Abstract
No clinical diagnostic support tool can help identify patients with LSS. Simple diagnostic tool may improve the accuracy of the diagnosis of LSS. The aim of this study was to develop a simple clinical diagnostic tool that may help physicians to diagnose LSS in patients with lower leg symptoms. Patients with pain or numbness of the lower legs were prospectively enrolled. The diagnosis of LSS by experienced orthopedic specialists was the outcome measure. Multivariable logistic regression analysis identified factors that predicted LSS; a simple clinical prediction rule was developed by assigning a risk score to each item based on the estimated beta-coefficients. From December 2002 to December 2004, 104 orthopedic physicians from 22 clinics and 50 hospitals evaluated 468 patients. Two items of physical examination, three items of patients' symptom, and five items of physical examination were included in the final scoring system as a result of multiple logistic regression analysis. The sum of the risk scores for each patient ranged from −2 to 16. The Hosmer–Lemeshow statistic was 11.30 (P = 0.1851); the area under the ROC curve was 0.918. The clinical diagnostic support tool had a sensitivity of 92.8% and a specificity of 72.0%. The prevalence of LSS was 6.3% in the bottom quartile of the risk score (−2 to 5) and 99.0% in the top quartile (12 to 16). We developed a simple clinical diagnostic support tool to identify patients with LSS. Further studies are needed to validate this tool in primary care settings.
Keywords: Lumbar spine, Lumbar spinal stenosis, Diagnosis
Background
Lumbar spinal stenosis (LSS) results from compression of the cauda equina or existing nerve roots and leads to substantial functional disability [12, 18, 20]. The diagnosis of symptomatic LSS has implications for treatment, since symptoms that are caused by nerve root compression may respond to either conservative treatments or decompressive surgery. Clinicians cannot rely solely on diagnostic imaging tests to make the diagnosis of LSS. Computed tomography and magnetic resonance imaging are often nonspecific [8], and there are discrepancies between clinical symptoms and imaging findings in lumbar spinal stenosis [1, 3], and these test results cannot determine whether symptoms arise from nerve root compression [4, 17, 22]. Thus, the clinical correlation between imaging test results and symptoms must ultimately be made on the basis of history and physical examination, both of which have not been extensively studied in LSS patients.
Symptoms of LSS are often chronic, frequently missed, and frequently misdiagnosed [11], resulting in severe disability or reduction in patients’ quality of life [10]. Possible reasons for this difficulty in making the diagnosis may include a lack of training in the recognition of this disorder or a failure of patients and/or their healthcare providers to discuss health problems during a health care visit. The patients’ symptoms, specific questions on history taking, and the findings on physical examination are all used to make the diagnosis of LSS in the primary care setting. A simple clinical support tool may assist primary care physicians to identify patients with LSS more readily; patients would then benefit from an appropriate therapeutic approach.
The objective of this study was to develop and evaluate a user-friendly clinical tool based on a scoring system for the diagnosis of LSS so as to deliver a better quality of care to LSS patients.
Methods
Study design and setting
We prospectively evaluated the association between the diagnosis of LSS and clinical information, including the history and physical examination of patients with low back pain, leg pain, or tingling of the legs. This study was performed in university hospitals, medical centers, and other hospitals and clinics affiliated with university hospitals or medical centers. We enrolled consecutive patients older than 20 years of age with primary symptoms of pain or numbness in the legs. We selected these symptoms, since patients with these symptoms are often misdiagnosed as having peripheral artery disease or are otherwise underdiagnosed even though they have LSS. Such patients would benefit from a diagnostic tool that would improve their quality of care. We excluded patients who have been treated by some medical practices within one year before examination. Patients with cervical myelopathy, previous surgery, and inflammatory disorders were also excluded. The study was approved by the institutional review board of each study institution as necessary. Written informed consent was obtained from the all patients.
