continuously.
The load/displacement record was used to calculate four parameters. The neutral zone of the specimen was defined as the displacement corresponding to an applied force of 0.2 N. The bending stiffness was determined as the slope of the most linear region of the load/displacement record. The yield point was determined as the first decrease of force value in the slope. The bending strength was determined as the maximum load carried by the specimen prior to failure.
Statistical analysis
Standard multi-variance analysis (ANOVA) followed by Tukey post hoc analysis was used to compare specimen group means (SYSTAT, v.5.2). All data sets were first tested for normal distribution using the Shapiro–Wilk test for normalcy.
Results
Histology
Morphology (Table 1)
Table 1.
MIL disorganization ratio of the annulus (mean ± SD)
| Group I | Group II | Group III | Group IV | |||
|---|---|---|---|---|---|---|
| Concave | Convex | Concave | Convex | Both sides | Both sides | |
| Annulus MIL ratio | 0.63 ± 0.06 | 0.64 ± 0.02 | 0.65 ± 0.05 | 0.66 ± 0.06 | 0.65 ± 0.05 | 0.64 ± 0.08 |
The nucleus pulposus and the disc space had restored a symmetric morphology between the concave and convex side at 3 weeks and 3 months. Disorganization of the annulus (determined by the MIL aspect ratio; Table 1) was not statistically different between the concave and the convex side of Group 1 (P = 0.9) and Group II (P = 0.55) discs.
Three weeks after removal of the bending apparatus (Group I) the middle and outer collagen layers of the concave side appeared acellular as compared to the convex side. Furthermore, the remaining cells in the inner concave annulus were rounded (some of them with multiple nuclei) and surrounded with Safranin-O positive matrix similar to chondrocytes. In some specimens, sparse chondrocyte appearing cells, were observed in the convex inner annulus (Fig. 2).
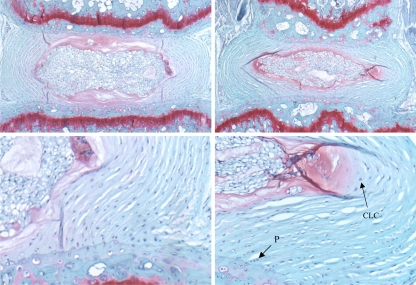
Fig. 2.
Safranin-O stained sections of sham disc (upper left, lower left) and concave side of the bent disc (upper right, lower right) at 3-week time point. Proliferation of fibroblasts (P) at the vertebral margin and chondrocyte-like cells (CLC) at the inner annulus are noted in the 3-week recovery discs (lower right)
Three months after removal of the bending apparatus (Group II) no cells were observed in the middle and outer annulus on the concave side. In the inner annulus there were some rounded cells not surrounded by matrix, some of these cells appeared with multiple nuclei. They were mainly present at the interface with the annulus and end plate and close to the lateral edge of the nucleus pulposus on the concave side (Fig. 3). In the convex annulus, two populations of cells were present: fibroblasts were present in the middle and outer annulus and chondrocytes were present in the inner (but fewer than in the same region in the concave annulus). Chondrocytes were present in the matrix between the nucleus pulposus and the inner annulus.
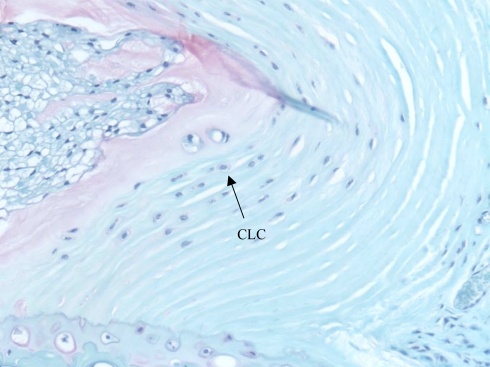
Fig. 3.
Safranin-O stained section of bent disc at 3-month time point. Inner and outer annulus is acellular. Chondrocyte-like cells (CLC) with proteoglycan matrix are present within the inner annulus
In the shams (Group III) and in the controls (group IV) fibroblasts were present in the inner, middle and outer annulus. Few rounded cells not surrounded with matrix (some of them with multiple nuclei) were present in the inner annulus. No cells were present in the matrix between the nucleus pulposus and the inner annulus as in Group II. The MIL ratio in the annulus was not statistically different between the sham group and the control group (P = 0.15) (Table 1).
