Abstract
Reduction of blood flow in compressed nerve roots is considered as one important mechanism of induction of neurogenic intermittent claudication in lumbar spinal canal stenosis. Vascular endothelial growth factor (VEGF) is a potent stimulator of angiogenesis, and is increased in expression in hypoxic conditions. The objective of this study was to examine if cauda equina compression affects motor function and induces expression of VEGF and angiogenesis. The cauda equina was compressed by placing a piece of silicone rubber into the L5 epidural space. Walking duration was examined by rota-rod testing. The compressed parts of the cauda equina and L5 dorsal root ganglion (DRG) were removed at 3, 7, 14, or 28 days after surgery, and processed for immunohistochemistry for VEGF and Factor VIII (marker for vascular endothelial cells). Numbers of VEGF-immunoreactive (IR) cells and vascular density were examined. Walking duration was decreased after induction of cauda equina compression. The number of VEGF-IR cells in the cauda equina and DRG was significantly increased at 3, 14, and 28 days after cauda equina compression, compared with sham-operated rats (P < 0.05). Vascular density in the cauda equina was not increased at any of the time points examined. Cauda equina compression decreased walking duration, and induced VEGF expression in nerve roots and DRG.
Keywords: Cauda equina compression, Intermittent claudication, Vascular endothelial growth factor, Angiogenesis
Introduction
Compression of the cauda equina by lumbar spinal canal stenosis is a major clinical problem associated with intermittent claudication. Some experimental studies have shown that compression of the cauda equina reduces blood flow in compressed nerve roots, and this is considered one important mechanism of induction of neurogenic intermittent claudication in lumbar spinal canal stenosis [1, 6, 9, 13, 14, 16, 23, 25].
It has also been reported that the chronically compressed cauda equina acquires resistance to additional compression [8]. Another study demonstrated reduction of nerve conduction velocity in chronically compressed cauda equina that recovered over time [15]. These findings suggest that vascular adaptation such as angiogenesis might occur in chronically compressed nerve roots, followed by recovery of nerve function and acquisition of tolerance.
Vascular endothelial growth factor (VEGF) is a potent stimulator of angiogenesis. Increase in hypoxia [3, 19], as in conditions of myocardial ischemia [2, 11], stimulates VEGF expression. Brain ischemia [4, 10], spinal cord ischemia [5], mechanical injury [20], and sciatic nerve ischemia [17] induce VEGF expression in vascular endothelial cells, glial cells, and neurons. We therefore hypothesized that cauda equina compression induces expression of VEGF in the compressed cauda equina and related dorsal root ganglion (DRG). The aim of this study was to examine if cauda equina compression induces expression of VEGF. In addition, we examined whether angiogenesis occurred in the compressed cauda equina.
Materials and methods
A total of 72 adult male Sprague-Dawley rats (200–250 g) were used in this study. All animal experiments conformed to the regulations of the Animal Research Committee of Fukushima Medical University and accorded with the Guidelines on Animal Experiments at Fukushima Medical University and the Japanese Government Animal Protection and Management Law (No. 105).
Surgical procedures
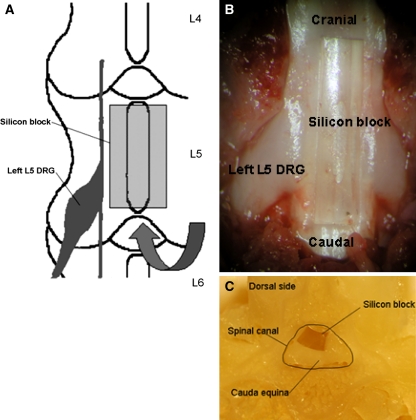
Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and placed in prone position. A skin incision was made over the spinal midline, and the lamina of L5 and L6 were exposed with the aid of a microscope. The ligamentum flavum was removed between L5 and L6. A piece of silicon block (length: 4.0 mm, width: 1 mm, thickness: 0.9 mm) [18] was placed into the epidural space under the L5 vertebra (n = 35) (Fig. 1). The silicon block occupied about a half AP-diameter of the spinal canal (Fig. 1c). After surgery, the incision was closed. In sham-operated rats, silicone was not placed in the L5 epidural space (n = 35).