We collected the following information for the current alalysis: age (<60, 60–70, ≥70 years), gender, months from onset (quartiles), leg numbness or pain, back pain, intermittent claudication, bilateral plantar numbness, exacerbation of symptoms when standing up, improvement of symptom when bending forward, symptoms related to cauda equina syndrome, no history of diabetes, history of hypertension, history of hyperlipidemia, peripheral circulation (poor, good), straight leg raising (SLR) test, symptoms induced by having patients bend forward, symptoms induced by having patients bend backward, abnormality on manual muscle testing, any sensory disturbance, abnormal Achilles tendon reflex, abnormal patellar tendon reflexes. Poor peripheral circulation was defined as a dorsalis pedis artery that was not easily palpable or, if the blood pressure of the legs was measured, an ankle brachial index of less than 0.9 [2, 5, 16]. Orthopedic staff physicians in each institution took a history, did a physical examination, and ordered lumbar X-rays and magnetic resonance imaging evaluation (MRI) based on a standardized protocol. The history taken by the physicians included information on the type and distribution of patients’ symptoms (such as leg pain and low back pain), the posture that attenuated or worsened these symptoms, and comorbidities, such as diabetes or peripheral artery disease. The physical examination included the ankle brachial index and various tests that are thought to identify dysfunction in the lumbopelvic region [21]. Patients then had lumbar X-rays and MRIs. We allowed all enrolled patients to have diagnostic imaging studies. Each participating physician recorded the clinical and diagnostic test information on a standardized report form, and then sent the form to the study coordinator, an experienced orthopedic surgeon, who verified the information on the form and the diagnosis.
Outcome measure
In the absence of a universally accepted gold standard for LSS, the impression of expert clinicians provides a reasonable method of establishing a clinical diagnosis [9]. Such an approach has been adopted in the development of classification criteria for rheumatic diseases, which, like LSS, cannot be defined by a single laboratory measurement [2, 13].
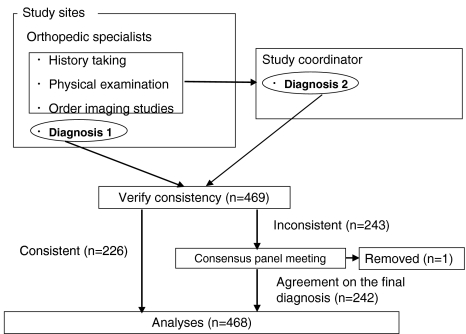
The following steps were taken to reach a final diagnosis for each of the enrolled patients (Fig. 1). In the first step, at each institution the orthopedic physician who saw a patient made the clinical diagnosis based on the history, physical examination, and radiographic findings. In addition, to verify the diagnosis made by each physician, the study coordinator also made a diagnosis for each patient based on the clinical information and a copy of the diagnostic imaging studies. Interobserver agreement (physicians in each institution and one panel member) was assessed by calculating agreement ratios and the kappa statistic [5].
Fig. 1.
Flow chart showing how the diagnosis of LSS was determined. Diagnosis 1 denotes the diagnosis made by an orthopedic physician at each study site, and diagnosis 2 denotes the diagnosis made by the study coordinator. Of the 469 patients enrolled in this study, the diagnoses of 226 cases were consistent. Inconsistencies in the remaining 243 cases were resolved by a consensus panel meeting. Only one case was removed from the analysis because no agreement could be reached on the final diagnosis
As there was a substantial discrepancy in the diagnoses [interobserver agreement rate on the diagnosis of LSS was 60.8%, and kappa was 0.261 (95%CI, 0.185–0.336)], we created a second step for the making the diagnosis. We formed a consensus panel that would meet and resolve any discrepancies. The consensus panel consisted of 10 expert physicians with extensive clinical experience of LSS; all panel members were either professors or associate professors at university hospitals or chiefs of departments of orthopedics at teaching hospitals in Japan. For each case in which there was a discrepancy in the diagnosis in the first step, each panel member scored the probability of LSS on a scale from 1 to 4 (lowest = 1, highest = 4) based on the clinical information and the imaging studies. Then, the mean score for each patient was calculated. If a patient’s mean score was equal to or above 3, the diagnosis was confirmed as LSS; if the mean score was equal to or below 2, the case was regarded as non-LSS. When the mean score was between 2 and 3, consensus panel members discussed why there was a discrepancy, and after a thorough discussion a final diagnosis was made. If there was no agreement on the diagnosis, the case was removed from the analysis.