Cell density (Table 2; Fig. 4)
Table 2.
Annulus cell density (mean ± SD)
| Group I | Group II | Group III | Group IV | |||
|---|---|---|---|---|---|---|
| Concave | Convex | Concave | Convex | Both sides | Both sides | |
| Cell density (cells/mm2) | 435 ± 355 | 681 ± 220 | 279 ± 94 | 795 ± 85 | 959 ± 72 | 988 ± 225 |
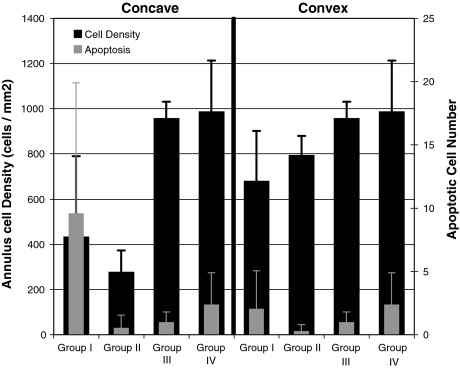
Fig. 4.
Cell density and apoptotic cell number for convex and concave portions of the test discs. Cell density in the concave annulus was reduced for Group II specimens (as compared to the convex side for the same Group or either side for Groups III and IV). There were more apoptotic cells in the concave annulus for Group I as compared to the convex side for that Group
In Group I, cell density was not statistically different between the concave annulus and the convex annulus (P = 0.5), but there was a trend of fewer cells in the concave annulus.
In Group II, cell density in the concave annulus was 65% less than in the convex side (P = 0.0004). Cell density in the concave annulus was 71% less than in the sham group (P = 0.0001) and 72% less than in the control group (P = 0.0001). There was a trend that concave annulus had fewer cells after 3 months of recovery than after 3 weeks of recovery (P = 0.8).
Cell density in the convex annulus in Group II was not statistically different between the shams (P = 0.28) and the controls (P = 0.18).
Cell density was not statistically different between the sham group and the control group (P = 0.98).
Apoptosis (Table 3; Fig. 4)
Table 3.
Number of apoptotic cells within the mid-sagittal section (mean ± SD)
| Concave annulus | Convex annulus | P value | |
|---|---|---|---|
| Group I | 9.6 ± 10.3 | 2.05 ± 3 | 0.003 |
| Group II | 0.55 ± 1 | 0.3 ± 0.5 | 0.56 |
In Group I, the number of apoptotic cells was statistically greater in the concave annulus than in the convex annulus (P = 0.003; Table 3). This is consistent with the histologic observations noted above: the concave side appeared more acellular. The number of apoptotic cells in the concave side was not statistically different between the inner and middle annulus (P = 0.78); between the inner and outer annulus (P = 0.9); or between the middle and outer annulus (P = 0.7).
In Group II, the number of apoptotic cells was not statistically different between the concave and convex annulus (P = 0.56).
Between sham and normal group the number of apoptotic cells was not statistically different (P = 0.12) respectively 1 ± 0.8 (DS) and 2.4 ± 2.5 (DS). In all groups very few apoptotic cells were seen in the nucleus pulposus.
Aggrecan gene expression
Three weeks after the removal of the bending apparatus (Group I), aggrecan gene expression was present in the growth plate, in the nucleus pulposus and in the annulus. Aggrecan gene expression in the annulus was greater and more diffuse on the convex side than in the concave side (where it was more likely seen in the inner annulus).
Three months after the removal of the bending apparatus (Group II), aggrecan gene expression was present in the growth plate, in the nucleus pulposus and was sparse in the convex annulus and located close to the end plate. In the concave annulus, aggrecan expression was present in the inner annulus close to the lateral edge of the nucleus pulposus. In this area, aggrecan gene expression appeared increased as compared to the opposite edge of the nucleus pulposus.