Fig. 1.
a The surgical procedure for induction of cauda equina compression. A silicon block (length: 4.0 mm, width: 1 mm, thickness: 0.9 mm) was placed into the epidural space under the L5 vertebra through the interlaminar space between L5 and L6. b This is an anatomic photo coinciding with a. Laminectomy was performed for showing a place of silicon block. c This photo shows a cross sectional image of the spinal canal with the silicon block
Behavioral testing
A total of 18 rats were included in this part of the study. In the compression and sham-operated groups, 9 rats of each group were used to measure the duration of walking on a treadmill (Rota-rod test), which had a circular column with a diameter of 125 mm [24, 27]. The rats were trained on the treadmill for 3 days before surgery. Rotation speed was initially ten rotations per minute, and was increased five rotations per minute every 1 min. Walking time until the rat fell off the rotating rod was measured five times for each animal at 1 day before surgery, and 1, 3, 7, 14, 21, and 28 days after surgery. The interval between each test was 15 min. The mean of five trials were calculated for each rat. Walking times are expressed as mean standard deviation (SD).
Immunohistochemistry
A total of 54 rats (Compression group: n = 27, sham group: n = 27) were included in this part of the study. At 3, 7, 14 and 28 days after surgery, 6–9 rats of each group were deeply anaesthetized by inhalation of ether and underwent intracardiac perfusion with 200 ml saline followed by 300 ml of paraformaldehyde in 0.1 M phosphate buffer saline (PBS). The compressed part (L5) of the cauda equina, and left L5 dorsal root ganglion were removed, post-fixed overnight in the same fixative, and dehydrated overnight in 20% sucrose. Transverse frozen sections of cauda equina and DRG were cut on a cryostat and thaw-mounted on slides. Sections were immunostained with a rabbit polyclonal antibody for VEGF (1:100; NeoMarkers, Fremont, CA) and a rabbit polyclonal antibody for Factor VIII, marker for endothelial cells (1:100; Chemicon, Temecula, CA) as a primary antibody, a biotinylated antibody for rabbit IgG as a secondary antibody (1:200; Vector Laboratories, Burlingame, CA), the avidin-biotin peroxidase complex (Vector Laboratories), and 0.02% 3,3′ diaminobenzidine dihydrochrolide as chromogen. Then, tissue sections were dehydrated, xylene-treated, and cover-slipped.
Numbers of VEGF-immunoreactive cells
VEGF-immunoreactive (IR) cells were counted in cauda equina and DRG. VEGF-IR cells in cauda equina were counted in five fields with the highest number of positive cells per section at 400× magnification, with three sections per rat. The mean of fifteen fields was calculated for each rat. Numbers of VEGF-IR cells are expressed as mean ± SD. VEGF-IR neurons and all neurons in DRG were counted in three sections per rat. Percentages of VEGF-IR neurons in DRG were calculated, and are shown as the mean ± SD. The investigator counting the number of VEGF-IR cells in cauda equina and DRG was blind to the experimental procedure.
Vascular density
Factor VIII-positive blood vessels in cauda equina were counted in five sections per rat. The cross-sectional area of cauda equina was calculated by imaging analysis software (KS100, Carl Zeiss, German), and vascular density was expressed as number of blood vessel cells per square millimeter. The mean of five sections was calculated for each rat. Results are expressed as mean ± SD vascular density of the cauda equina. The investigator counting the number of blood vessel cells was blind to the experimental procedure.
Statistical analysis
Statistical analysis of walking duration was performed by repeated-measures ANOVA followed by the unpaired t-test. For VEGF-positive cells and vascular density, the unpaired t-test was performed. P values less than 0.05 were considered significant.
Results
No wound infection or obvious limb paralysis was observed after cauda equina compression or in sham-operated rats.
Walking time
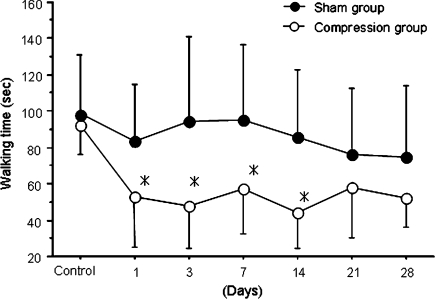
Walking time was decreased after cauda equina compression, with significant differences observed from day 1 to day 14 compared with sham-operated rats (P < 0.05), (Fig. 2).