Statistical analysis
The diagnostic support tool was developed in two steps. Step 1 was designed to identify a subgroup of patients who were likely to have LSS and therefore needed additional investigation. In this first step, we tested the all the variables collected for this study. When we derived the model for the scoring system, we did not include variables from the MRI studies, because it is not practical to obtain MRI in all patients who are complaining low back and leg symptoms. Each questionnaire item was evaluated using simple logistic regression, and the odds ratio was calculated.
In the next step of developing the clinical decision support tool, factors with a P value less than 0.2 on the univariate analyses in step 1, as well as the variables that we thought clinically important from our experience as orthopedic specialists, were included in the stepwise multiple logistic regression model. We identified the significant (P < 0.05) predictors of a final diagnosis of LSS, and removed any variable that had a p-value more than 0.05 in the final model. Using a regression coefficient-based scoring system, a score-based prediction rule for a final diagnosis of LSS was developed for each step based on the results of the multivariable logistic regression equations. To generate a simple integer-based point score for each predictor variable, scores were assigned by dividing the β-coefficient by two-fifths of the sum of the two smallest coefficients in the model and rounding up to the nearest integer. The overall risk score for each patient was calculated by summing the scores of each component.
The discrimination ability of the models was assessed by the area under the receiver operating characteristic (ROC) curve, and the calibration was evaluated by using the Hosmer–Lemeshow chi-square statistic (P > 0.05 for all models); P equal or greater than 0.05 supports the goodness of fit. Discriminatory power is the ability to identify which patients are likely to have an outcome; An area of 1.00 under the ROC curve indicates perfect discrimination whereas an area of 0.50 indicates complete absence of discrimination. The calibration was also evaluated by comparing the prevalence of LSS in the risk score quartiles. To examine the performance of the support tool, we calculated the sensitivity, the specificity, and the likelihood ratios for positive and negative results. The positivity criterion for the presence of LSS was defined as the point with the highest sum of sensitivity and specificity.
Results
A total of 104 orthopedic surgeons from 22 clinics and 50 hospitals in various sites of Japan evaluated 469 patients from December 2002 to December 2004. The patients’ mean age was 64.2 (range 20–96) years, and 45.9% were male. Of the total 469 participants, the diagnoses were consistent between two observers in 226 participants; of these, 126 cases were diagnosed as having LSS. The consensus panel discussed the 243 cases that were given initially discrepant diagnoses. Mean scores of 61 cases were equal or above 3, then the diagnosis of these patients was confirmed as LSS; mean scores of 166 patients were equal or below 2, the case was regarded as non LSS. Mean scores of 15 cases were between 2 and 3, then consensus panel members discussed why the discrepancy was raised, and after careful discussion final diagnosis was made; of these 15 cases, 11 cases were diagnosed as having LSS. The consensus panel did not reach agreement in one case; this case was removed from the analysis. Thus, 468 cases were included in the current analysis. The overall prevalence of LSS was 47.4%. Other diagnoses included: lumbar disc herniation (17.7%), diabetic neuropathy (2.8%), and peripheral artery disease (8.3%). In 23.7% of patients, no specific diagnosis other than “not LSS” was made.
In step 1, on univariate analysis, the following variables had a P value less than 0.2 (Table 1): age, onset; symptoms including presence of pain or numbness of the legs, presence of low back pain, presence of bilateral plantar numbness, urinary disturbance, presence of numbness in the perineal region, exacerbation of symptoms when standing up, improvement of symptoms when bending forward, presence of symptoms related to the cauda equina syndrome; cormorbidity, including absence of diabetes, hypertension, and hyperlipidemia; physical examination, including good peripheral artery circulation, a positive straight leg raising test, symptoms induced by having patients bend forward, symptoms induced by having patients bend backward, abnormal Achilles tendon reflex, and abnormal patellar tendon reflex (Table 2).
Table 1.
Participants’ demographic characteristics
| Variables | (n = 469) | |
|---|---|---|
| Age (mean ± SD years) | 65.2 ± 13.7 | |
| Gender (male) | 45.9% | |
| Clinical impressions of patient condition | N | % |
| LSS | 222 | 47.3 |
| LDHa | 83 | 17.7 |
| Diabetic neuropathy | 13 | 2.8 |
| Peripheral artery disease | 39 | 8.3 |
| Otherb | 111 | 23.7 |
| Undetermined | 1 | 0.1 |
aLumbar disc herniation
bUnknown or unspecified, but regarded as non LSS
Table 2.