In the sham and control groups, aggrecan gene expression was present in the growth plate and in the nucleus pulposus. Very little expression was present in the annulus and was located close to the end plate.
Collagen type II gene expression
In Group I, collagen type II gene expression was present in the growth plate and in the end plate. Expression was less intense in the concave side (limited to the inner annulus) than in the convex side where expression was diffuse throughout the annulus.
In Group II, collagen type II gene expression was present in the growth plate and in the end plate. In the concave annulus, collagen type II gene expression was only present in the inner annulus. By contrast, the collagen type II gene expression was more diffuse in the convex annulus and mostly in the outer part.
In the shams, the collagen type II gene expression was present in the growth plate and in the annulus, primarily in the outer part.
In the control group (IV), expression was similarly located, but less intense than in group III.
Mechanical testing (Table 4)
Table 4.
Bending test data (mean ± SD)
| Bent disc | Sham disc | P value | |
|---|---|---|---|
| Neutral zone (mm) | 0.89 ± 0.2 | 1.16 ± 0.18 | 0.14 |
| Yield point (N) | 25.12 ± 12.2 | 17.2 ± 15.01 | 0.44 |
| Strength (N) | 26.5 ± 13.4 | 21.8 ± 11.4 | 0.63 |
| Stiffness (N) | 4.77 ± 1.21 | 2.66 ± 2.04 | 0.10 |
None of the mechanical parameters were statistically different between the concave and sham annulus. After 1 week of bending the mean bending angle was 42 ± 5.5°. After 3 months of recovery (group I) there was a trend of a stiffer concave annulus as compared to sham group, but this did not reach the level of significance (P = 0.1).
Discussion
In this study, we investigated whether the deleterious effects of hyperphysiologic bending were permanent or reversible after restoration of a normal mechanical environment. Our results demonstrate cell death in the concave annulus induced by bending was not recovered at either 3 weeks or 3 months. In addition, the cells in the inner, concave annulus after 3 weeks or 3 months appeared to change from a fibroblast phenotype (as observed in sham and control annulus) to a chondrocyte phenotype. However, the annular organization recovered to a normal baseline value, which is likely due to the restoration of normal tissue stress due to maintained nuclear volume and cellularity.
Cell density in discs subjected to 1 week of bending and 3 months of recovery (group II) was 65% less in the concave annulus than in the convex annulus. Also, cell density in the convex annulus was not statistically different from those in sham discs and control discs. These results reaffirm that excessive pressure (concave annulus), as opposed to excessive distortion (convex annulus), or immobilization is detrimental to annular cells. These results are consistent with observations of human scoliotic discs in which there is a decrease in cellularity in the concave side [4]. Further, these data demonstrate that cellularity in this avascular tissue is slow to recover after acute trauma. While our load application was relatively long (1 week), other studies demonstrate that annular cell death can be triggered by shorter durations of compression (3 h) [2]. Consequently, imbalances between rates of cellular injury and repair may lead to damage accumulation, with long-term detrimental consequences for annular integrity.
TUNEL-positive cells were still detectable on the concave annulus 3 weeks after load removal. This may be an indication of ongoing detrimental cellular effects bending. Conversely, the lingering presence of fragmented DNA may be because there is no mechanism of active clearance (e.g. by macrophages) given their location in an immunoprotected site. The number of TUNEL-positive cells was, however, reduced at the 3-month time point. This, along with the presence of empty cellular lacunae, demonstrate that degradative processes eliminate cells in the absence of systemic immunity. Because dying cells are not rapidly cleared, the TUNEL assay may identify DNA fragments from cells undergoing necrosis [21]. However, as part of other investigations, we have demonstrated that apoptosis, via a caspase-9-dependent mitochondrial pathway, is the predominate death mode in this mouse disc loading model [17].