Fig. 2.
Time course of changes in walking time. Results are the mean ± standard deviation of walking duration. There were significant differences between the compression and sham-operated groups at days 1, 3, 7, and 14 after surgery (*P < 0.05)
VEGF-IR cells
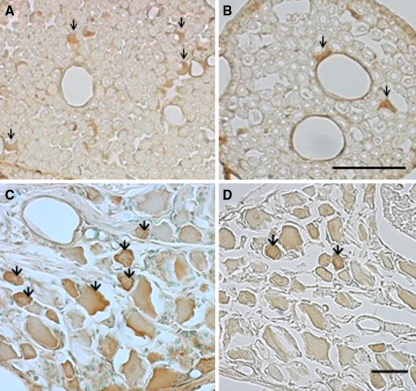
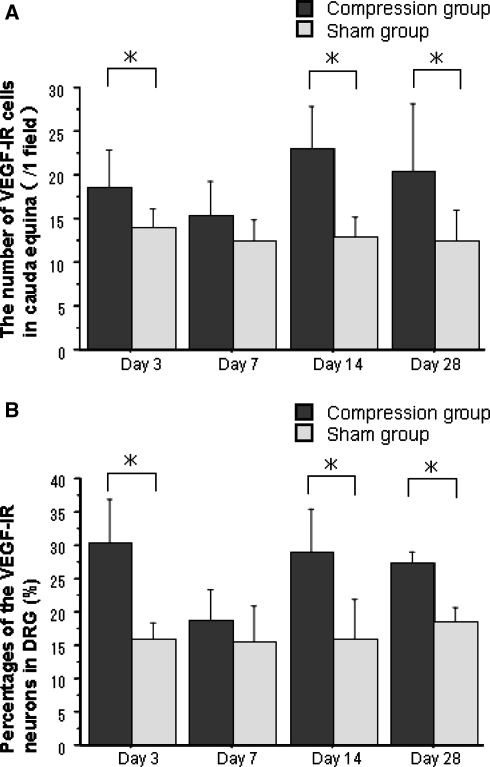
In the cauda equina, Schwann-like cells around axons exhibited immunoreactivity for VEGF, (Fig. 3a, b). The VEGF-IR cell number was significantly increased in the cauda equina at 3, 14, and 28 days after cauda equina compression compared with the sham-operated group (P < 0.05). However, there were no significant differences in number of VEGF-IR cells between the two groups at 7 days after surgery, (Fig. 4a). In the DRG, DRG neurons exhibited immunoreactivity for VEGF (Fig. 3c, d). The number of VEGF-IR neurons in the compression group was increased at 3, 14, and 28 days after surgery compared with the sham-operated group (P < 0.01). However, there were no significant differences in number of VEGF-IR neurons between the two groups at 7 days after surgery (Fig. 4b)
Fig. 3.
Photomicrographs demonstrating VEGF-immunoreactive cells in the cauda equine (a, b) and DRG (c, d) in compression group (a, c) and sham-operated group (b, d) at day 28 after surgery. Schwann-like cells in the cauda equina and DRG neurons exhibited immunoreactivity for VEGF (arrows). Scale bar 50 μm
Fig. 4.
Histogram presenting numbers of VEGF-IR cells in the cauda equine (a) and percentages of VEGF-IR neurons in the DRG (b). In the compression group, numbers of VEGF-IR cells in the cauda equina and percentages of VEGF-IR neurons in DRG were significantly increased at days 3, 14, and 28 after surgery compared with the sham-operated group (*P < 0.05). Results are the mean ± standard deviation
Vascular density
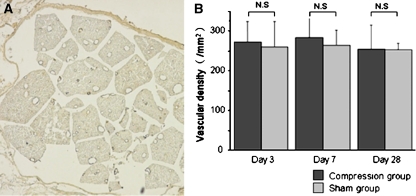
Vascular endothelial cells in the cauda equina exhibited immunoreactivity for Factor VIII, (Fig. 5a). There were no significant differences in vascular density between the compression and sham-operated groups at 3, 7, or 28 days after surgery (Fig. 5b).