Univariate analyses for factors from the MD and MRI data sheets associated with a diagnosis of LSS
| LSS (−), n = 246 | LSS (+), n = 222 | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <60 | 37.9% | 15.3% | Reference | ||
| 60–70 | 23.5% | 24.8% | 2.61 | 1.53–4.44 | <0.001 |
| >70 | 31.8% | 64.4% | 4.66 | 2.89–7.49 | <0.001 |
| Gender (female) | 45.5% | 46.4% | 1.04 | 0.72–1.49 | 0.851 |
| Onset | 0.003 | ||||
| 1st quartile (<1 month) | 30.1% | 18.5% | Reference | ||
| 2nd quartile (1–5 months) | 25.6% | 23.9% | 1.52 | 0.90–2.58 | 0.121 |
| 3rd quartile (6–12 months) | 23.6% | 25.2% | 1.74 | 1.03–2.96 | 0.040 |
| 4th quartile (≥13 months) | 17.5% | 30.6% | 2.85 | 1.66–4.90 | <0.001 |
| Missing data | 3.3% | 1.8% | 0.90 | 0.26–3.18 | 0.873 |
| Symptoms | |||||
| Leg pain or numbness (+) | 44.7% | 57.7% | 1.68 | 1.17–2.43 | 0.005 |
| Low back pain (+) | 58.5% | 66.2% | 1.39 | 0.95–2.02 | 0.088 |
| Intermittent claudication (+) | 22.0% | 82.0% | 16.18 | 10.25–25.53 | <0.001 |
| Bilateral plantar numbness (+) | 12.6% | 27.0% | 2.57 | 1.59–4.15 | <0.001 |
| Urinary disturbance (+) | 2.0% | 14.0% | 7.82 | 2.99–20.50 | <0.001 |
| Numbness of perineal region (+) | 1.2% | 4.5% | 3.82 | 1.04–14.07 | 0.044 |
| Exacerbation of symptoms when standing up | 29.7% | 68.0% | 5.04 | 3.40–7.47 | <0.001 |
| Improvement of symptoms when bending forward | 8.1% | 51.8% | 12.14 | 7.17–20.58 | <0.001 |
| Symptoms related to cauda equina syndromea | 0.8% | 5.9% | 7.59 | 1.69–34.01 | 0.008 |
| Comorbidity | |||||
| Diabetes (−) | 82.9% | 83.3% | 1.03 | 0.63–1.67 | 0.97 |
| Hypertension (+) | 23.6% | 41.9% | 2.34 | 1.57–3.48 | <0.001 |
| Hyperlipidemia (+) | 7.3% | 13.1% | 1.90 | 1.03–3.53 | 0.041 |
| Physical examination | |||||
| Peripheral artery circulation | |||||
| Badb | 19.5% | 14.9% | Reference | ||
| Good | 57.7% | 73.9% | 1.68 | 1.02–2.76 | 0.041 |
| Missing data | 22.8% | 11.3% | 0.65 | 0.34–1.24 | 0.191 |
| Straight leg raising test positive | 32.9% | 16.7% | 0.41 | 0.26–0.63 | <0.001 |
| Symptoms induced by having patients bend forward (+) | 37.0% | 17.6% | 0.36 | 0.24–0.56 | <0.001 |
| Symptoms induced by having patients bend backward (+) | 45.5% | 69.8% | 2.77 | 1.89–4.05 | <0.001 |
| Abnormal manual muscle strength testc | 6.9% | 9.5% | 1.41 | 0.72–2.74 | 0.315 |
| Sensory disturbance | |||||
| (−) | 56.9% | 49.5% | Reference | ||
| (+)d | 37.8% | 44.6% | 1.00 | 0.99–1.01 | 0.762 |
| Missing data | 5.3% | 5.9% | 1.27 | 0.57–2.86 | 0.559 |
| Achilles tendon reflex | |||||
| Normal | 54.5% | 32.4% | Reference | ||
| Abnormale | 43.5% | 66.2% | 2.56 | 1.75–3.74 | <0.001 |
| Missing data | 2.0% | 1.4% | 1.12 | 0.26–4.81 | 0.882 |
| Patellar tendon reflex | |||||
| Normal | 70.3% | 63.5% | Reference | ||
| Abnormale | 28.5% | 36.5% | 1.42 | 0.96–2.10 | 0.078 |
| Missing data | 1.2% | 0.0% | – | – | – |
aA burning sensation around the buttocks and/or intermittent priapism associated with walking
bAnkle brachial index (API)<0.9, or diminished pulsation of dorsalis pedis artery or posterior tibial artery
cMMT ≤3, Strength was graded from 0 (no movement) to 5 (normal) at the knee extensors, ankle dorsiflexors, and plantar flexors, and extensor hallucis longus
dHypoesthesia, analgesia, or hyperalgesia at the medial knee, dorsal foot, plantar foot, and perineal lesion