Along with changes in cell viability, we observed changes in cell phenotype in the inner concave annulus. Three weeks after bending removal, these cells appeared chondrocytic, being rounded, surrounded by Safranin-O positive matrix, and positive for aggrecan and collagen II gene expression. This change in phenotype remained at the 3-month time point. By contrast, at all time points, cells in the convex annulus had fibroblastic appearance, elongated and parallel to the collagen layers. These observations are consistent with reports of cell plasticity in tendons [23]; fibroblasts are present in regions of tensile stress whereas chondrocytes are typically present in areas experiencing compressive stress. Yet, the mechanism by which cells respond to their mechanical environment are poorly understood.
The appearance of chondrocytes in the inner annulus could be explained by three different mechanisms. First, metaplasia of fibroblasts into chondrocytes could have occurred. For example, studies in tendon have demonstrated that fibroblasts subjected to compressive stress can differentiate into chondrocytes [5, 18, 23]. Furthermore, Lipson [9] found chondrocytes in human herniated disc, with evidence of fibroblast metaplasia that may be due to compressive stress in these discs. That we observed only chondrocytes within the inner annulus after 3 months of recovery (a period during which tensile stress is restored) suggests metaplasia of inner annular fibroblasts due to compressive stress was not the predominant mechanisms.
A second mechanism could be that fibroblasts were preferentially injured by compression in the concave annulus, and that a subpopulation of chondrocytes was spared. This supposes that two different cell populations (fibroblasts and chondrocytes) co-exist throughout the normal annulus. This is supported by our observation that in control and sham groups, collagen type II gene expression was expressed at a low level throughout the annulus. However, on routine staining, chondrocytes were only sparsely present in the inner annulus. This observation is consistent with that of Mason [13]. In the outer annulus the magnitude of the compressive stress is higher than in the inner annulus in a bent disc [25] and static compressive stress has been reported either to enhance (low stress) or decrease (high stress) chondrocytes metabolism [6, 7, 10]. In our study, the magnitude of the compressive stress applied in the outer annulus had led to both fibroblastic and chondrocytic cell death. Conversely, in the inner annulus, were magnitude of compressive stress was less, only the fibroblastic cells died while the chondrocytes remained present.
A third potential mechanism is chondrocyte migration from the end plate. In support of this, chondrocytes were more numerous in regions close to the end plate (where chondrocytes are normally present) and close to the edge of the nucleus pulposus in the inner concave annulus. Chondrocyte migration from the end plate has been reported for rabbit discs [8]. Alternatively, the chondrocyte-like cells may have arisen from the nucleus since in some specimens cells in the edge of the matrix around the nucleus pulposus were very similar to the inner chondrocytes (Fig. 3). The nucleus pulposus contains notochordal cells, but whether these cells can differentiate into chondrocytes is unclear. In the human nucleus pulposus chondrocyte-like cells have been found among notochordal cells population suggesting potential transdifferentiation [16, 22]. In any event, we believe that compressive stress supports a chondrocyte phenotype in the inner annulus, via differentiation or migration (from the end plate or from the nucleus pulposus). The remaining acellular outer annular region is slow to recover: we were unable to demonstrate recellularization at 3 months after bending removal.
Annular organization was not statistically different between the concave and the convex annulus, either 3 weeks or 3 months after the removal of bending. Consistent with this, we observed no differences in mechanical behavior. We have previously reported that collagen layers of the concave annulus were disorganized after 1 week of bending while that of the convex annulus was unaffected [3]. We have also reported that the inner annulus continues to degrade for at least 1 month after a 1-week application of symmetric disc compression that adversely effects nuclear cellularity and volume [11]. Taken together, these results support the contention that annular tension (which is dependent on nuclear volume and pressure) is important for maintaining tissue integrity. Bending induces transient compression on the concave annulus without the detrimental nuclear effects, as suggested by restoration of normal nuclear and disc shape after bending removal. Since the preserved nucleus can restore annular stress, annular morphology is recovered when bending is removed. That this occurs in acellular regions suggests a passive process. This observation is consistent with historical data demonstrating that collagen tension retards enzymatic and thermal denaturation and may have a thermodynamic basis [1]. Therefore, nuclear pressure and annular tension appear to be mutually dependent for maintaining disc architecture; in regions where the annulus is chronically compacted, matrix is degraded because collagen fibers are no longer required to resist tensile stress. This form of Wolff’s law appears to contribute to inner annular disorganization that invariably follows nuclear depressurization in the early stages of disc degeneration.