Fig. 5.
a Photomicrographs demonstrating Factor VIII-immunoreactive blood vessels in the cauda equina of sham-operated rat at day 3 after surgery. Blood vessels are visible after immunostaining for factor VIII. Scale bar 200 μm. b Histogram presenting vascular density in the cauda equina. There were no significant differences between the compression and sham-operated groups at any time point. Results are the mean ± standard deviation of vascular density
Discussion
In the present investigation the walking time decreased after cauda equina compression from day 1 to day 14. Expression of VEGF in the cauda equina and DRG was found after cauda equina compression. VEGF-IR cells in the cauda equina and DRG were increased at 3, 14, and 28 days after cauda equina compression, compared with sham-operated rats. These findings suggest that single-level compression of the cauda equina by insertion of a silicone block can reduce walking capacity and induce VEGF expression in cauda equina and DRG.
In the subacute phase, e.g., day 7 after compression, no increase in VEGF-IR cells was observed. VEGF expression in cauda equina and DRG thus appeared to be induced not only by acute compression, but also by chronic, continuous compression of cauda equina. In this study, cauda equina compression was induced by placing a silicone block into the L5 epidural space. When the block was inserted into the epidural space, acute compression of the cauda equina occurred. This limits the interpretation of the data since the onset of clinical spinal canal stenosis is much more gradual. The increase in VEGF-IR cells at day 3 might have been a direct effect of the compression being acute. However, the increase in VEGF expression at 14 or 28 days after cauda equina compression might have been due to chronic, continuous compression of the cauda equina, since the increase in VEGF expression at day 3 had disappeared by day 7, and was observed again at day 14 and day 28. These findings suggest that chronic cauda equina compression induces VEGF expression in compressed cauda equina and related DRG. In this animal model, the L5 DRG was not directly compressed, but the nerve root proximal to the L5 DRG was indirectly compressed by silicone block with decrease of spinal canal volume. Some early studies showed that VEGF expression in DRG is induced by axonal injury, and that VEGF is axonally transported and released at the site of axonal injury [21, 22]. The expression of VEGF we observed in DRG might have resulted from compression of the nerve root proximal to DRG, and VEGF may have acted at the site of compression of cauda equina. However, in our immunohistochemical examination, axons in the cauda equina did not show immunoreactivity for VEGF. Therefore, further studies are needed to explain the axonal transport of VEGF. Vascular density in the cauda equina was not increased at any of the time points examined after cauda equina compression. VEGF expression alone was observed in the compressed part of cauda equina and DRG, and was not followed by angiogenesis. It is possible that a longer period of observation associated with significant recovery of walking ability is needed to detect angiogenesis. In our animal model, cauda equina blood flow might have been reduced during walking exercise, since walking time was significantly decreased. However, cauda equina blood flow was not examined in this study. More severe compression might thus be needed to reduce blood flow enough to induce angiogenesis. On the other hand, while VEGF was originally discovered as an angiogenic factor, recent studies have indicated that it also has direct neurotrophic effects on the central and peripheral nervous systems. VEGF stimulates axonal outgrowth and enhances the survival of superior cervical and DRG neurons [21, 22], promotes neurogenesis in vitro and in vivo [7], and improves neuronal function and decreases degeneration after experimental spinal cord injury [26]. Another study suggested that endogenous VEGF has effects on neurons [12]. Therefore, expression of VEGF itself might be related to neuronal adaptation to cauda equina compression, via angiogenic as well as neurotrophic effects. Additional studies are needed to clarify the roles played by VEGF in the compressed cauda equina.
Acknowledgments
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The authors thank Kenji Ono, Kazuo Sasaki and Satosi Sato for their technical support.