eAbsence or low response of deep reflexes
In step 2, on stepwise multivariable logistic regression analysis, we include all variables with a P value less than 0.2. However, since we thought that the history of diabetes was important for the diagnosis of LSS, since diabetic neuropathy is one of the differential diagnoses of LSS, we included the absence of diabetes in the model, even though it had a P value greater than 0.2. Thus, the following variables were included as independent predictors in the multivariable model with a P value less than 0.05: age, absence of diabetes, intermittent claudication, exacerbation of symptoms when standing up, improvement of symptoms when bending forward, symptoms induced by having patients bend forward, symptoms induced by having patients bend backward, good peripheral artery circulation, abnormal Achilles tendon reflex, and positive SLR test (Table 3). For the final model, the Hosmer–Lemeshow statistic was 11.30 (P = 0.1851), which indicates good calibration. To develop a simple clinical diagnostic tool from the results of step 2, an integer score derived from the β-coefficient was assigned to the identified risk factors. For each patient, all applicable risk score values were summed up to attain a total risk score for the patient. The sum of the risk scores for each patient ranged from −2 to 16.
Table 3.
Multivariable predictors of a diagnosis of LSS and the associated risk scoring system
| Characteristic | Regression β-coefficient | 95% CI | Risk score assigned* |
|---|---|---|---|
| History | |||
| Age (years) | |||
| 60–70 | 0.91 | 0.09–1.73 | 1 |
| >70 | 1.36 | 0.60–2.11 | 2 |
| Absence of diabetes | 0.93 | 0.19–1.68 | 1 |
| Symptoms | |||
| Intermittent claudication (+) | 2.43 | 1.82–3.04 | 3 |
| Exacerbation of symptoms when standing up | 1.27 | 0.65–1.89 | 2 |
| Symptom improvement when bending forward | 2.09 | 1.36–2.82 | 3 |
| Physical examination | |||
| Symptoms induced by having patients bend forward | −0.91 | −1.61 to −0.22 | −1 |
| Symptoms induced by having patients bend backward | 0.90 | 0.31–1.49 | 1 |
| Good peripheral artery circulation | 1.96 | 1.14–2.77 | 3 |
| Abnormal Achilles tendon reflex | 1.03 | 0.40–1.66 | 1 |
| SLR test positive | −1.12 | −1.87 to −0.37 | −2 |
* Scores were assigned by dividing the β-coefficient by the absolute value of two-fifths of the sum of the two smallest coefficients in the model. Hosmer–Lemeshow statistics, 11.30 (P = 0.1851)
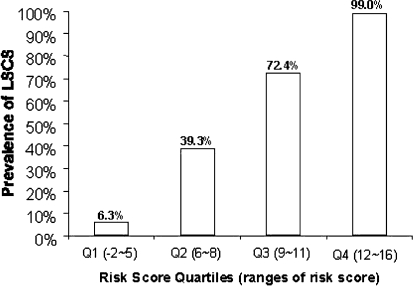
Table 4 presents the model performance indices. The area under the ROC curve was 0.918; thus the model had good discriminatory power (Fig. 1). The positivity cut-off point was defined as 7, since the sum of the sensitivity and the specificity was the highest at that cut-off point. Given that the positivity criterion for risk score was greater than 7, the clinical diagnostic support tool had a sensitivity of 92.8% and a specificity of 72.0%. The prevalence of LSS increased as the risk score increased; LSS prevalences were 6.3% in the first quartile (−2 to 5), 39.3% in the second quartile (6 to 8), 72.4% in the third quartile (9 to 11), 99.0% in the fourth quartile (12 to 16); these results suggest good calibration of the model (Fig. 2).