In conclusion, we have shown that the deleterious, cellular effects of high compressive stress applied on the concave annulus remain after restoration of normal disc loading: disc annulus was unable to recover normal cellularity after 3 months of recovery. Yet, restoration of normal mechanics supported maintenance of normal annular architecture. This finding suggests that cellular injury may be an important and independent phenomenon to matrix damage. If this is true, then disc injury criteria may need to be modified to include the cellular effects of what has previously be considered non-injurious loading. Also, our data highlight the need for therapeutic agents to enhance the rate of repair in this avascular tissue.
References
- 1.Bass EC, Wistrom EV, Diederich CJ, Nau WH, Pellegrino R, Ruberti J, Lotz JC. Heat-induced changes in porcine annulus fibrosus biomechanics. J Biomech. 2004;37(2):233–240. doi: 10.1016/j.jbiomech.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Chin JR, Lotz JC (2001) Cell death and matrix gene expression are upregulated in intervertebral discs during recovery from short durations of moderate static compression. In: Forty-seventh annual meeting of the Orthopaedic Research Society
- 3.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the intervertebral disc. Spine J. 2001;1(4):239–245. doi: 10.1016/S1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 4.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22(24):2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Giori NJ, Beaupré GS, Carter DR. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res. 1993;11(4):581–591. doi: 10.1002/jor.1100110413. [DOI] [PubMed] [Google Scholar]
- 6.Handa T, Ishihara H, Ohshima H, Osada R, Isuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22(10):1085–1091. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, McNally DS, Urban JPG, Hall AC. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80(3):839–846. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- 8.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28(10):982–990. doi: 10.1097/00007632-200305150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lipson SJ. Metaplastic proliferative fibrocartilage as an alternative concept to herniated intervertebral disc. Spine. 1988;13(9):1055–1060. doi: 10.1097/00007632-198809000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Liu GZ, Ishihara H, Osada R, Kimura T, Tsuji H. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine. 2001;26(2):134–141. doi: 10.1097/00007632-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23(23):2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Mason RM, Palfrey AJ. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2(4):333–338. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Yonenobu K, Ono K. Experimental cervical spondylosis in the mouse. Spine. 1991;16(10 Suppl):S495–S500. doi: 10.1097/00007632-199110001-00008. [DOI] [PubMed] [Google Scholar]
- 15.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br. 2002;84(2):289–294. doi: 10.1302/0301-620X.84B2.11937. [DOI] [PubMed] [Google Scholar]
- 16.Pritzker K. Aging and degeneration in the lumbar intervertebral disc. Orth Clin Nor Am. 1977;8(1):65–77. [PubMed] [Google Scholar]
- 17.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164(3):915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scapinelli R, Little K. Observations on the mechanically induced differentiation of cartilage from fibrous tissue. J Pathol. 1970;101:85–91. doi: 10.1002/path.1711010203. [DOI] [PubMed] [Google Scholar]
- 19.Tiball JG, Albrecht DE (1998) Regulation of apoptosis by cellular interactions with the extracullular matrix. In: Wiley-Liss
- 20.Torgerson W, Dotter W. Comparative Roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58-A(6):850–853. [PubMed] [Google Scholar]
- 21.Tornusciolo DRZ, Schmidt RE, Roth KA. Simultaneous detection of TDT-mediated dUTP-biotin nick end-labeling (TUNEL)-positive cells and multiple immunohistochemical markers in single tissue sections. BioTechniques. 1995;19:800–805. [PubMed] [Google Scholar]
- 22.Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14(2):359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 23.Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/S0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- 24.Wada E, Ebara S, Saito S, Ono K. Experimental spondylosis in the rabbit spine. Overuse could accelerate the spondylosis. Spine. 1992;17(3 Suppl):S1–S6. doi: 10.1097/00007632-199203001-00001. [DOI] [PubMed] [Google Scholar]
- 25.White AA, Panjabi MM (1990) Clinical Biomechanics of the Spine. In: Second JB (eds) Lippincott Co., Philadelphia