References
- 1.Baker AR, Collins TA, Poter RW, et al. Laser doppler study of procaine cauda equina blood flow. The effect of electrical stimulation of the rootlets during single and double site, low pressure compression of the cauda equina. Spine. 1995;20:660–664. doi: 10.1097/00007632-199503150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Banai S, Chandra M, Lazarovici, et al. Upregulation of vascular endothelial growth factor expression induced by myocardial ischemia: implication for coronary angiogenesis. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Abe K, Suzuki H, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Sakurai M, Abe K, et al. Expression of angiogenic factor in rabbit spinal cord after transient ischemia. Neuropathol Appl Neurobiol. 1999;25:63–71. doi: 10.1046/j.1365-2990.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Jespersen SM, Hansen ES, Hoy K, et al. Two-level spinal stenosis in minipig. Hemodynamic effect of exercise. Spine. 1995;20:2765–2773. doi: 10.1097/00007632-199512150-00020. [DOI] [PubMed] [Google Scholar]
- 7.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi S, Konno S, Kayama S, et al. Increased resistance to acute compression injury in chronically compressed spinal nerve roots: an experimental study. Spine. 1996;21:2544–2550. doi: 10.1097/00007632-199611150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Konno S, Arai I, Otani K, et al. Effects of beraprost sodium on canine cauda equina function and blood flow using a chronic spinal cord compression model. J Spinal Disord. 2001;14:336–338. doi: 10.1097/00002517-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs Z, Ikezaki K, Samoto K, et al. VEGF and flt: expression time kinetics in rat brain infarction. Stroke. 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga T, Warltier DC, Tessmer J, et al. Expression of VEGF, Angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ. 2003;285:H352–H358. doi: 10.1152/ajpheart.00621.2002. [DOI] [PubMed] [Google Scholar]
- 12.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and Autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 13.Olmarker K, Rydevik B, Holm S, et al. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res. 1989;7:817–823. doi: 10.1002/jor.1100070607. [DOI] [PubMed] [Google Scholar]
- 14.Ooi Y, Mita F, Satoh Y. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine. 1990;15:544–549. doi: 10.1097/00007632-199006000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Otani K, Kayama S, Mao GP, et al. Tolerance to acute compression injury and recovery of nerve function in chronically compressed spinal nerve root: experimental study. J Orthop Sci. 1997;2:266–270. doi: 10.1007/BF02489047. [DOI] [Google Scholar]
- 16.Otani K, Kikuchi S, Konno S, et al. Blood flow measurement in experimental chronic cauda equina compression in dogs: changes in blood flow at various conditions. J Spinal Disord. 2001;14:343–346. doi: 10.1097/00002517-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Pola R, Gaetani E, Flex A, et al. Peripheral nerve ischemia: apolipoprotein E deficiency results in impaired functional recovery and reduction of associated intraneural angiogenic response. Exp Neurol. 2003;184:264–273. doi: 10.1016/S0014-4886(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 18.Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis. Relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Shweiki D, Itin D, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate Hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 20.Skold M, Cullheim S, Hammarberg H, et al. Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur J Neurosci. 2000;12:3675–3686. doi: 10.1046/j.1460-9568.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- 21.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816X.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Olmarker K, Holm S, et al. Double-level cauda equina compression: an experimental study with continuous monitoring of intraneural blood flow in the porcine cauda equina. J Orthop Res. 1993;11:104–109. doi: 10.1002/jor.1100110112. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi N, Konno S, Kikuchi S. Histologic and functional study on cauda equina adhesion induced by multiple level laminectomy. Spine. 2003;28:4–8. doi: 10.1097/00007632-200301010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Takenobu K, Katsube N, Marsala M, et al. Model of neuropathic intermittent claudication in the rat: methodology and application. J Neursci Meth. 2001;104:191–198. doi: 10.1016/S0165-0270(00)00342-3. [DOI] [PubMed] [Google Scholar]
- 26.Widenfalk J, Lipson A, Jubran M, et al. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neurosci. 2003;120:951–960. doi: 10.1016/S0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi K, Murakami M, Takahashi K, et al. Behavioral and morphologic studies of the chronically compressed cauda equina: experimental model of lumbar spinal stenosis in the rats. Spine. 1999;9:845–851. doi: 10.1097/00007632-199905010-00003. [DOI] [PubMed] [Google Scholar]