Table 4.
Performance indices of the clinical prediction rule
| Index | Estimates |
|---|---|
| Sensitivitya | 0.928 |
| Specificitya | 0.720 |
| Likelihood ratioa | |
| Positive test resulta | 3.31 |
| Negative test resulta | 0.1 |
| Area under the ROC curve | 0.918 |
aGiven the positivity criteria for the total risk score ≥7
Fig. 2.
Incidence of LSS stratified by risk score quartiles. Quartile 1 represents a risk score of −2 to 5, quartile 2 represents a risk score of 6 to 8, quartile 3 represents a risk score of 9 to 11, and quartile 4 represents a risk score of 12 to 17
Discussion
The purpose of a clinical prediction rule is to improve the accuracy of diagnosis [14, 16]. The rule we developed was designed to help non-orthopedic specialists to identify patients with LSS. The prevalence of degenerative spine disease will increase with the continued aging of the population [15]. This will require not only orthopedic specialists to develop greater expertise in diagnosing LSS, but also non-specialists will need to have screening tools for this condition. The diagnostic support tool we developed is simple and easy to use, and thus our results indicate that self-reported symptoms and medical history are useful, and thus this tool may be useful even for non orthopedic specialists to identify patients with LSS.
The presence of a narrowed spinal canal on radiographic imaging does not define LSS [1, 3, 4, 8, 22]. No significant correlation is found between the area of the dural sac in axially loaded CT and the clinical symptoms of spinal stenosis [22]. Confusing clinical findings resembling spinal stenosis are relatively common in patients who have mild or no narrowing of the spinal canal on CT [22]. Symptomatic lumbar spondylosis, peripheral arterial disease (PAD), and peripheral neuropathy must all be considered in the differential diagnosis of LSS. Since both symptomatic lumbar spondylosis and LSS are caused by the aging process, the differential diagnosis may be difficult. The primary distinguishing symptom of LSS seems to be a predominance of leg symptoms. Therefore, in this study, we studied patients whose primary symptoms were pain or numbness of the legs.
Several limitations of this study should be noted. First, there is no good objective reference standard for diagnosing LSS; it is essentially a clinical diagnosis. Imaging studies such as computed tomography (CT) or MRI showing compression of nerve root are insufficient for making the diagnosis of LSS, since false positive and false negative results are well documented [4, 7, 22]. MRI and CT findings of LSS using current qualitative methods result in significant variation of image interpretation [19]. However, in the absence of valid objective criteria, expert opinion is a reasonable strategy for making the diagnosis of a clinical syndrome [9], and has been used in a variety of disorders [2, 13]. In our current study, when there was an inconsistency in diagnosis between the first two observers, the inconsistency was resolved by a consensus panel consisting of 10 orthopedic specialists, and such an expert panel should provided a sufficiently accurate reference standard for the current analysis. Second, this study was performed primarily in hospitals, such as university hospitals, medical centers, and other hospitals, while LSS is prevalent in the primary care setting, where it is often underdiagnosed or misdiagnosed. The diagnostic support tool that we developed may play an important role in such settings, though our scoring system has yet to be validated in the further research; in this research, primary care physician use our tool and evaluate the usability of our tool, and the sensitivity, specificity, and discriminatory power should also be evaluated. Pulse palpation is not sensitive for the detection of peripheral artery disease compared to ankle brachial index. It was reported that more than two thirds of the patients with peripheral artery disease of either the left or right leg had a detectable pulse, especially in overweight patients [6]. Although our results suggested that palpation of dorsalis pedis or ankle blood pressure less than 0.9 is useful to predict LSS, caution should be made for our diagnostic tool to be applied to overweight patients.
Despite these limitations, this is the first report of the development of a diagnostic support tool for LSS. Using this tool, it is possible to accurately diagnose patients with LSS. We expect that use of the tool in primary care will improve the accuracy of diagnosis, thus leading to improved quality of patient care.
Conclusions
We developed a simple clinical diagnostic support tool to identify patients with LSS.
References
- 1.Amundsen T, Weber H, Lilleas F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine. 1995;20:1178–1186. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 3.Beattie PF, Meyers SP, Stratford P, Millard RW, Hollenberg GM. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine. 2000;25:819–828. doi: 10.1097/00007632-200004010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 5.Chalmers I. Underreporting research is scientific misconduct. Jama. 1990;263:1405–1408. doi: 10.1001/jama.263.10.1405. [DOI] [PubMed] [Google Scholar]
- 6.Collins TC, Suarez-Almazor M, Peterson NJ. An absent pulse is not sensitive for the early detection of peripheral arterial disease. Fam Med. 2006;38:38–42. [PubMed] [Google Scholar]
- 7.Coulier B, Devyver B, Ghosez JP. Severe underestimation of lumbar spinal stenosis by supine imaging. Clin Radiol. 2003;58:167–169. doi: 10.1053/crad.2002.1096. [DOI] [PubMed] [Google Scholar]
- 8.Graaf I, Prak A, Bierma-Zeinstra S, Thomas S, Peul W, Koes B. Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine. 2006;31:1168–1176. doi: 10.1097/01.brs.0000216463.32136.7b. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein AR. Clinical epidemiology: the architecture of clinical research. WB Saunders: Philadelphia; 1985. [Google Scholar]
- 10.Goldman SM, Funk JD, Christensen VM. Spinal stenosis. A common cause of podiatric symptoms. J Am Podiatr Med Assoc. 1997;87:117–124. doi: 10.7547/87507315-87-3-117. [DOI] [PubMed] [Google Scholar]
- 11.Goldman SM. Diabetic peripheral neuropathy and spinal stenosis: prevalence of overlap and misdiagnosis. An introductory report. Diabet Med. 2004;21:394–396. doi: 10.1111/j.1464-5491.2004.01128.x. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson A, Rydberg T, Sterner G, Melander A. Pharmacokinetics of glibenclamide and its metabolites in diabetic patients with impaired renal function. Eur J Clin Pharmacol. 1998;53:429–435. doi: 10.1007/s002280050403. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Liang MH. Classification criteria revisited. Arthritis Rheum. 1991;34:1228–1230. doi: 10.1002/art.1780341004. [DOI] [PubMed] [Google Scholar]
- 14.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. Jama. 1997;277:488–494. doi: 10.1001/jama.277.6.488. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence JS. Disc degeneration. Its frequency and relationship to symptoms. Ann Rheum Dis. 1969;28:121–138. doi: 10.1136/ard.28.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. Jama. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 17.Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS. Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet. 1986;2:1366–1367. doi: 10.1016/S0140-6736(86)92008-8. [DOI] [PubMed] [Google Scholar]
- 18.Spengler Degenerative stenosis of the lumbar spine. J Bone Joint Surg Am. 1987;69:305–308. [PubMed] [Google Scholar]
- 19.Stafira JS, Sonnad JR, Yuh WT, Huard DR, Acker RE, Nguyen DL, Maley JE, Ramji FG, Li WB, Loftus CM. Qualitative assessment of cervical spinal stenosis: observer variability on CT and MR images. AJNR Am J Neuroradiol. 2003;24:766–769. [PMC free article] [PubMed] [Google Scholar]
- 20.Stucki G, Liang MH, Lipson SJ, Fossel AH, Katz JN. Contribution of neuromuscular impairment to physical functional status in patients with lumbar spinal stenosis. J Rheumatol. 1994;21:1338–1343. [PubMed] [Google Scholar]
- 21.Waddell G, Somerville D, Henderson I, Newton M. Objective clinical evaluation of physical impairment in chronic low back pain. Spine. 1992;17:617–628. doi: 10.1097/00007632-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wiesel SW, Tsourmas N, Feffer HL, Citrin CM, Patronas N. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549–551. doi: 10.1097/00007632-198409000-00003. [DOI] [PubMed] [Google Scholar]